Hydrogels Based on Dynamic Covalent and Non Covalent Bonds: A Chemistry Perspective
Abstract
:1. Introduction
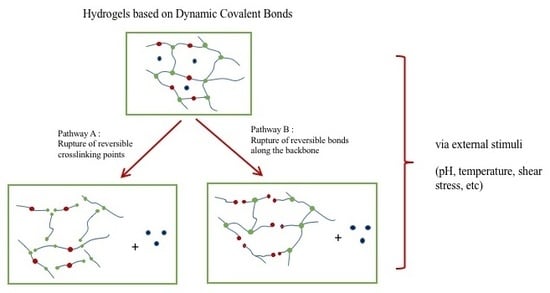
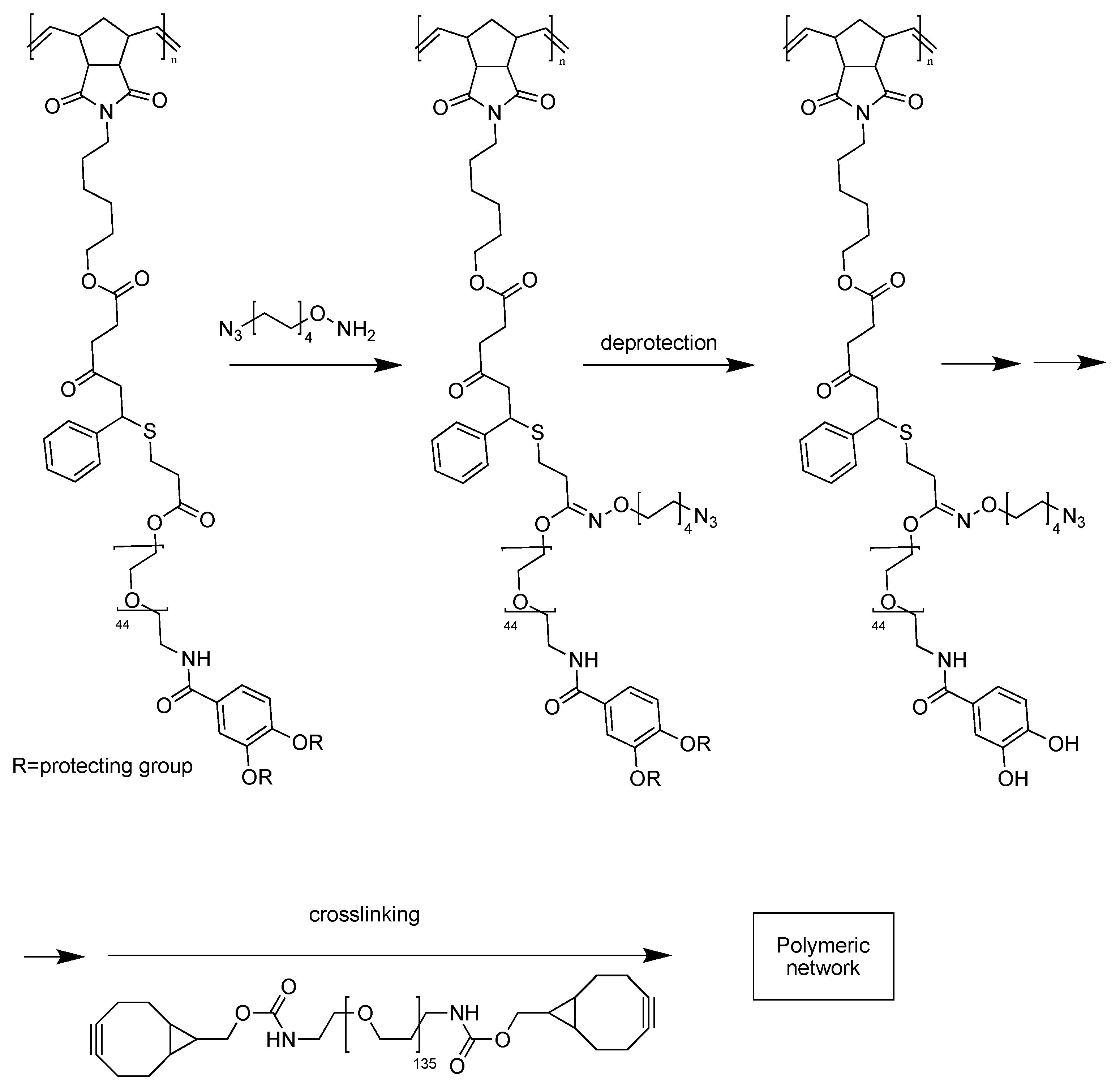
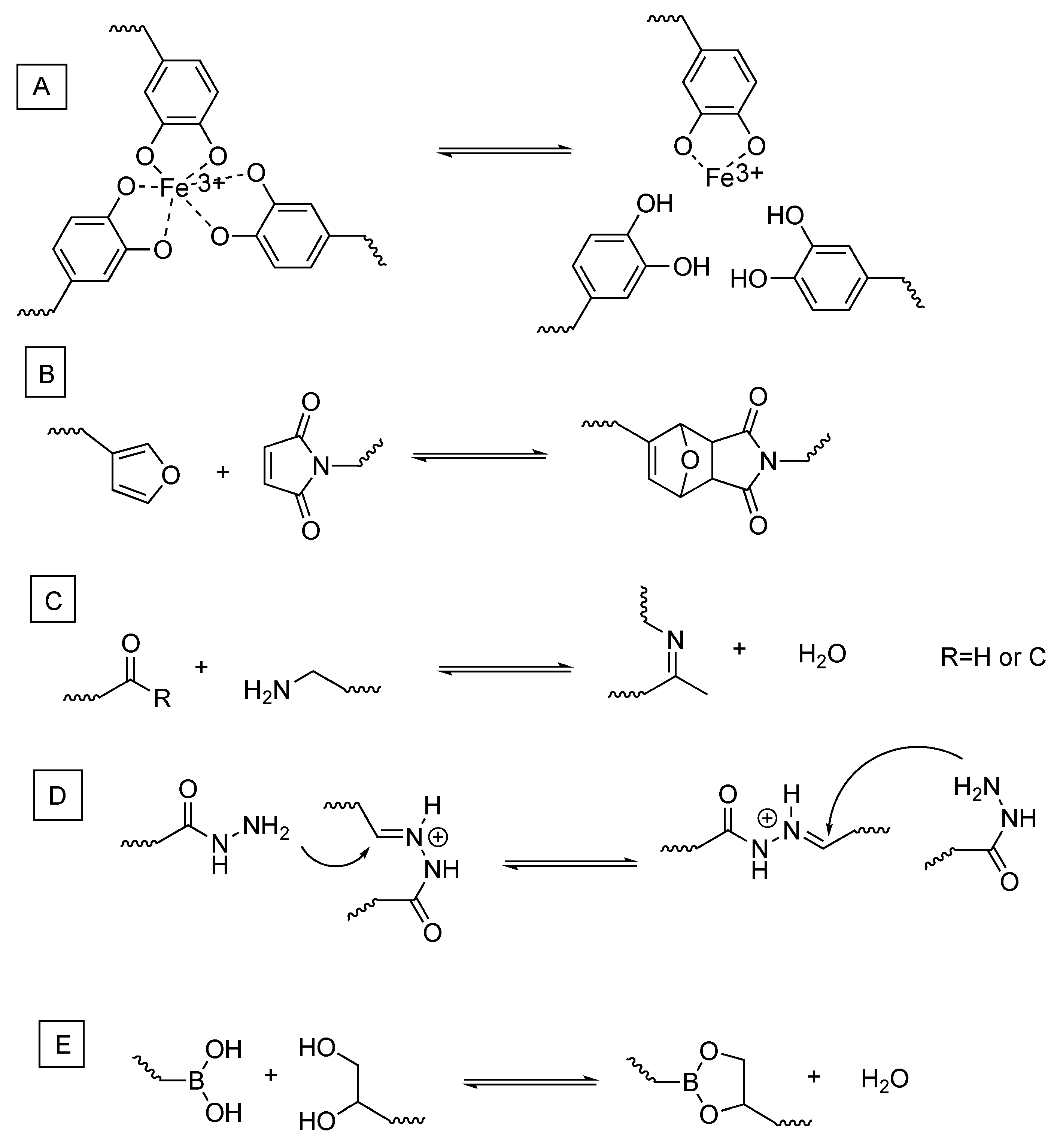
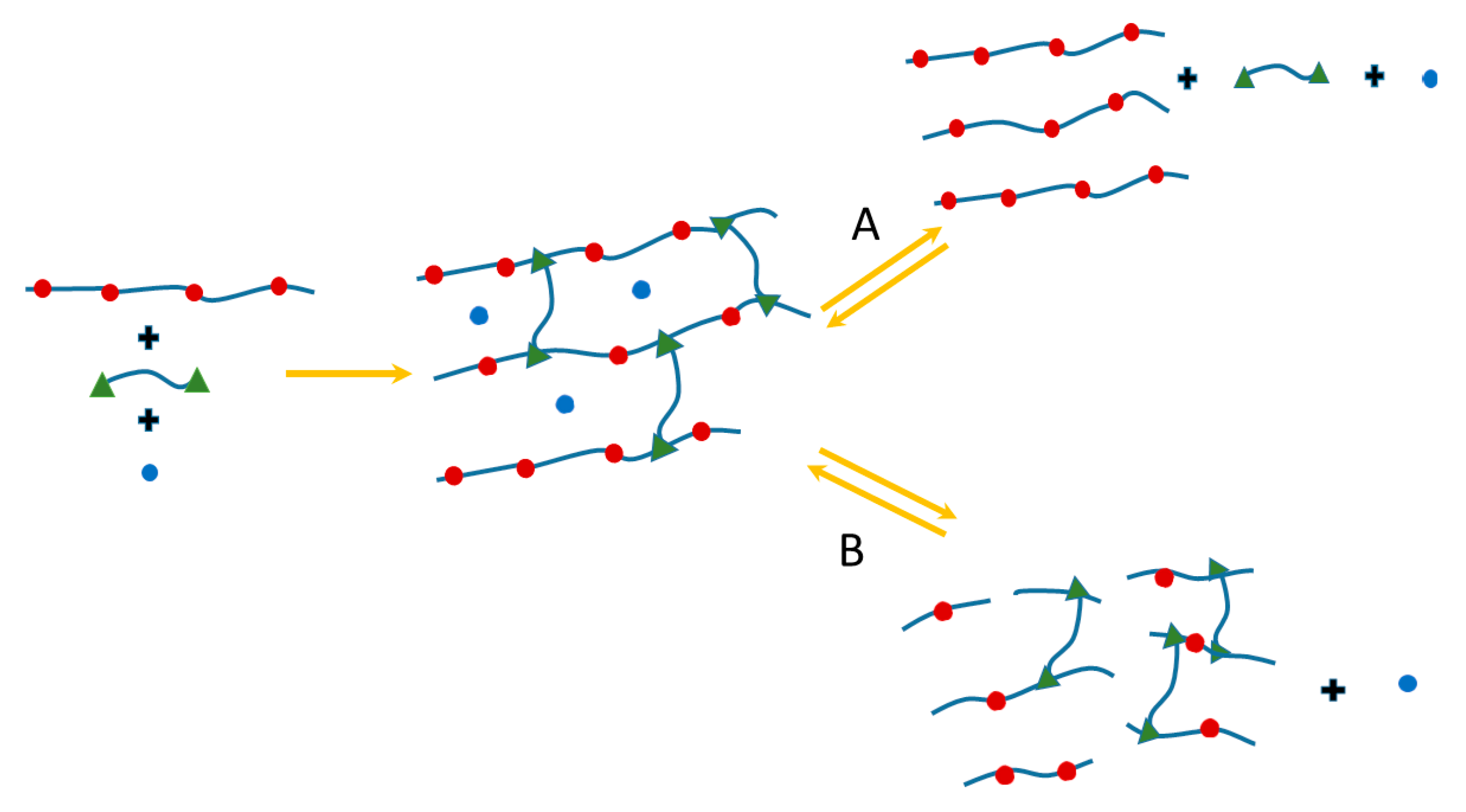
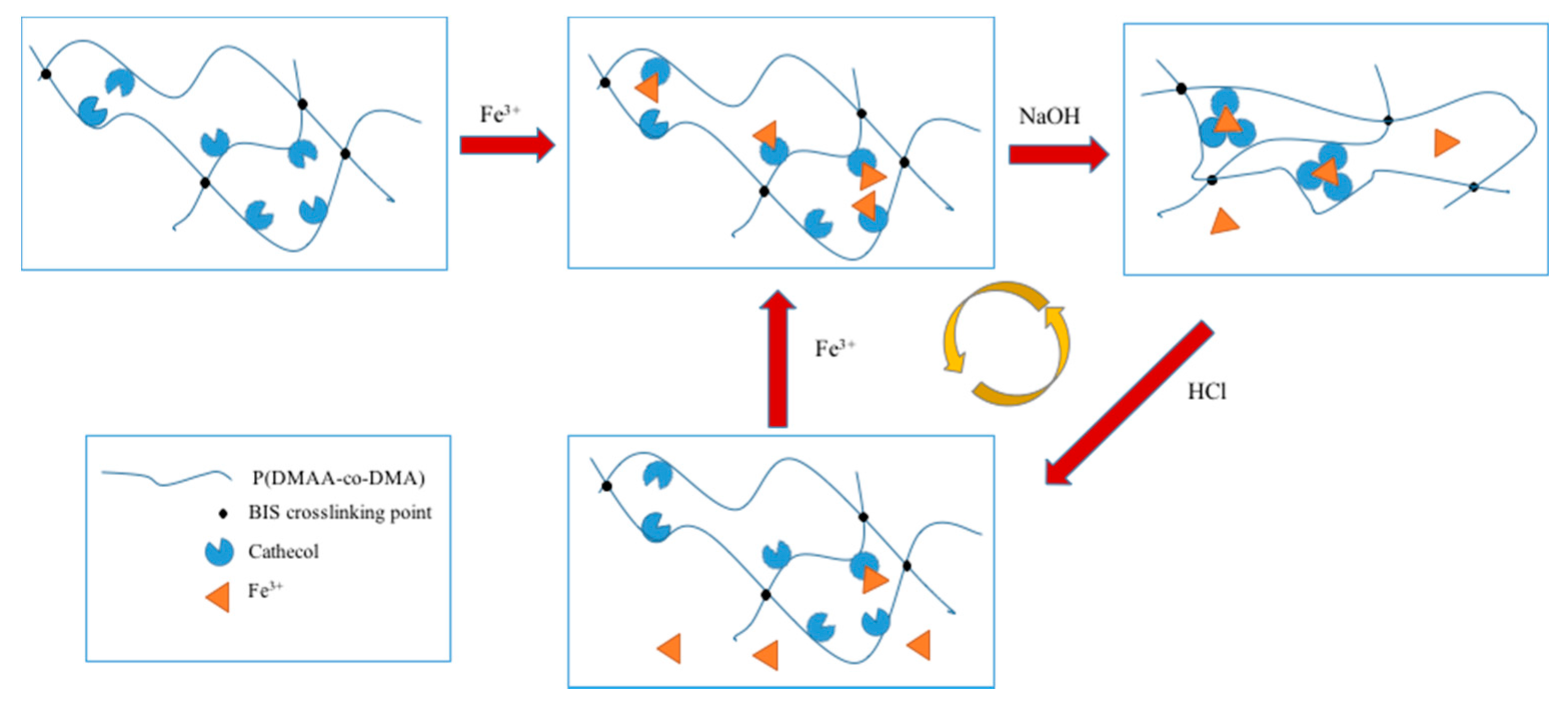
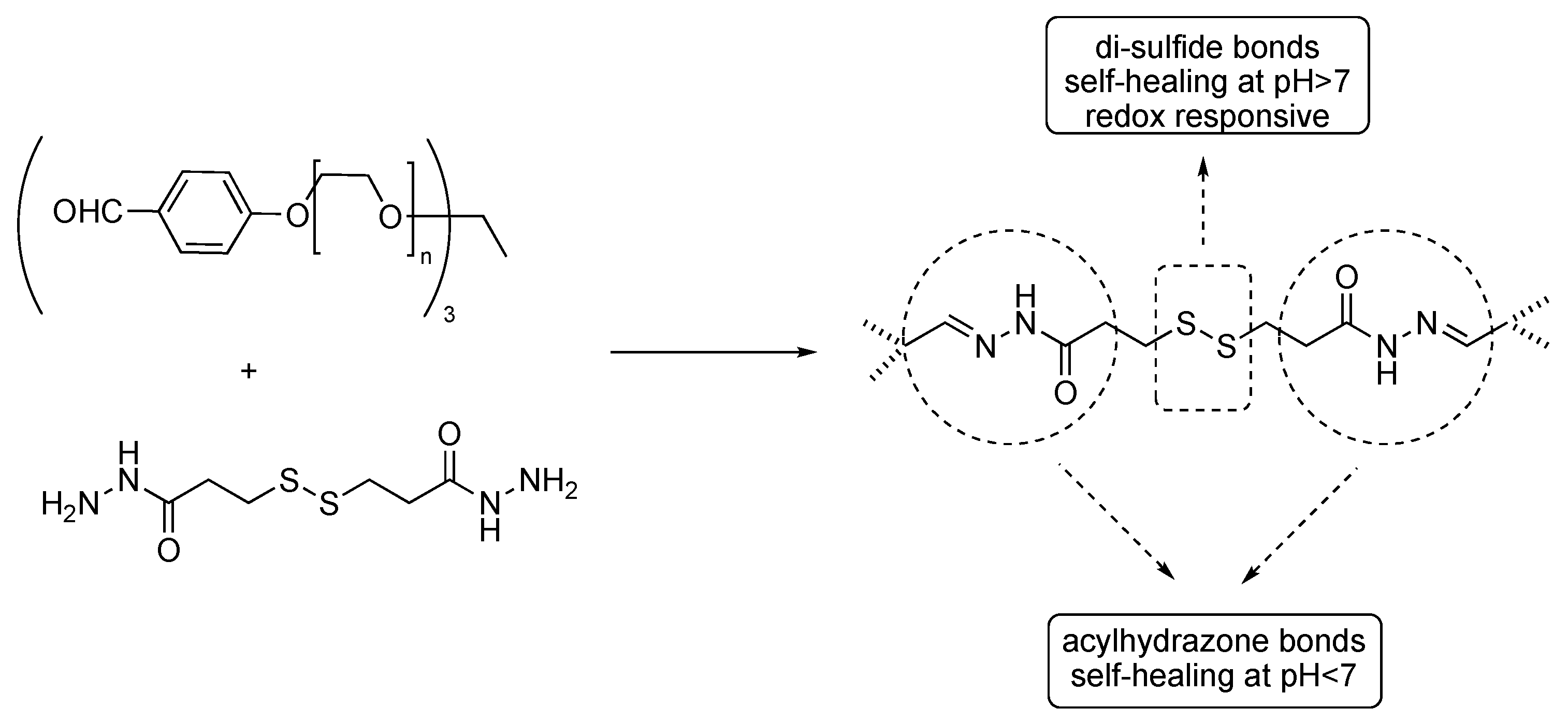
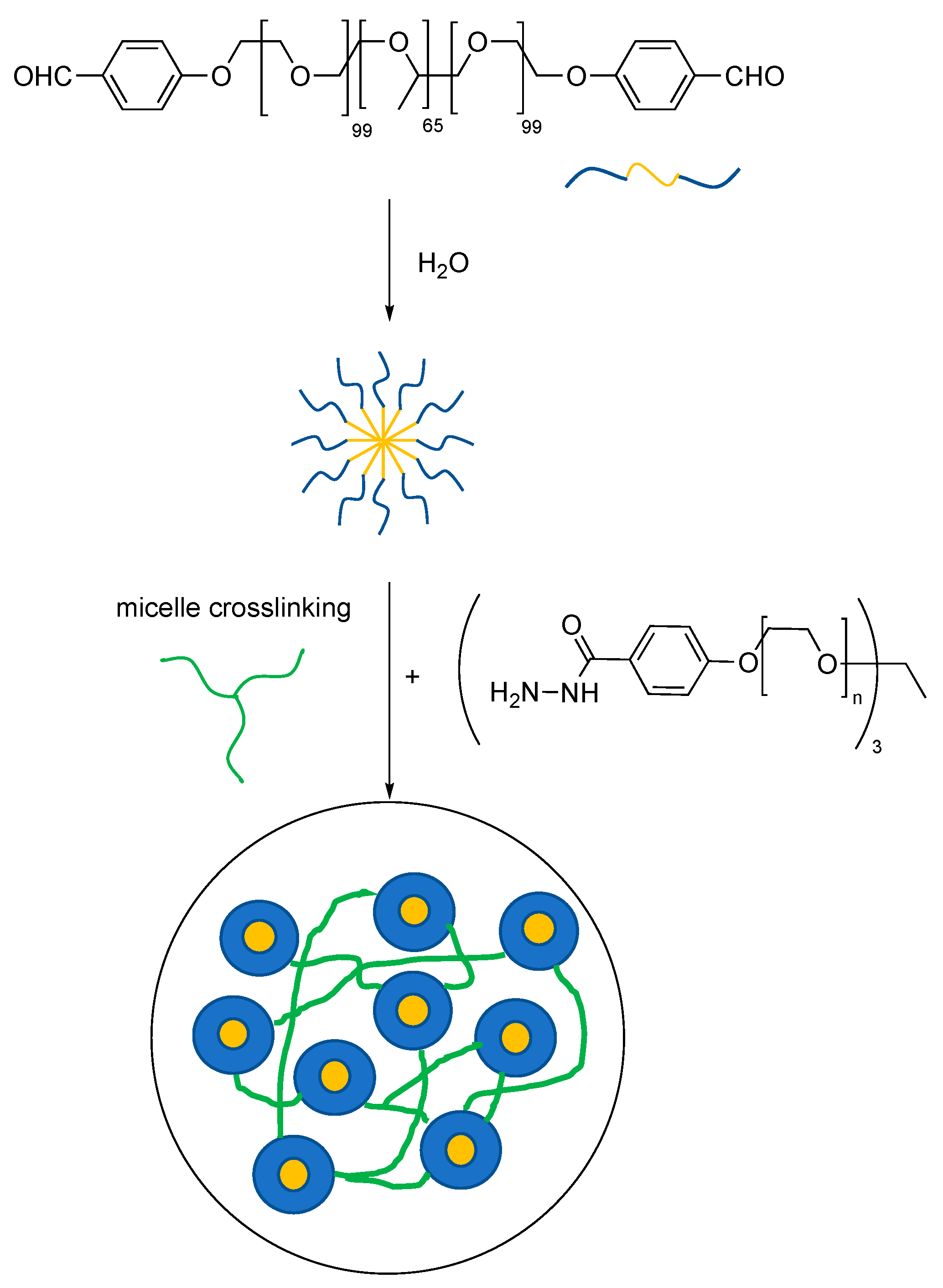
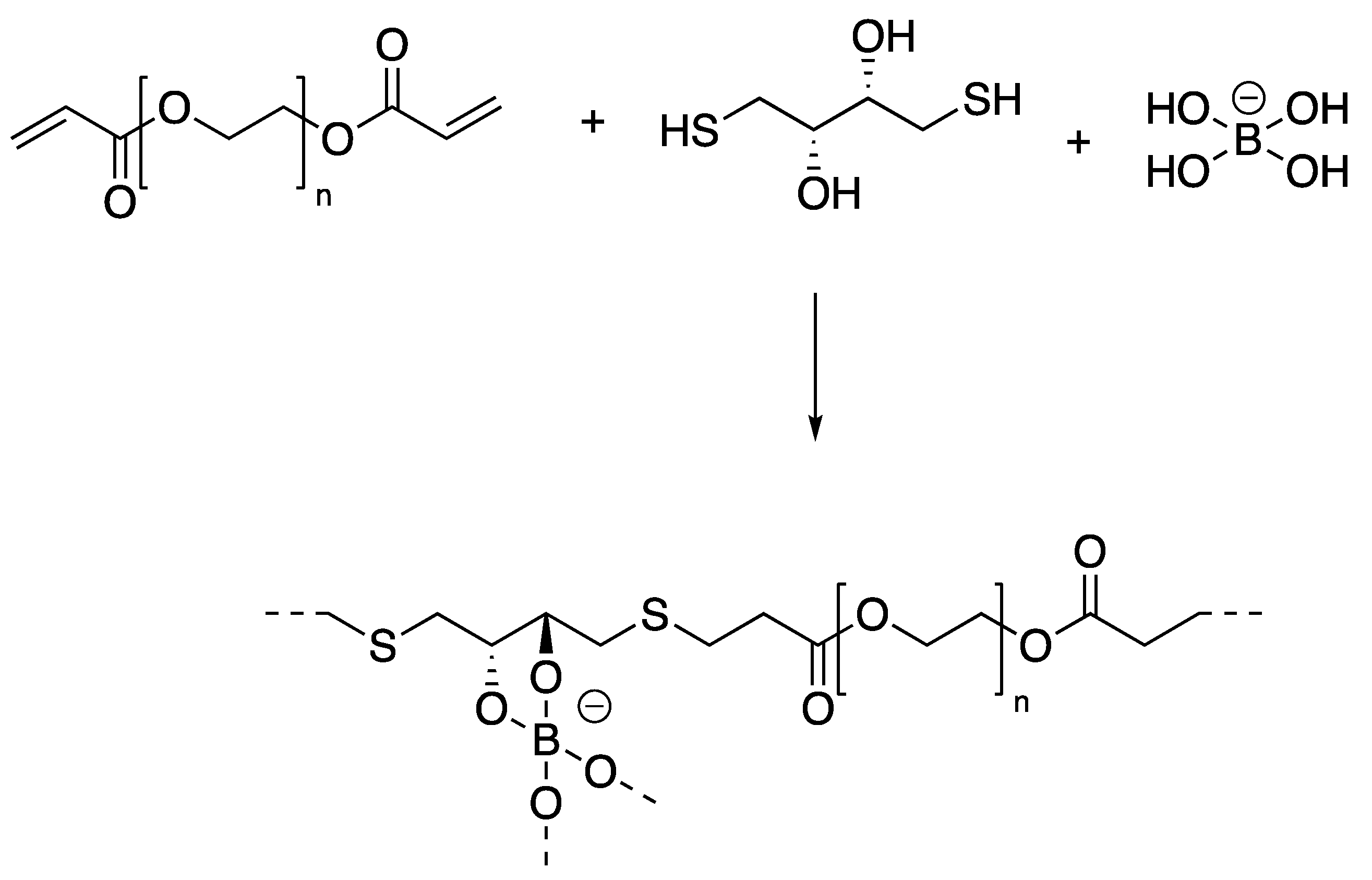
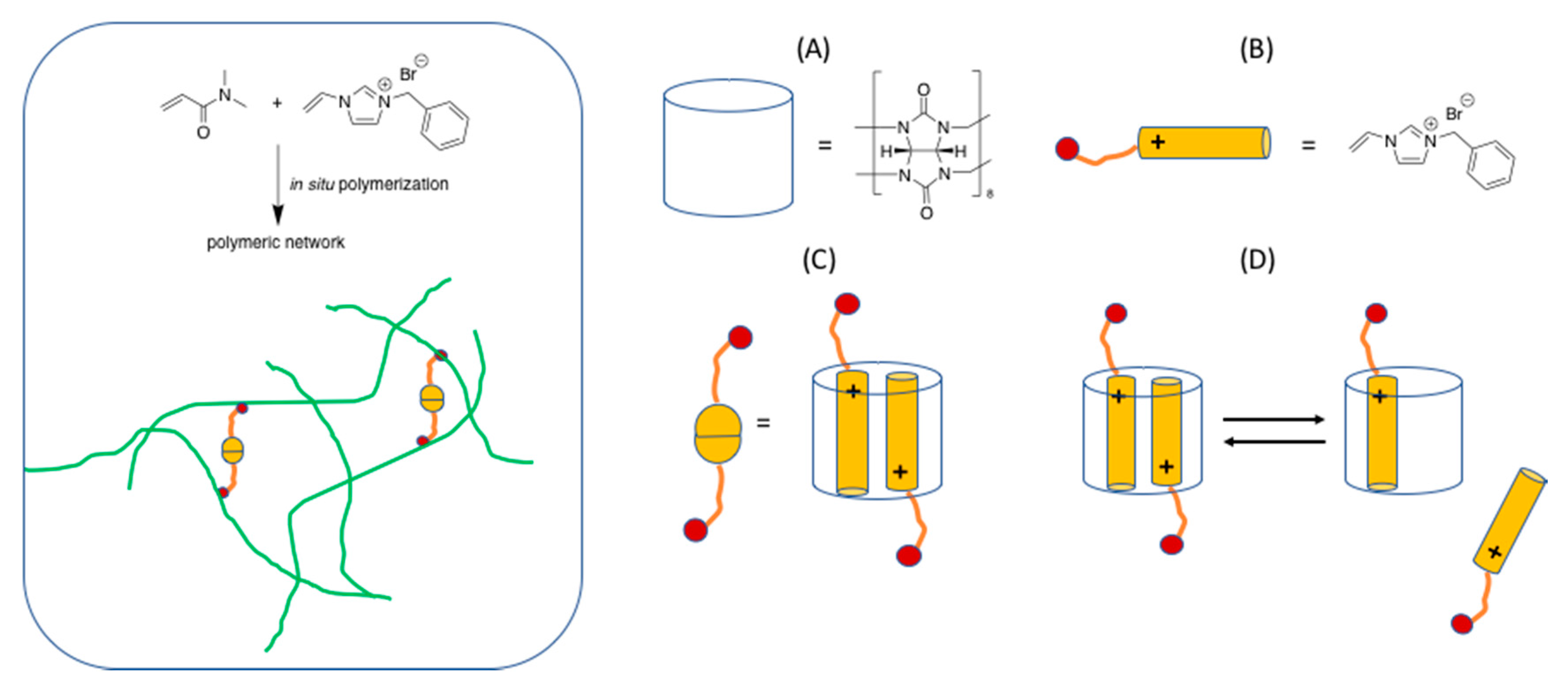
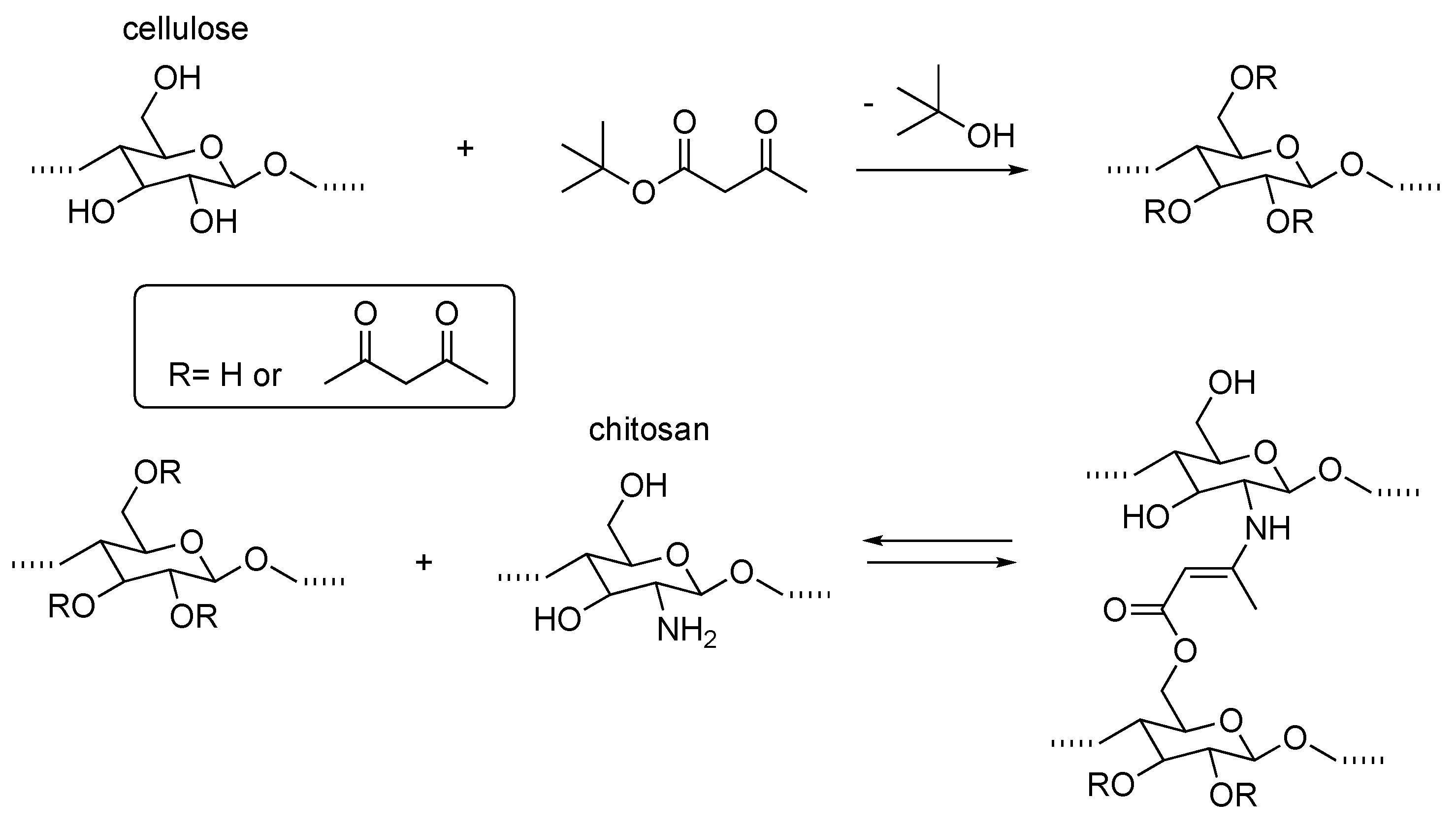
2. Dynamic Hydrogels Based on Reversible (Covalent) Interactions
3. Conclusions and Future Perspectives
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yin, Y.; Liu, Y.; Lin, L.; Liu, M. Design and fabrication of functional hydrogels through interfacial engineering. Chin. J. Polym. Sci. 2017, 35, 1181–1193. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, K.; Jiao, T.; Xing, R.; Hong, W.; Zhang, L.; Zhang, Q.; Peng, Q. Preparation of graphene oxide-polymer composite hydrogels via thiol-ene photopolymerization as efficient dye adsorbents for wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 668–676. [Google Scholar] [CrossRef]
- Piatkowski, M.; Janus, L.; Radwan-Praglowska, J.; Raclavsky, K. Microwave-enhanced synthesis of biodegradable multifunctional chitosan hydrogels for wastewater treatment. Express Polym. Lett. 2017, 11, 809–819. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, J.; Li, Y.; Wu, J.; Yu, F.; Chen, Y. An aptamer-patterned hydrogel for the controlled capture and release of proteins via biorthogonal click chemistry and DNA hybridization. J. Mater. Chem. B 2017, 5, 5974–5982. [Google Scholar] [CrossRef]
- Yang, D.H.; Seo, D.I.; Lee, D.; Bhang, S.H.; Park, K.; Jang, G.; Kim, C.H.; Chun, H.J. Preparation and evaluation of visible-light cured glycol chitosan hydrogel dressing containing dual growth factors for accelerated wound healing. J. Ind. Eng. Chem. 2017, 53, 360–370. [Google Scholar] [CrossRef]
- Xu, L.; Cooper, R.C.; Wang, J.; Yeudall, W.A.; Yang, H. Synthesis and Application of Injectable Bioorthogonal Dendrimer Hydrogels for Local Drug Delivery. ACS Biomater. Sci. Eng. 2017, 3, 1641–1653. [Google Scholar] [CrossRef] [PubMed]
- Gregoritza, M.; Messmann, V.; Abstiens, K.; Brandl, F.P.; Goepferich, A.M. Controlled Antibody Release from Degradable Thermoresponsive Hydrogels Cross-Linked by Diets-Alder Chemistry. Biomacromolecules 2017, 18, 2410–2418. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Cao, X.; Du, J.; Wang, G.; Chen, X. Multifunctional Hydrogel with Good Structure Integrity, Self-Healing, and Tissue-Adhesive Property Formed by Combining Die Is Alder Click Reaction and Acylhydrazone Bond. ACS Appl. Mater. Interfaces 2015, 7, 24023–24031. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Bai, X.; Liu, H.; Ning, P.; Wang, Z.; Gao, C.; Ni, B.; Liu, M. An injectable and self-healing hydrogel with covalent cross-linking in vivo for cranial bone repair. J. Mater. Chem. B 2017, 5, 3739–3748. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, H.; Trinh, P.; Heilshorn, S.C.; Yang, F. Elastin-like protein-hyaluronic acid (ELP-HA) hydrogels with decoupled mechanical and biochemical cues for cartilage regeneration. Biomaterials 2017, 127, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Ono, R.J.; Lee, A.L.Z.; Voo, Z.X.; Venkataraman, S.; Koh, B.W.; Yang, Y.Y.; Hedrick, J.L. Biodegradable Strain-Promoted Click Hydrogels for Encapsulation of Drug-Loaded Nanoparticles and Sustained Release of Therapeutics. Biomacromolecules 2017, 18, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Derry, M.J.; Mable, C.J.; Ning, Y.; Armes, S.P. Using Dynamic Covalent Chemistry to Drive Morphological Transitions: Controlled Release of Encapsulated Nanoparticles from Block Copolymer Vesicles. J. Am. Chem. Soc. 2017, 139, 7616–7623. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Zhang, Y.; He, L.; Wu, S.; Li, B.; Li, Y. Fabrication of nanoemulsion-filled alginate hydrogel to control the digestion behavior of hydrophobic nobiletin. LWT-Food Sci. Technol. 2017, 82, 260–267. [Google Scholar] [CrossRef]
- Slegeris, R.; Ondrusek, B.A.; Chung, H. Catechol- and ketone-containing multifunctional bottlebrush polymers for oxime ligation and hydrogel formation. Polym. Chem. 2017, 8, 4707–4715. [Google Scholar] [CrossRef]
- Fu, S.; Dong, H.; Deng, X.; Zhuo, R.; Zhong, Z. Injectable hyaluronic acid/poly(ethylene glycol) hydrogels crosslinked via strain-promoted azide-alkyne cycloaddition click reaction. Carbohydr. Polym. 2017, 169, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Guaresti, O.; Garcia-Astrain, C.; Palomares, T.; Alonso-Varona, A.; Eceiza, A.; Gabilondo, N. Synthesis and characterization of a biocompatible chitosan-based hydrogel cross-linked via ‘click’ chemistry for controlled drug release. Int. J. Biol. Macromol. 2017, 102, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Marin, L.; Ailincai, D.; Morariu, S.; Tartau-Mititelu, L. Development of biocompatible glycodynameric hydrogels joining two natural motifs by dynamic constitutional chemistry. Carbohydr. Polym. 2017, 170, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Selegard, R.; Aronsson, C.; Brommesson, C.; Danmark, S.; Aili, D. Folding driven self-assembly of a stimuli-responsive peptide-hyaluronan hybrid hydrogel. Sci. Rep. 2017, 7, 7013. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Carstensen, H.; Hoelzl, K.; Lunzer, M.; Li, H.; Hilborn, J.; Ovsianikov, A.; Ossipov, D.A. Dynamic Coordination Chemistry Enables Free Directional Printing of Biopolymer Hydrogel. Chem. Mater. 2017, 29, 5816–5823. [Google Scholar] [CrossRef]
- Tsurkan, M.V.; Jungnickel, C.; Schlierf, M.; Werner, C. Forbidden Chemistry: Two-Photon Pathway in [2 + 2] Cycloaddition of Maleimides. J. Am. Chem. Soc. 2017, 139, 10184–10187. [Google Scholar] [CrossRef] [PubMed]
- Casuso, P.; Odriozola, I.; Perez-San Vicente, A.; Loinaz, I.; Cabanero, G.; Grande, H.; Dupin, D. Injectable and Self-Healing Dynamic Hydrogels Based on Metal(I)-Thiolate/Disulfide Exchange as Biomaterials with Tunable Mechanical Properties. Biomacromolecules 2015, 16, 3552–3561. [Google Scholar] [CrossRef] [PubMed]
- Barcan, G.A.; Zhang, X.; Waymouth, R.M. Structurally Dynamic Hydrogels Derived from 1,2-Dithiolanes. J. Am. Chem. Soc. 2015, 137, 5650–5653. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Shan, M.; Li, B.; Wu, G. Injectable dual redox responsive diselenide-containing poly(ethylene glycol) hydrogel. J. Biomed. Mater. Res. Part A 2017, 105, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, D.E.; Mahon, C.S.; Fulton, D.A. Thermoresponsive Dynamic Covalent Single-Chain Polymer Nanoparticles Reversibly Transform into a Hydrogel. Angew. Chem. Int. Ed. 2013, 52, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Wu, S.; Wang, S.; Xiong, Y.; Li, Y.; Li, B.; Deng, H.; Du, Y.; Xiao, L.; Shi, X. A dynamic and self-crosslinked polysaccharide hydrogel with autonomous self-healing ability. Soft Matter 2015, 11, 3971–3976. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Hill, M.R.; Sumerlin, B.S. Self-healing hydrogels containing reversible oxime crosslinks. Soft Matter 2015, 11, 6152–6161. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.; Liontos, G.; Avgeropoulos, A.; Voulgari, E.; Avgoustakis, K.; Tsitsilianis, C. Injectable Hydrogel: Amplifying the pH Sensitivity of a Triblock Copolypeptide by Conjugating the N-Termini via Dynamic Covalent Bonding. ACS Appl. Mater. Interfaces 2016, 8, 17539–17548. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sui, X.; Xu, H.; Zhang, L.; Zhong, Y.; Mao, Z. Self-Healing Polysaccharide Hydrogel Based on Dynamic Covalent Enamine Bonds. Macromol. Mater. Eng. 2016, 301, 725–732. [Google Scholar] [CrossRef]
- Chang, R.; Wang, X.; Li, X.; An, H.; Qin, J. Self-Activated Healable Hydrogels with Reversible Temperature Responsiveness. ACS Appl. Mater. Interfaces 2016, 8, 25544–25551. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, D.; Paul, A.; Cai, L.; Enejder, A.; Yang, F.; Heilshorn, S.C. Covalently Adaptable Elastin-Like Protein-Hyaluronic Acid (ELP-HA) Hybrid Hydrogels with Secondary Thermoresponsive Crosslinking for Injectable Stem Cell Delivery. Adv. Funct. Mater. 2017, 27, 1605609. [Google Scholar] [CrossRef]
- Yang, X.; Liu, G.; Peng, L.; Guo, J.; Tao, L.; Yuan, J.; Chang, C.; Wei, Y.; Zhang, L. Highly Efficient Self-Healable and Dual Responsive Cellulose-Based Hydrogels for Controlled Release and 3D Cell Culture. Adv. Funct. Mater. 2017, 27, 1703174. [Google Scholar] [CrossRef]
- Wang, P.; Deng, G.; Zhou, L.; Li, Z.; Chen, Y. Ultrastretchable, Self-Healable Hydrogels Based on Dynamic Covalent Bonding and Triblock Copolymer Micellization. ACS Macro Lett. 2017, 6, 881–886. [Google Scholar] [CrossRef]
- Yang, T.; Ji, R.; Deng, X.; Du, F.; Li, Z. Glucose-responsive hydrogels based on dynamic covalent chemistry and inclusion complexation. Soft Matter 2014, 10, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Szopinski, D.; Wu, Y.; Luinstra, G.A.; Theato, P. Toward Self-Healing Hydrogels Using One-Pot Thiol-Ene Click and Borax-Diol Chemistry. ACS Macro Lett. 2015, 4, 673–678. [Google Scholar] [CrossRef]
- Seidler, C.; Ng, D.Y.W.; Weil, T. Native protein hydrogels by dynamic boronic acid chemistry. Tetrahedron 2017, 73, 4979–4987. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Ma, R.; Xu, Y.; Zhang, Y.; Li, B.; An, Y.; Shi, L. A G-Quadruplex Hydrogel via Multicomponent Self-Assembly: Formation and Zero-Order Controlled Release. ACS Appl. Mater. Interfaces 2017, 9, 13056–13067. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Huang, J.; Wang, T.; Sun, W.; Tong, Z. Rapid shape memory and pH-modulated spontaneous actuation of dopamine containing hydrogels. Chin. J. Polym. Sci. 2017, 35, 1297–1306. [Google Scholar] [CrossRef]
- Deng, G.; Li, F.; Yu, H.; Liu, F.; Liu, C.; Sun, W.; Jiang, H.; Chen, Y. Dynamic Hydrogels with an Environmental Adaptive Self-Healing Ability and Dual Responsive Sol-Gel Transitions. ACS Macro Lett. 2012, 1, 275–279. [Google Scholar] [CrossRef]
- Chen, J.; Su, Q.; Guo, R.; Zhang, J.; Dong, A.; Lin, C.; Zhang, J. A Multitasking Hydrogel Based on Double Dynamic Network with Quadruple-Stimuli Sensitiveness, Autonomic Self-Healing Property, and Biomimetic Adhesion Ability. Macromol. Chem. Phys. 2017, 218, 1700166. [Google Scholar] [CrossRef]
- Collins, J.; Nadgorny, M.; Xiao, Z.; Connal, L.A. Doubly Dynamic Self-Healing Materials Based on Oxime Click Chemistry and Boronic Acids. Macromol. Rapid Commun. 2017, 38, 1600760. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, J.; Luo, Q.; Zhu, J.; Zou, H.; Gao, Y.; Wang, L.; Xu, J.; Dong, Z.; Liu, J. Dual stimuli-responsive supramolecular pseudo-polyrotaxane hydrogels. Soft Matter 2013, 9, 4635–4641. [Google Scholar] [CrossRef]
- Liu, J.; Tan, C.S.Y.; Lan, Y.; Scherman, O.A. Toward a Versatile Toolbox for Cucurbit[n]uril-Based Supramolecular Hydrogel Networks through In Situ Polymerization. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3105–3109. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Ma, Q.; Yu, H.; Zhang, Y.; Yan, Z.; Liu, F.; Liu, C.; Jiang, H.; Chen, Y. Macroscopic Organohydrogel Hybrid from Rapid Adhesion between Dynamic Covalent Hydrogel and Organogel. ACS Macro Lett. 2015, 4, 467–471. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, X.; Ren, H.; Meng, X.; Qiu, D. Reinforcement of polyacrylamide hydrogel with patched laponite-polymer composite particles. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 268–273. [Google Scholar] [CrossRef]
- Inal, M.; Isiklan, N.; Yigitoglu, M. Preparation and characterization of pH-sensitive alginate-g-poly(N-vinyl-2-pyrrolidone)/gelatin blend beads. J. Ind. Eng. Chem. 2017, 52, 128–137. [Google Scholar] [CrossRef]
- Wang, L.; Hui, X.; Geng, H.; Ye, L.; Zhang, A.; Shao, Z.; Feng, Z. Synthesis and gelation capability of mono- and disubstituted cyclo(L-Glu-L-Glu) derivatives with tyramine, tyrosine and phenylalanine. Colloid Polym. Sci. 2017, 295, 1549–1561. [Google Scholar] [CrossRef]
- Nunes, C.S.; Rufato, K.B.; Souza, P.R.; de Almeida, E.A.M.S.; da Silva, M.J.V.; Scariot, D.B.; Nakamura, C.V.; Rosa, F.A.; Martins, A.F.; Muniz, E.C. Chitosan/chondroitin sulfate hydrogels prepared in [Hmim][HSO4] ionic liquid. Carbohydr. Polym. 2017, 170, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Yesilyurt, V.; Webber, M.J.; Appel, E.A.; Godwin, C.; Langer, R.; Anderson, D.G. Injectable Self-Healing Glucose-Responsive Hydrogels with pH-Regulated Mechanical Properties. Adv. Mater. 2016, 28, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Czarnecki, S.; Rossow, T.; Seiffert, S. Hybrid Polymer-Network Hydrogels with Tunable Mechanical Response. Polymers 2016, 8, 82. [Google Scholar] [CrossRef]
- Raza-Karimi, A.; Khodadadi, A. Mechanically Robust 3D Nanostructure Chitosan-Based Hydrogels with Autonomic Self-Healing Properties. ACS Appl. Mater. Interfaces 2016, 8, 27254–27263. [Google Scholar] [CrossRef] [PubMed]
- Iftime, M.; Morariu, S.; Marin, L. Salicyl-imine-chitosan hydrogels: Supramolecular architecturing as a crosslinking method toward multifunctional hydrogels. Carbohydr. Polym. 2017, 165, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Wang, M.; Chang, H.; Xu, F.; Yang, J. A Self-Healing Cellulose Nanocrystal-Poly(ethylene glycol) Nanocomposite Hydrogel via Diels-Alder Click Reaction. ACS Sustain. Chem. Eng. 2017, 5, 6167–6174. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picchioni, F.; Muljana, H. Hydrogels Based on Dynamic Covalent and Non Covalent Bonds: A Chemistry Perspective. Gels 2018, 4, 21. https://doi.org/10.3390/gels4010021
Picchioni F, Muljana H. Hydrogels Based on Dynamic Covalent and Non Covalent Bonds: A Chemistry Perspective. Gels. 2018; 4(1):21. https://doi.org/10.3390/gels4010021
Chicago/Turabian StylePicchioni, Francesco, and Henky Muljana. 2018. "Hydrogels Based on Dynamic Covalent and Non Covalent Bonds: A Chemistry Perspective" Gels 4, no. 1: 21. https://doi.org/10.3390/gels4010021