1. Introduction
The production and storage of energy obtained from renewable sources, the reduction of CO
2 emissions into the atmosphere and its re-use are currently some of the main challenges for mankind. Artificial photosynthesis has been used in attempts to mimic the natural water splitting process and to use of the sunlight as an energy source to produce molecules that can be used as feedstock and for energy storage [
1,
2]. Therefore, efforts to develop renewable, carbon-neutral fuel sources have led to a new research and development focus on sunlight-driven water-splitting systems [
3]. Among these systems, photo-electrochemical (PEC) water splitting devices are used to exploit renewable sources, i.e., water and sunlight, to produce O
2 and H
2. Moreover, the generated hydrogen can also be used to reduce CO
2 for the production of either other C-containing fuels or high added-value chemicals (i.e., CO in syngas, CH
4, methanol and ethanol among others) [
4].
Many studies have been conducted with the aim of increasing the solar-to-hydrogen (STH) or solar-to-fuel (STF) conversion efficiencies of PEC devices [
5,
6,
7]. Most of them have been focused on improving the design of photo-electrocatalysts (semiconductors such as TiO
2 and ZnO, BiVO
4, Fe
2O
3, WO
3, Cu
2O/CuO) [
8,
9,
10] and catalysts (i.e., Pt, or Ir, Ru, Co or Mn oxides, etc.) [
11]; on the electrode structure modification (from the nano- to micro-scopic level) [
12,
13], on changes in the operative conditions (i.e., pressure, temperature, kind of electrolyte and ion concentrations ) [
14,
15] and on the development of different cell configurations (i.e., with a single photo-electrode, tandem photosystems with a Z scheme, photovoltaic (PV)-PEC photosystems and buried PV photo-systems without photo-electrodes) [
5,
6].
As far as PEC reactors are concerned, two-compartment PEC cells are generally preferred, from both the cost and safety viewpoints. In such a system, the O
2, produced through water oxidation at the anode, is separated directly from the reduction products (i.e., H
2, CO, etc.) that form at the cathode, due to the presence of a proton exchange membrane (PEM), which is used for both H
+ transport and to separate the anodic and cathodic chambers. The housing of such a reactor should have a transparent window, to allow sunlight irradiation, and current collectors, which can either be integrated with the electrodes or not. The overall cell potential of the electrolysis cells that has to be applied to a PEC electrolyzer can be calculated as the sum of: the minimum thermodynamic potential required for the total cell reaction (E°: Nernst potential); the kinetic overpotentials associated with the anodic and cathodic reactions at the catalyst surfaces (i.e., η
Ox, for O
2 evolution and η
Red for H
2 evolution or for the formation of other desired CO
2-reduction products, such as syngas [
16]); the concentration overpotential (η
conc) and the ohmic resistance losses (η
ohm), which depends on both the electrical contacts and the conductivity of the electrocatalyst substrates) [
3,
17]. η
Ox, η
Red and η
ohm depend on the electrode materials and electron transport, but η
conc, which is governed by the proton transport in the electrolyte, has been investigated less than the former ones.
Proton conduction is often more critical than electron conduction, since high ionic conductivity is difficult to achieve [
5]. Unlike polymeric exchange membrane (PEM) electrolyzers, in which the catalysts are closely coupled to the PEM forming membrane-electrode-assemblies (MEA), the liquid and PEM electrolytes in most PEC devices are both media for H
+ transport. In fact, there are few examples of transparent, conductive and porous electrode supports that can be used to form MEA in PEM photo-electrolyzes. However, in a recent work [
12], thin transparent quartz sheets, covered by fluorine-doped tin oxide (FTO), were laser-drilled and used as supports of both TiO
2 and Pt nanoparticles, thus constituting electrodes that were sandwiched with a Nafion membrane to form a transparent MEA for water splitting and H
2 production. In other works, photocatalysts and catalysts (e.g., TiO
2 and Pt powders) have been embedded with a suitable amount of conductive carbon black powder in Nafion membranes to form a MEA for H
2 production [
18]. Therefore, since liquid electrolytes generally have a lower level of conductivity than solid ones; the distance and the pathway for proton transport between electrode surfaces are important aspects in the design of PEC cells. Nocera and co-workers [
19] obtained a 2.5% STF conversion efficiency using a monolithic and wireless H
2 production device, in which protons generated at the front of the cell had to move around the electrode to reach the back, while a 4.7% STF conversion efficiency was obtained by employing a wired PEC system with facing electrodes and a shorter proton transport distance.
Microfluidic flow cells (MFCs) are suitable for use as PEM photo-electrolyzers and can be designed to minimize dead volumes and optimize retention times. MFCs have been demonstrated, in several experimental studies, to be effective reactors and versatile analytical tools for water splitting for H
2 production [
12,
20] and for the electro-reduction of CO
2 [
16,
21]. However, not much modelling work has been reported in the literature on such systems, although the electrolyte distribution at the device inlets and the fluid dynamics of the liquid electrolyte inside the electrode chambers both have a great influence on η
conc and on the performance of a PEC device. On one hand, they need to be optimized in order to guarantee interaction between the reactants (i.e., water, CO
2) and the catalytic surfaces. On the other hand, the bubbles of gaseous products (i.e., O
2, H
2, CO, etc.), which tend to remain attached to the electrocatalyst surface, need to be removed, in order to avoid a reduction in the reaction rate, due to a decrease in the effective active area and in the reactant concentration [
22]. Moreover, the influence of the solar incident flux on the flow field and temperature distribution can also have an effect on the (photo) catalyst efficiency, reaction rates and on the final PEC reactor performance. In some recent works, theoretical models have been developed to analyze the formation and accumulation of bubbles on BiVO
4 porous photocatalyst and Pt catalyst surfaces, and their influence on the photoelectric response (as a consequence of the O
2 and H
2 production rates) for different applied potentials under dark and light conditions [
22,
23]. In one of these models, which employs a percolation approach, it was shown that the photocurrent density decreases over the time due to bubbles generation, under static flow conditions at a fixed applied bias [
22]. It was also found that if the applied bias is raised, the current density increases proportionally, and as a result, a larger concentration of molecular oxygen covers the anodic electrode surface with bubbles in a shorter time. However, under light conditions, the critical value of the electrode coverage factor (β
c) increased for higher bias potentials [
22]. In the other model, the formation of bubbles has been modeled by using a kinetic equation at the interface, considering the adsorption mechanism, where the adsorption term, hypothesized to be proportional to the current density, describes the formation of the bubbles on the electrode, whereas the desorbing term takes into account the detachment of the bubbles from the electrode [
23]. Qureshi et al. [
21] performed numerical simulations of a two-chamber PEC reactor, in which both transport phenomena (i.e., Navier-Stokes equation), and an energy equation for the electrolyte and radiative transfer equation were included, and they found that both the H
2 production rate and STH efficiency were enhanced by increasing the solar incident flux from 500 to 2000 W/m
2, considering an Fe
2O
3 photoanode and a Pt cathode as a case study. Wu et al. [
21] presented a steady-state isothermal model for the electrochemical reduction of CO
2 to CO in a microfluidic flow cell, which integrates charge, mass, and momentum transport with electrochemistry for both the cathode and anode, in order to determine the kinetic parameters at different flow rates while varying the applied cell potentials.
Nevertheless, simulations from a macroscopic point of view focused on the optimization of the design of PEC devices have not been developed. CFD is a useful computing tool that uses numerical analysis to solve and investigate problems that involve fluid flows [
24,
25,
26]. It simulates the interaction of liquids and gases with surfaces defined by boundary conditions [
27]. This method has already been used to simulate photo-electrochemical reactors for hydrogen production [
28], and other types of fuel cells, such as solid oxide [
29] or redox cells [
30]. Multiphase problems can also be dealt with in CFD simulations, and they allow bubbles inside a liquid medium to be simulated [
31]. In this study, CFD simulations have been carried out to assess the most efficient design of a microfluidic PEC cell.
3. Results and Discussion
3.1. Hydrodynamics Inside the Photoelectrochemical Cells
The purpose of this study was to establish which hydrodynamic conditions favor a homogeneous liquid distribution inside the wall where a catalyst is located. Mixing is not required. Laminar behavior can therefore be expected to be the most suitable for this purpose. However, stagnant zones should be avoided.
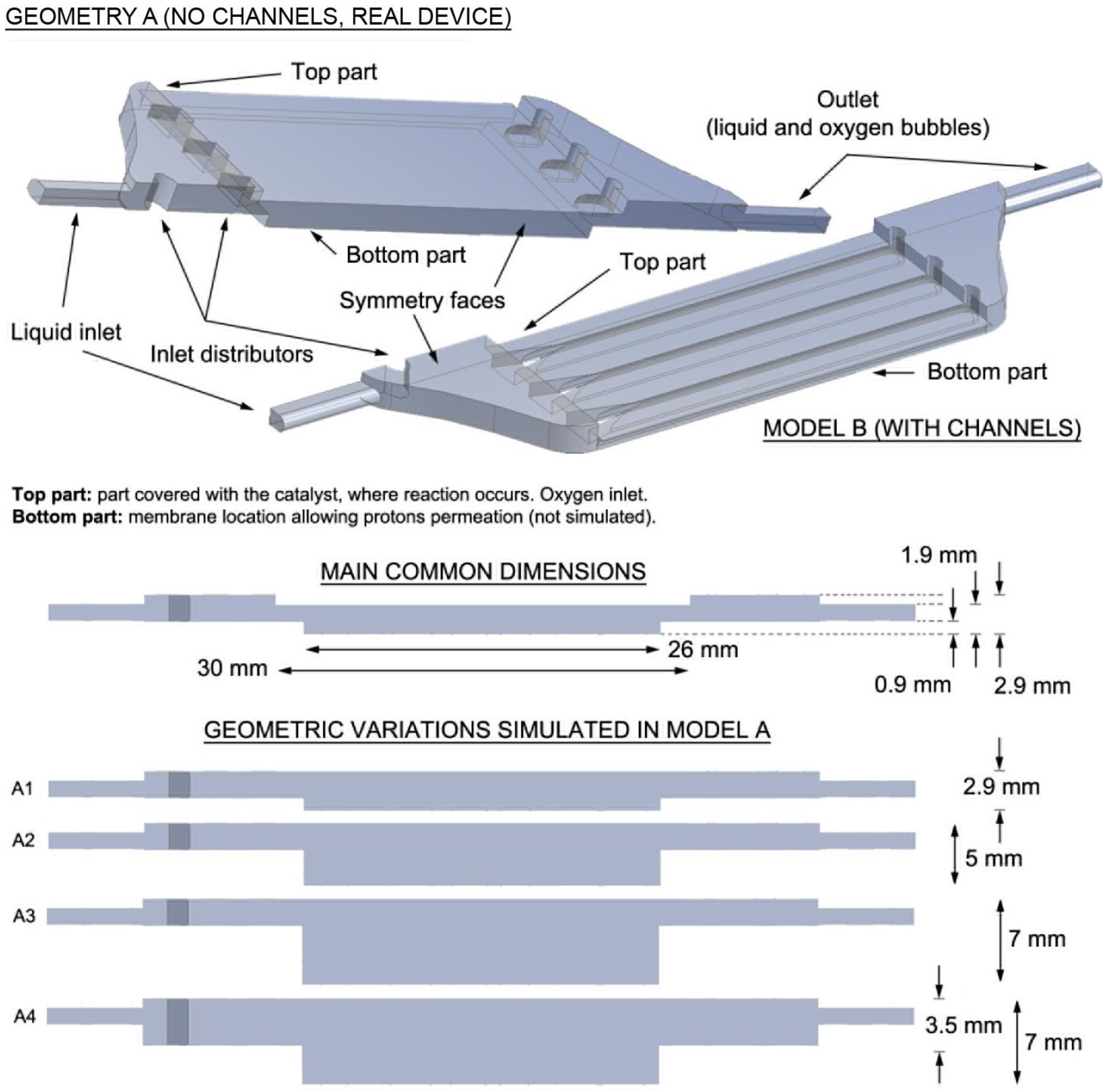
3.1.1. Geometry A of PEC Cell (Geometry without Channels)
Two parameters were studied for geometry A: inlet velocities and height of the central chamber. The inlet velocity parameter was first considered using the original height of the module. The height parameter was then analyzed with the velocity chosen at the end of the study.
Influence of the Inlet Velocity on Geometry A
Velocities of 0.1, 0.5, 1.0 and 2.0 m/s were considered for this study.
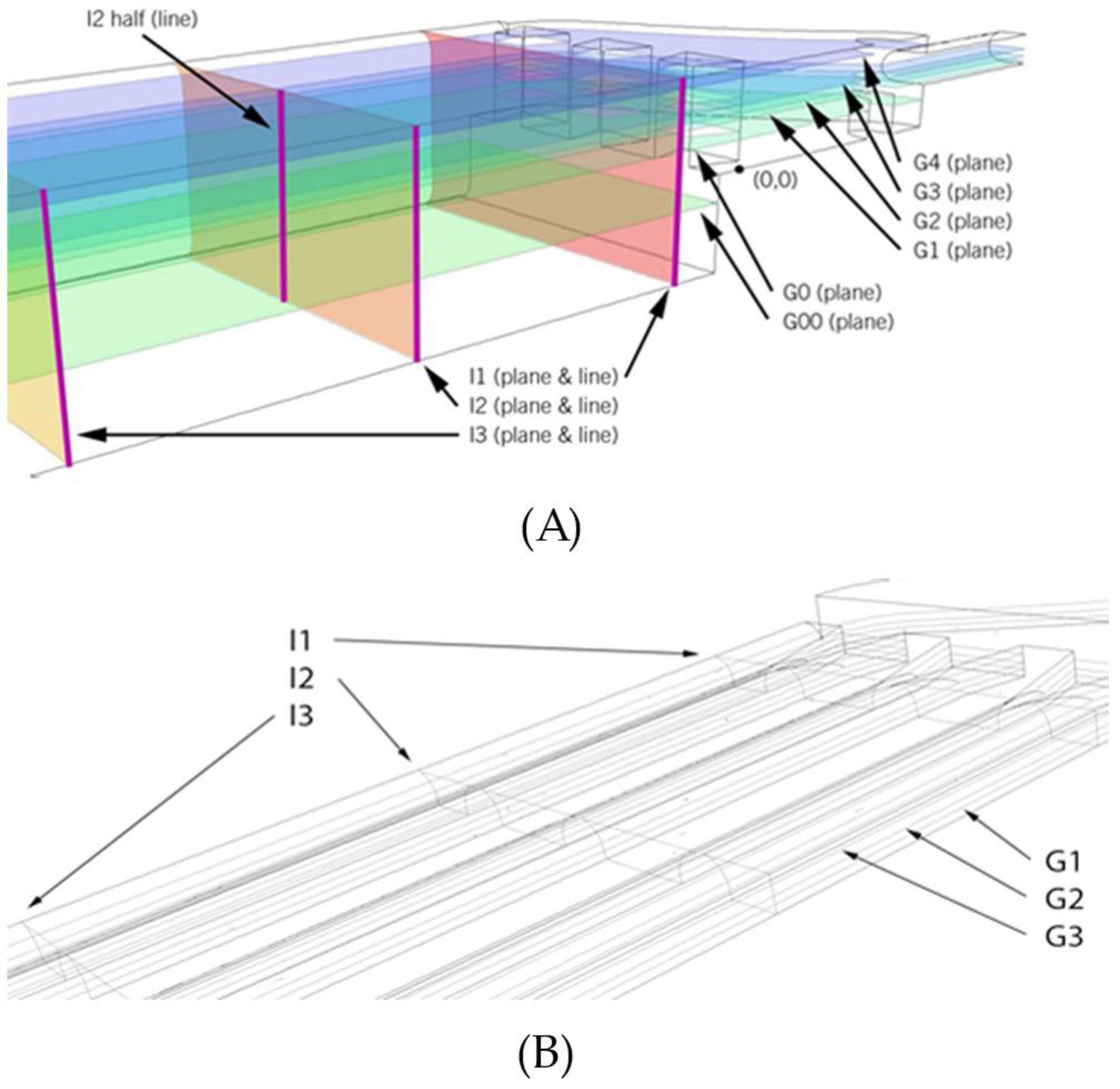
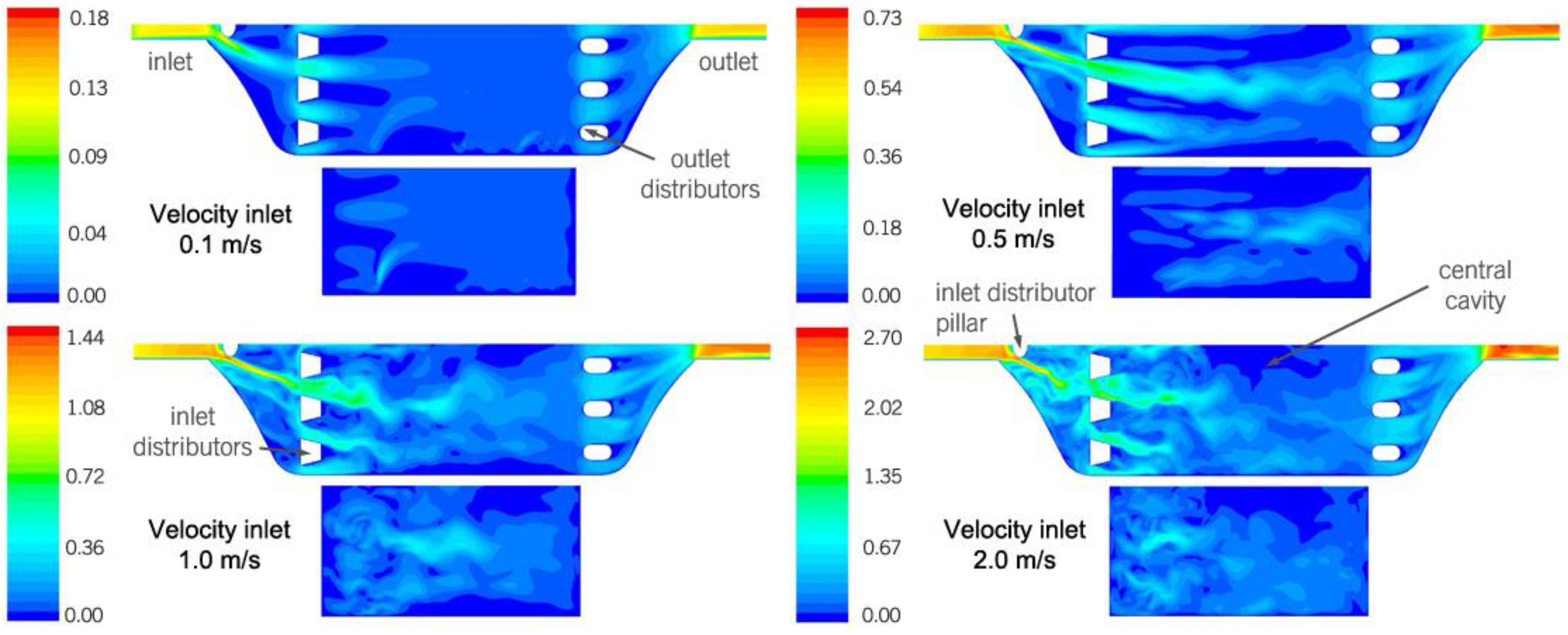
Figure 4 shows the velocity contours for the two planes parallel to the ground: one located at mid-height of the bottom cavity (G0 in
Figure 2) and the other at mid-height of the central cavity (G2 in
Figure 2).
The central cavity figures show that, in general, the flow inside the central cavity is influenced very little by the distributors at the inlet. This lack of influence can be observed at 0.1 m/s, due to the low velocity: the distributors do not produce significant jets. It can also be observed at 1.0 and 2.0 m/s, due to the high transient nature of the flow, which produces significant velocity magnitudes in all directions, and this in turn reduces the effect of the jets. Only at 0.5 m/s, at which a limited occurrence of the two abovementioned phenomena can be observed, does the influence of the jets produced by the distributors become the most important factor. Clear streamlines, produced by the distributors, can then be observed. The recorded videos confirm this transient behavior.
According to
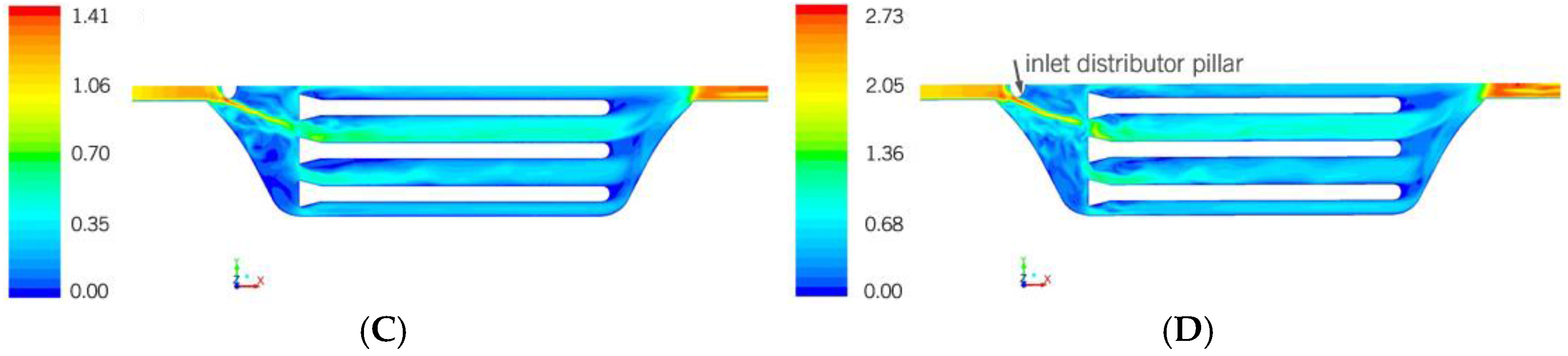
Figure 4, another characteristic that deserves mentioning is the influence of the rounded pillar in front of the inlet designed to distribute the flow. It produces a preferential flow path that goes directly toward the inlet of a certain microchannel. The magnitude of the preferential path depends on the velocity, but only at 0.5 m/s, is the influence clear.
The bottom cavity figures show that there is only a slight influence of the flow along the module in that part. The only effect that influences the flow is the transient behavior, which is significant for all of the studied inlet velocities, except at 0.1 m/s. There is in fact a clear tendency of a stagnant flow near the bottom of the cavity.
Table 3 shows the numerical mean results for the I and G planes, as well as for the entire volume (the mean velocity of all the PEC cell elements of the geometry).
In general, the ratio between the inlet velocity and the mean volume velocity is about 7. Standard deviations are included to show how uniform the velocities are in each plane.
A clear difference can be observed between the 0.1 m/s case and the others for the I planes (parallel to the inlet plane). In the first case, the velocity magnitudes in the three planes are almost the same, but important differences can be observed for the other cases. Plane I1 always shows a greater velocity magnitude than I2 and I3. Moreover, it has the largest standard deviation, because of the remarkable influence of the initial distributors and the rounded pillar. It should be noted that the mean velocities at these planes are not the same, because the magnitude considers all the velocity components.
As far as the G planes are concerned (parallel to the ground plane), plane G2 shows the largest velocity in all cases, because of the direct influence of the inlet. No large differences can be observed between the velocity magnitudes of the G1, G2 and G3 planes. However, a significant velocity divergence can be observed when the velocity magnitude of these planes is compared with the velocity of G0. In all the cases, the velocity magnitude in G0 is much lower, thus indicating that a clear stagnant region exists in the bottom cavity. This hypothesis is also supported by the fact that the standard deviations of the G0 planes are much lower than the others.
The results pertaining to the stagnant zones show that the cases with lower inlet velocities exhibit larger areas, with velocities below 10% of the mean, that is, near the lateral walls and behind the distributors. The case of the inlet velocity of 0.5 m/s is in particular different, due to the influence of the preferential jet caused by the rounded pillar in front of the inlet. Overall, stagnation is never more important in the cases with larger inlet velocities. A final consideration that affects all the analysis pertains to the transient nature of the flow, which produces variations of the flow over time.
Influence of the Geometry
Simulations were performed to establish the influence of the geometry of the PEC cell, as indicated in
Figure 1. Different heights inside the cell were considered in order to simulate possible changes on the dimension of the cell that could be necessary due to fabrication issues or to guarantee a good robustness of the device (i.e., to avoid the bending of the cell caused by its heating with the sunlight). The differences in height led to changes in the total cell volumes. The volume of the original model was 984 mm
3. Volumes of 1351, 2060, 2736 and 2990 mm
3 were adopted for A1, A2, A3 and A4, respectively. In order to analyze the results, the planes and edges shown in
Figure 2 were considered.
In all the cases, the inlet velocity was 0.1 m/s. On the basis of the conclusions on the influence of the velocity (previous section), this was considered to be an optimum velocity.
The results show that there are no clear differences in the flow distribution along the direction of the fluid flow. The preferential path created by the rounder pillar is slightly more stressed. Moreover, the magnitude of the vectors decrease as the volume of the PEC cell becomes larger, although their direction does not vary significantly.
Relevant differences can instead be observed in the perpendicular direction (along the planes parallel to the inlet plane). There are very few differences between the original model A and the A1 geometry, where only the central cavity height was increased. However, when the height of the bottom cavity is increased (A2 and A3), significant differences in the velocity magnitude can be observed, although there is still a similarity between the original cases, A1 and A2, as far as the boundary layers at the top and bottom walls are concerned. On the other hand, the situation changes significantly in A3, where a significant layer of stagnant fluid can be observed over the bottom surface.
The A4 flow characteristics are different, and show similarities with and differences from the other cases. The velocity magnitude range is much lower than in the other cases. In the other cases, there are zones of up to approx. 1.8 × 10−2 m/s, but the maximum velocity in A4 is around 1 × 10−2 m/s. This lower velocity makes the flow more stagnant, and leads to fewer differences in the magnitude and in the decrease of the stagnant layer over the bottom surface (which is naturally also due to the difference in geometry).
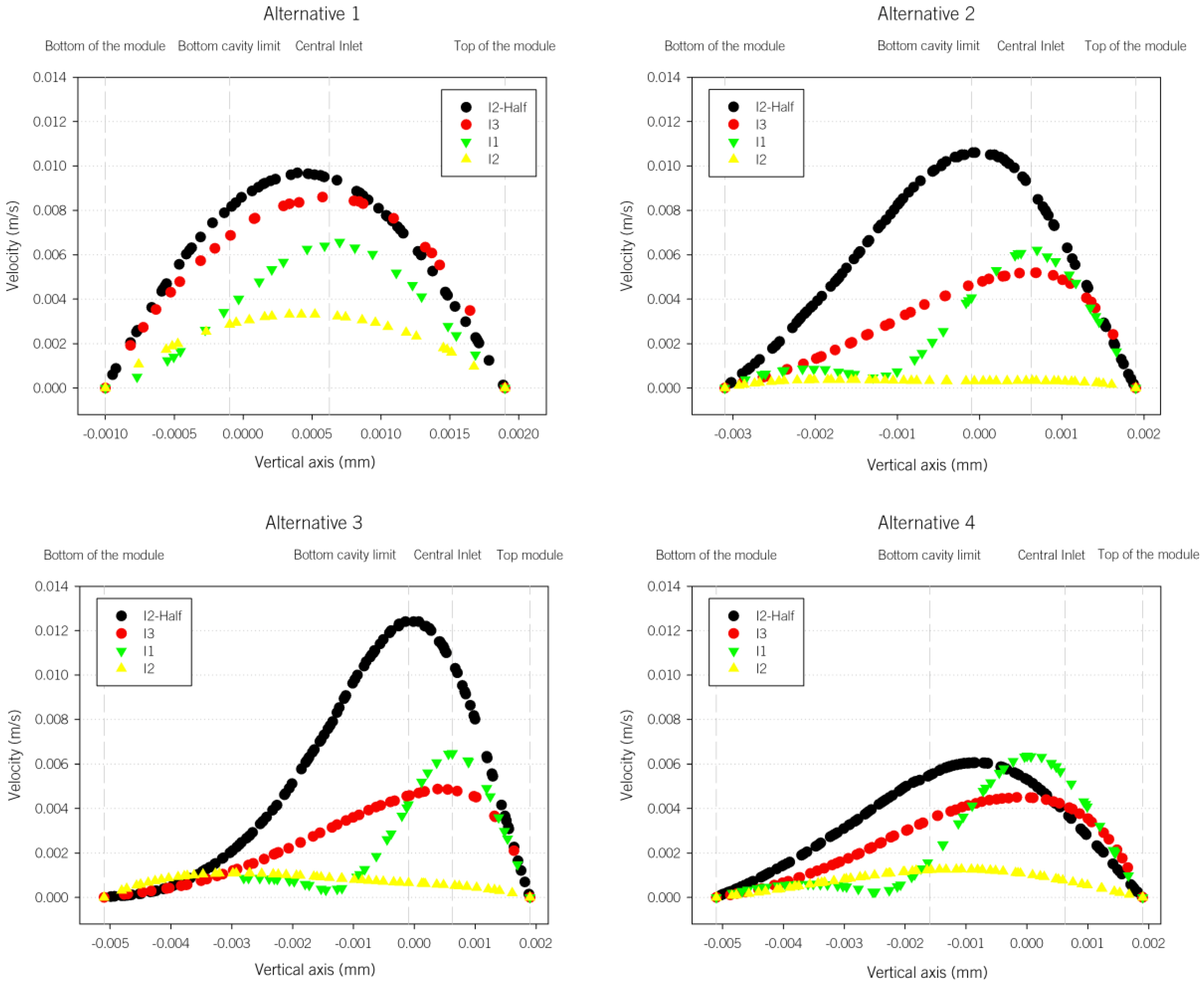
In order to further check the abovementioned differences,
Figure 5 shows the velocity profiles along the vertical direction for the 4 alternatives and in four different lines, as indicated in
Figure 2. It can clearly be observed that the lowest velocity magnitudes are in the alternative 4 (A4). In addition, the parabolic shapes in A1 are lost in the other cases, as the total height increases.
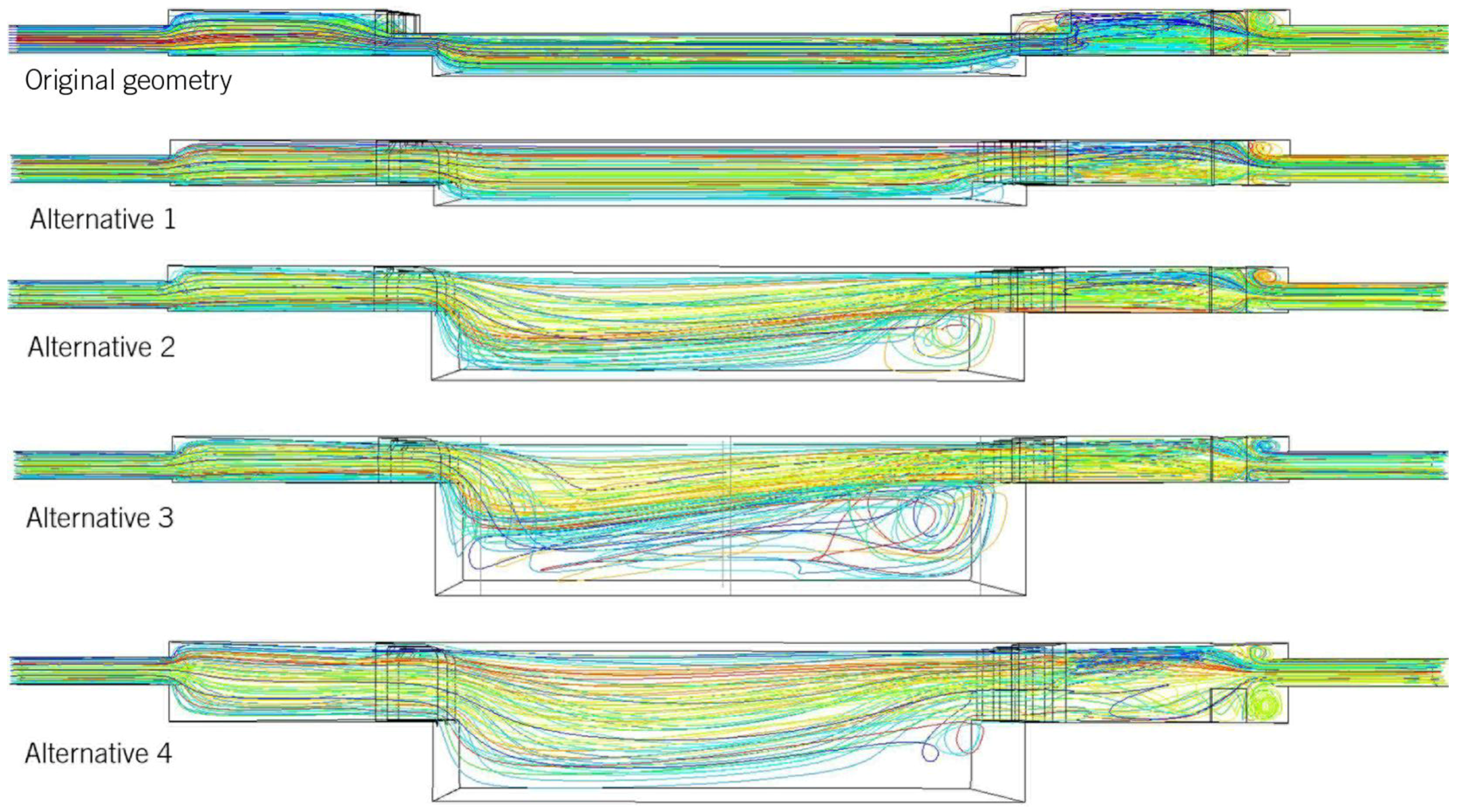
Figure 6 shows the pathlines of several configurations in order to assess the importance of the stagnation in the alternative geometries.
In general, it can be observed that there is almost no stagnation in the original geometry or in A1. The first indications that stagnation occurs can be observed in A2, but it is particularly evident in A3, where more stagnation occurs. The change in geometry in A4 makes this phenomenon decrease.
Table 4 shows the mean velocities and standard deviations for each plane for the different cases and for the entire volume (the mean velocity of all the PEC cell elements of the geometry A indicated in
Figure 2).
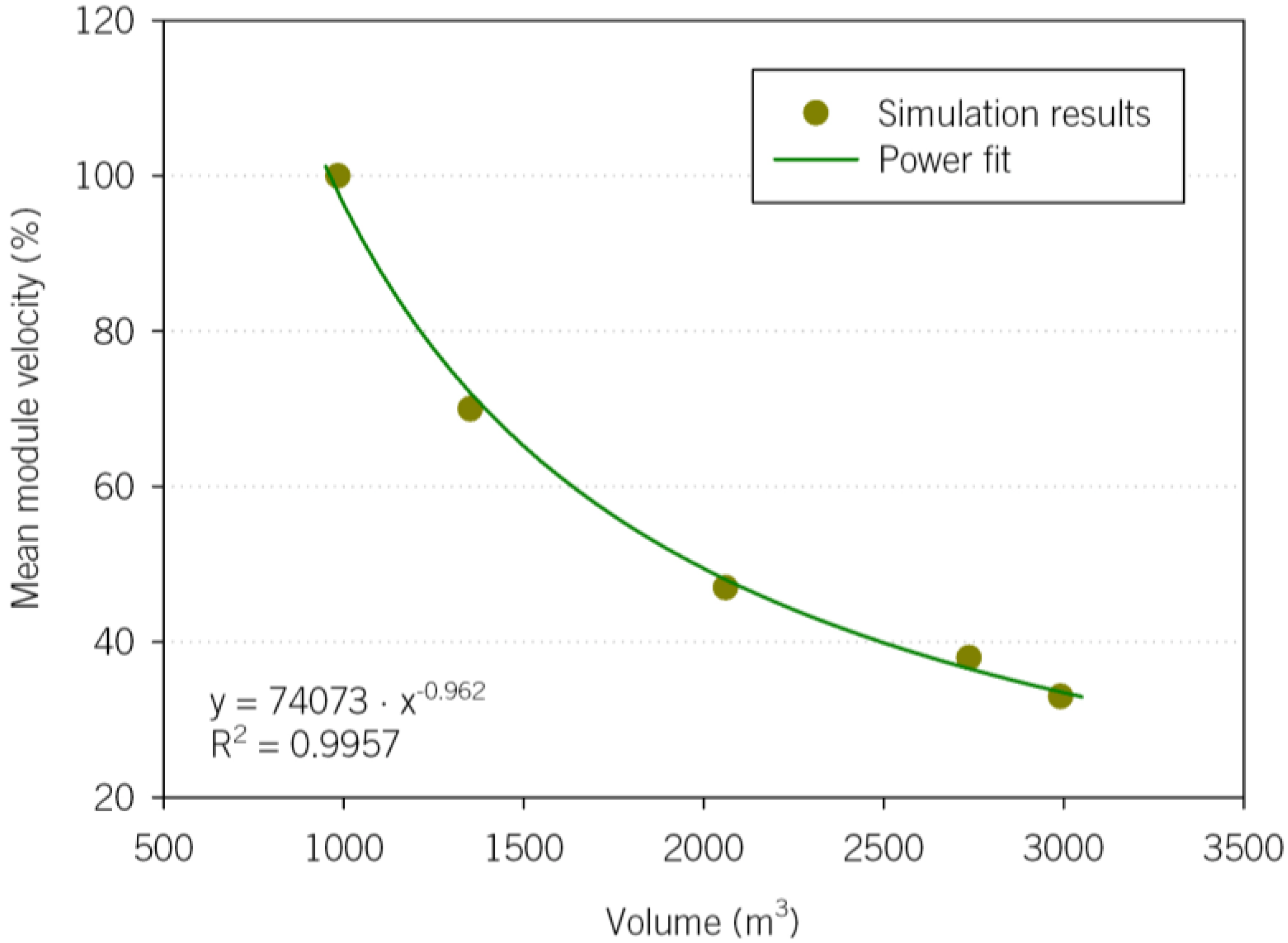
The mean volume velocity results decrease as the PEC cell model becomes larger; A4 in fact shows the lowest values. The mean velocity of A1 is 70% of the original case, and is 47%, 38% and 33% for A2, A3 and A4, respectively. The standard deviations show how different the velocities in the plane are (calculated from the velocities at each plane cell).
Figure 7 shows that a power regression is the best function that correlates the mean velocity inside the module and its volume.
The results regarding the I planes are proportional to the original case, but also show the abovementioned overall velocity reduction as the PEC cell cavity becomes larger. In all the cases, the I1 plane has the largest velocity magnitudes and standard deviations, due to the inlet effect.
The results pertaining to the G planes clearly show that the planes located at the height closest to the inlet one have the largest velocities. The velocity differences among the G planes are also clear. For example, the ratios between G2 and G00 range from 5.8 to 8.5. It is also interesting to note that, while the maximum velocity is located at G3 for the A1, A2 and A4 cases, it is located at G1 for A3.
Influence of the Initial Distributors
The previous results pointed out the critical effects that the initial flow distributors introduce. These distributors are needed to avoid direct streamlines from the inlet to the outlet, and to avoid stagnation inside the cell. Several geometries were considered and simulated to check their impact on flow spreading inside the cell.
Figure 8 shows the different velocity contours for the different designs. The results show that a good design can be obtained by combining two rows of distributors aligned according to
Figure 7D. This would facilitate flow homogenization, although a slight increment of the pressure drop would also be produced.
3.1.2. Geometry B (Geometry with Channels)
The only variable that was studied in this model of PEC cell was velocity. The considered velocities were 0.1, 0.5, 1 and 2 m/s, as in geometry A.
In this case, and unlike geometry A, the flow is much more constrained because of the presence of the microchannels. There is less difference in velocity inside the module, and the flow continues to develop along the microchannels until the inlet velocity exceeds 1 m/s. Thus, an advantage of this PEC cell model is that the stagnant zones are minimized, although the contact surface area of the module could also be lower, because the channel wall width can cover a part of the catalyst surface area limiting the water oxidation reaction on such area.
The results pertaining to the transient behavior of the fluid show that the flow is still stationary in all the module parts for 0.5 m/s. A clear transient zone can be observed at the inlet cavity for 1 m/s, but the flow remains stationary inside the microchannels. The flow is significantly transient for 2 m/s, and has not developed in any of the microreactor zones, including the microchannels (although the magnitude differs because of the microchannels).
A common characteristic of all the cases is the preferential fluid path (
Figure 9). The rounded pillar in front of the inlet creates a jet that goes directly toward the inlet of a microchannel. The magnitude of the preferential path depends on the velocity. The faster the fluid is injected, the greater the difference between the channel flow distributions. The effect is not so obvious for 0.1 m/s, but it starts to be significant from 0.5 onwards.
The results concerning the direction perpendicular to the fluid flow show that the fluid is developed for 0.1 m/s in all the microchannels, and the velocities are similar. Significant differences start to occur between the different microchannels for 0.5 m/s. Non-developed fluid flow symptoms also arise as non-symmetric shapes appear in the velocity magnitude contours. The abovementioned situation can clearly be observed for 1 m/s, and it increases even more for 2 m/s. An important change occurs between 0.5 and 1 m/s.
Some numerical data were extracted from the simulation at the different representative control planes defined in
Figure 2B.
Table 5 shows the mean velocities and standard deviations of each plane, for the different studied inlet velocities, and for the entire volume (i.e., the mean velocity of all the cell elements of the geometry).
The ratio between the inlet velocity and the mean volume velocity is about 4 (almost the half of the values observed for the geometry A), with a decreasing tendency as the inlet velocity increases. This tendency is similar to the behavior that was observed for geometry A, but the mean velocity values for model A are almost one order of magnitude lower than those for geometry B. This indicates that model A shows a more stagnant tendency because of the bottom cavity. Thus, as far as this feature is concerned, model B is better.
It can be noted, for the I planes (parallel to the inlet plane), that although I2 and I3 always show the same velocity, I1 is large for all the cases, except for the one related to 0.1 m/s. This confirms the change in the fluid behavior between 0.1 and 0.5 m/s. It should be noted that the mean velocities at these planes are not the same, because the magnitude considers all the velocity components.
As far as the G planes are concerned (parallel to the ground plane), the G2 plane shows the greatest velocity in all the cases, as it corresponds to the central one inside the microchannels. A difference can also be observed between 0.1 m/s and the others: although the other velocities at G1 and G3 are similar and lower than G2, G3 is closer to G2 than to G1 for 0.1 m/s. It should be pointed out that, in all the cases, G1 (the plane closest to the bottom surface) does not have a significantly low velocity, compared with the mean velocity. Proportional values of the standard deviation were obtained for all the cases.
The results regarding the stagnant zones show that, as the inlet velocity decreases, more of the flow area is under a lower velocity magnitude than 10% of the mean. However, almost all the sectors where there are stagnant zones are at a certain distance from the microchannels where the reaction occurs. In other words, there are almost no stagnant zones in the region of interest. Fluid that is more stagnant can be observed in the microchannel zones for high velocities. Therefore, it is only in the case of 0.5 m/s, which no fluid is found under a 10% of the mean volume velocity in the zone. Nevertheless, it should be noted that, in absolute terms, stagnation is never important in the cases with higher inlet velocities. In all the studied cases pertaining to the microchannel zones there are no elements with a velocity magnitude below 0.005 m/s, although stagnant zones are observed in the region of the PEC cell where the channels are not present.
3.1.3. Summary of the Hydrodynamic Results
As far as geometry A is concerned, an inlet velocity of 0.5 m/s (6.4 L/h) should be avoided, because of the presence of a preferential jet in front of the inlet, produced by the round pillar. That behavior has consequences on all the module zones, including the bottom cavity, because more differences in the vortices and in the magnitude of the velocity are observed. An alternative solution could be to make some changes to the design of the rounded pillar. The case with the lowest inlet velocity (0.1 m/s) clearly offers more velocity homogeneity, in terms of magnitude and direction. This is the only studied situation in which this effect has emerged. However, this case can also suffer from a drawback: a very low fluid velocity magnitude (0.7 × 10−2 m/s in plane G0). The importance of this low magnitude should be evaluated, but its effect may not be so important. A low velocity may enhance the reaction rate, if the catalytic surface is not covered with the produced bubbles, which can limit the catalytic activity. In this case, an inlet velocity of 0.1 m/s (1.3 L/h) would be the optimum one. In the contrary case, an inlet velocity higher than 0.5 m/s should be selected (with the present pillar design).
There are no relevant differences in the hydrodynamic behavior of the flow inside the module between 1 and 2 m/s. However, the velocity magnitude obviously changes overall as the inlet velocity changes. Thus, a suitable velocity would be the one achieved after the inlet velocity is increased from 0.5 m/s to the value at which the rounded initial pillar effect diminishes (between 0.5 and 1 m/s).
As the volume of the module increases (geometry A and its alternatives A1 to A4), the overall mean velocity inside decreases (therefore the lowest decrement occurs for A1, see
Section 3.1.1 (
Influence of the Geometry)). As far as the flow distribution is concerned, A1 does not show any significant differences in hydrodynamics compared to the original case. Variances start to be noted for A2, and are observed in the vertical velocity profile as well as some stagnant zones in the corner parts of the bottom cavity of the module. This is the case of A3, where major differences are encountered, especially in the presence of stagnant fluid over the bottom wall of the module. A4, which has the same height as A3, but a different cavity distribution, mitigates the stagnation issue of A3, but reduces the mean velocity inside the geometry even more.
The results obtained for geometry B, considering the studied parameters, make it possible to state that a change in behavior can be observed between 0.5 and 1 m/s. Fluid uniformity is lost inside the microchannels at high velocities: different fluid distributions in the channels, a transient flow, a more undeveloped flux or a turbulence increment can be observed. For these reasons, working at velocities lower than 0.5 m/s (6.4 L/h) may be adequate, if the coverage of the catalytic surface with the formed bubbles is low. Another reason for recommending working below 0.5 m/s is connected to avoiding the effect of the initial distributor located just after the inlet, which causes a jet to form. At high velocities, the jet continues to develop toward the entrance of a particular channel, and thus generates a preferential path. It produces a significant non-uniform flow distribution along all the microchannels. There are almost no stagnant zones and the effect of velocity is low.
3.2. Bubble Formation and Removal
Oxygen and protons are produced during the water splitting reaction. Oxygen forms as bubbles that quickly coalesce from the pores of the catalyst to the bulk solution (water) inside the cell. Because of the surface tension and wall adhesion forces, the oxygen bubbles have the tendency to remain attached to the catalyst wall [
22]. This makes the catalyst lose contact with the water. The consequence is that the water splitting reaction stops. In order to improve catalyst efficiency, it is necessary to remove the oxygen bubbles from the cell. Some ways of sweeping the bubbles out from the cell are: controlling the speed of the liquid and orienting the cell according to gravity.
Several simulations were performed to assess the bubble removal process considering the aforementioned factors, and the influence of the liquid velocity inside the cell and the gravity direction were studied. The influence of the oxygen production rate was also studied.
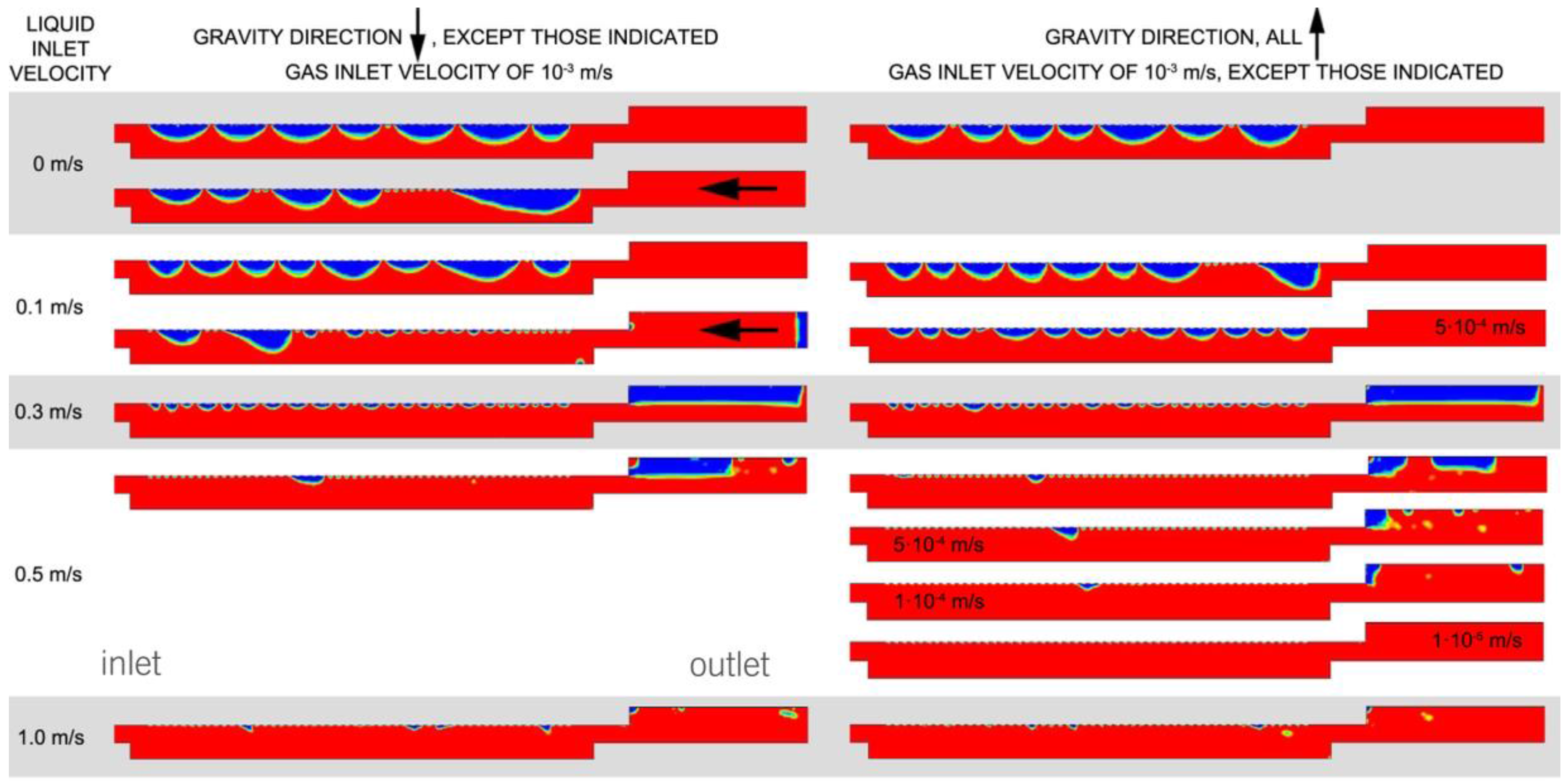
Figure 10 summarizes the obtained results. The results are expressed in terms of liquid and gas fraction related to the entire volume.
The results show three types of behavior that depend on the liquid inlet velocity. When the liquid is stagnant, all the oxygen remains in the cell after 1 s. Buoyancy on its own is not enough to detach the bubbles from the catalytic surface. Nevertheless, the results show that gravity could play an important role in facilitating bubble removal if used in the correct direction relative to the outlet. The accumulation of oxygen inside the cell, after 1 s, is still remarkable for a low liquid inlet velocity, except for the case where the gravity direction is more favorable (against the outlet). In this case, bubbles are removed. The bubbles are removed quickly and independently of the gravity direction for a liquid inlet velocity of 0.3 m/s and higher. The liquid velocity also ensures that the oxygen bubbles are removed just as they enter the cell, and this helps prevent coalescence. Thus, a high liquid velocity not only leads to better bubble removal, but also prevents large bubbles from forming, which instead occurs for a stagnant liquid and for low liquid inlet velocities.
Table 6 shows the mean numerical results of the oxygen fraction and the mean liquid velocities in the simulated surface. The results show that the gas fraction decreases when the velocity inside the cells increases, when gravity is opposite the flow direction and when less oxygen is produced.
3.2.1. Influence of the Liquid Velocity
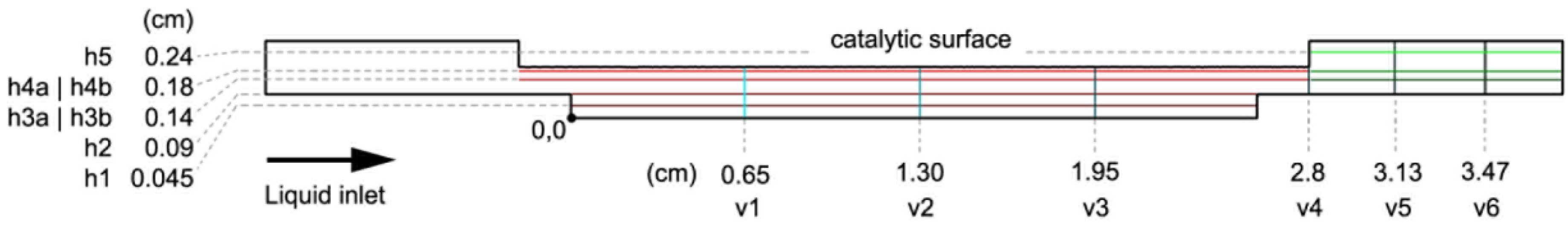
Five liquid velocity inlets were considered to assess the effects of the liquid velocity on bubbles removal: 0 (stagnant fluid), 0.1, 0.3, 0.5 and 1.0 m/s. The main monitored parameter was the oxygen fraction, and it was monitored in several control lines (as shown in
Figure 3).
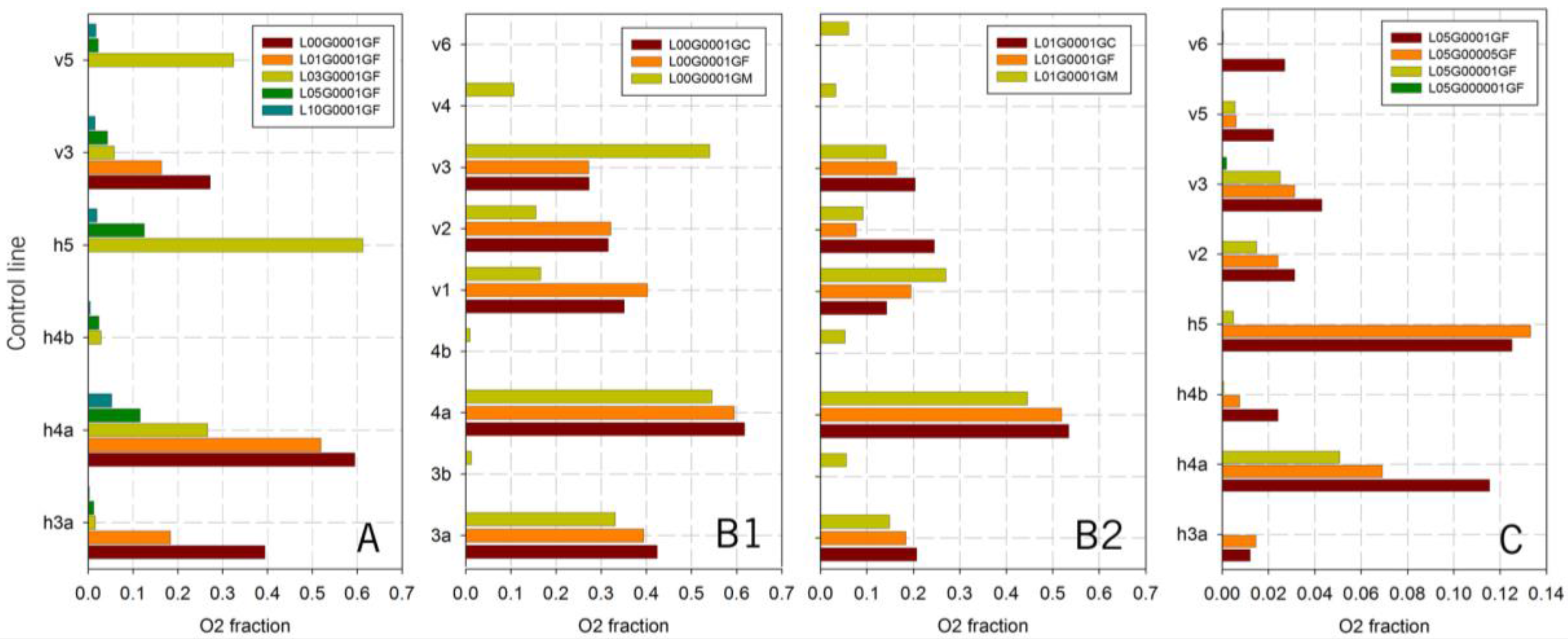
Figure 11A shows this parameter in different control lines as a function of the inlet velocity after one simulated second.
A notable influence of the inlet velocity can be observed for the h4a control line (the nearest to the catalyst surface). First, as the velocity increases, the oxygen fraction decreases in a non-linear manner. No great difference is observed from 0 to 0.1 m/s, while a difference can instead be observed from 0.1 to 0.3 m/s. A great difference can also be noted from 0.3 to 0.5 m/s, but not from 0.5 to 1.0 m/s. Thus, the 0.1 to 0.5 m/s range is the one that causes significant changes in the oxygen fraction and consequently in the growing of bubbles after their formation: almost no bubbles are removed below 0.1 m/s, while most of the bubbles are expulsed from 0.5 m/s onwards.
It can be observed that the oxygen fraction, in the h3a control line, drops to half when the liquid inlet velocity changes from 0 (a stagnant fluid) to 0.1 m/s. At 0.3 m/s, the oxygen fraction is almost 0, which means that no oxygen bubbles are in that zone. Therefore, there is no need to increase the velocity after 0.3 m/s is reached. Other control lines, such as h4b and v3, confirm this result. The oxygen fraction at 0.3 m/s is still high in the v5 and h5 control lines, but this is due to some stagnant oxygen remaining in the outlet part of the reactor (see h5) that is located outside the reactive zone.
Finally, it should be pointed out that the oxygen fraction in v5, h5 and h4b is zero for the stagnant fluid, because all the formed oxygen remains attached to the catalytic surface.
3.2.2. Influence of the Gravity Direction
Three gravity directions were considered to assess the effects of gravity on the removal of bubbles (GC, GF and GM, as previously described in
Section 2.1.2). GC (gravity with same direction as the gas inlet) was found to be the most unfavorable direction, as it facilitates the attachment of gas bubbles to the catalytic wall. The main monitored parameter once again was the oxygen fraction, which was analyzed in several control lines.
Figure 11B shows this parameter in different control lines as a function of the gravity direction, and for two different inlet liquid velocities: 0 (stagnant fluid) and 0.1 m/s. The obtained results show that gravity has almost no influence at higher velocities (the liquid velocity is the dominant variable).
In general, the differences pertaining to the gravity direction are not so large. The largest differences were found for the stagnant liquid fluid and the GM case. That gravity direction (i.e., contrary to the liquid inlet direction) is the one that clearly helped the bubbles exit the cell. This result was observed in the v1, v2, v3 and v4 control lines. The oxygen fraction in v1 and v2 was much lower for GM than for GC and GF (gravity with opposite direction as the gas inlet), while it was much higher in v3. This is because the bubbles in the GM case can be removed, but they did not do so in the GC and GF cases. In the GM case, not only did more bubbles reach v3 but they also reached v4, where the presence of oxygen was not detected for the GC and GF cases.
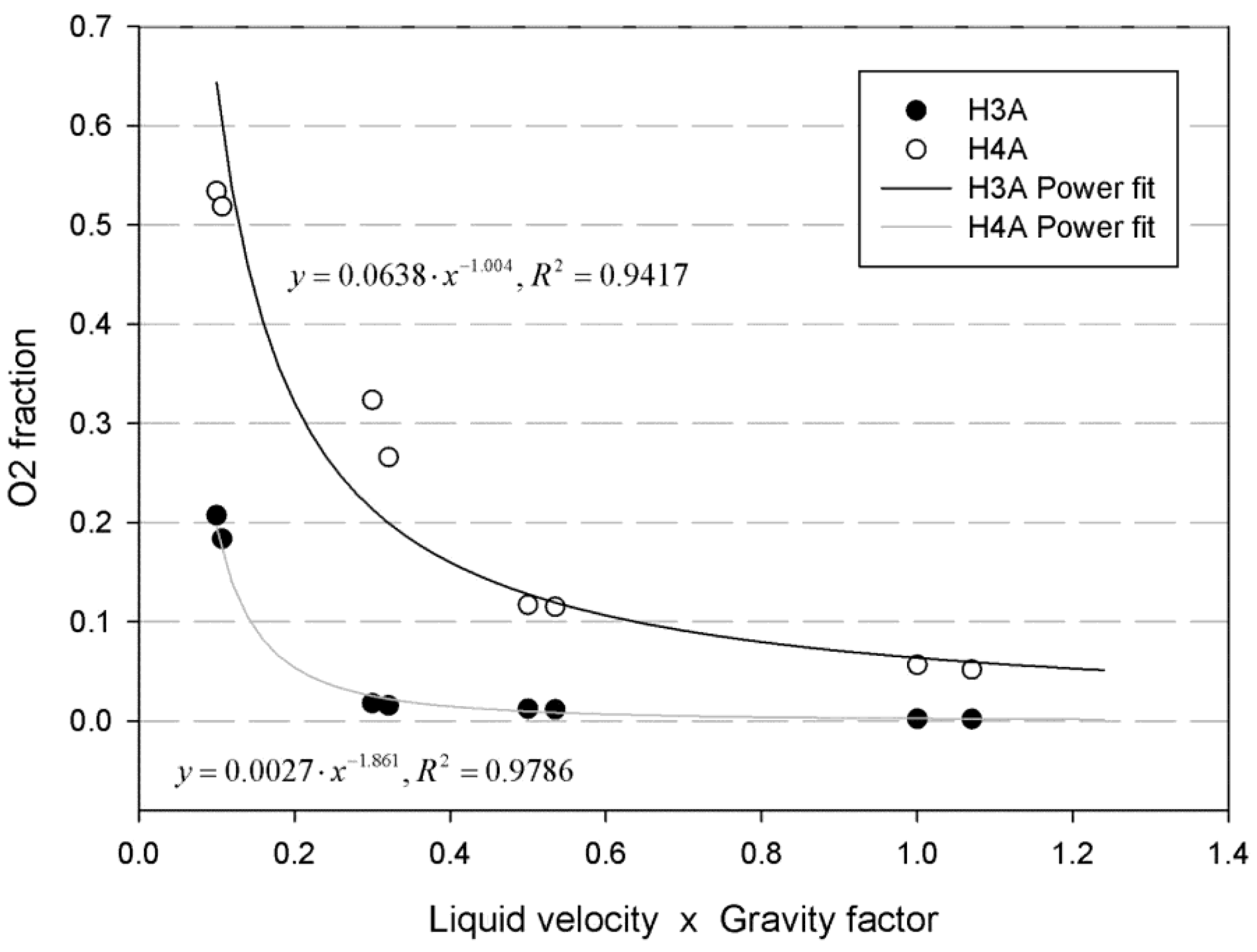
The liquid velocity inside the cell and gravity are the two main variables that can be set to affect the removal of bubbles. Curve fittings were made from the obtained results in order to check whether there was any kind of correlation between those parameters and the oxygen fraction in any of the key control lines. A factor obtained by multiplying the liquid inlet velocity by the gravity (set to 1 for the GC cases and 1.1 for the GF ones) was plotted against the oxygen fraction for the h3a and h4a control lines. The best correlation was obtained through the fitting with a power function, for which a R
2 higher than 0.94 was obtained, as
Figure 12 shows. This result confirmed the predominant effect of the liquid velocity.
3.2.3. Oxygen Production Rate
The oxygen production rate also influences the amount of oxygen remaining in the cell. This rate does not correspond to a process variable but to a process yield and, obviously, depends on the catalyst material. In order to consider different possible yields, five different gas inlet velocities were analyzed (1 × 10
−1, 1 × 10
−2, 1 × 10
−3, 5 × 10
−4, 1 × 10
−4 and 1 × 10
−5 m/s). The liquid inlet velocity and gravity direction were fixed at 0.5 m/s and GF, respectively.
Figure 11C shows the oxygen fractions for several control lines.
In general, the results show that no oxygen is produced at the lowest velocity (10−5 m/s) or within the simulated frame time. Relative differences were observed for the other cases, although they were not reflected in absolute values.
The h4a results (that is, for the control line nearest to the catalytic surface) show the influence of this variable and its non-linear behavior: there is a larger difference between 1 × 10−3 and 5 × 10−4 m/s (half velocity) than between 5 × 10−4 m/s and 1 × 10−4 m/s (five times lower). The results for the other control lines are similar, except for h5. This control line is located outside the reactive part, where the oxygen accumulates, as already mentioned above. The oxygen fraction has the largest values in the accumulation area, and they are very similar for the 1 × 10−3 and 5 × 10−4 m/s cases. There is almost no oxygen for the other situations or in this control line, because of the low oxygen production rate.
The high oxygen inlet velocity (1 × 10−1 and 1 × 10−2 m/s) as well as the conditions of a stagnant liquid accelerate the gas bubbles coalescence and cause the formation of large bubbles inside the cell. Oxygen leaves the cell at a steady state, but a large concentration (large bubbles) of oxygen remains inside the cell (at 0.52 s, the mean oxygen concentration on the 2D face is 0.70). In these conditions, the hypothesis of a constant oxygen inlet velocity cannot be considered reliable because once the catalytic surface is completely covered, no more oxygen will be produced. Nevertheless, the results are useful, because they indicate that although a large bubble could not increase inside the cell, it would remain attached to the catalytic surface without leaving the cell, despite the influence of gravity.
3.2.4. Comparison of the 2D and 3D Simulation Results
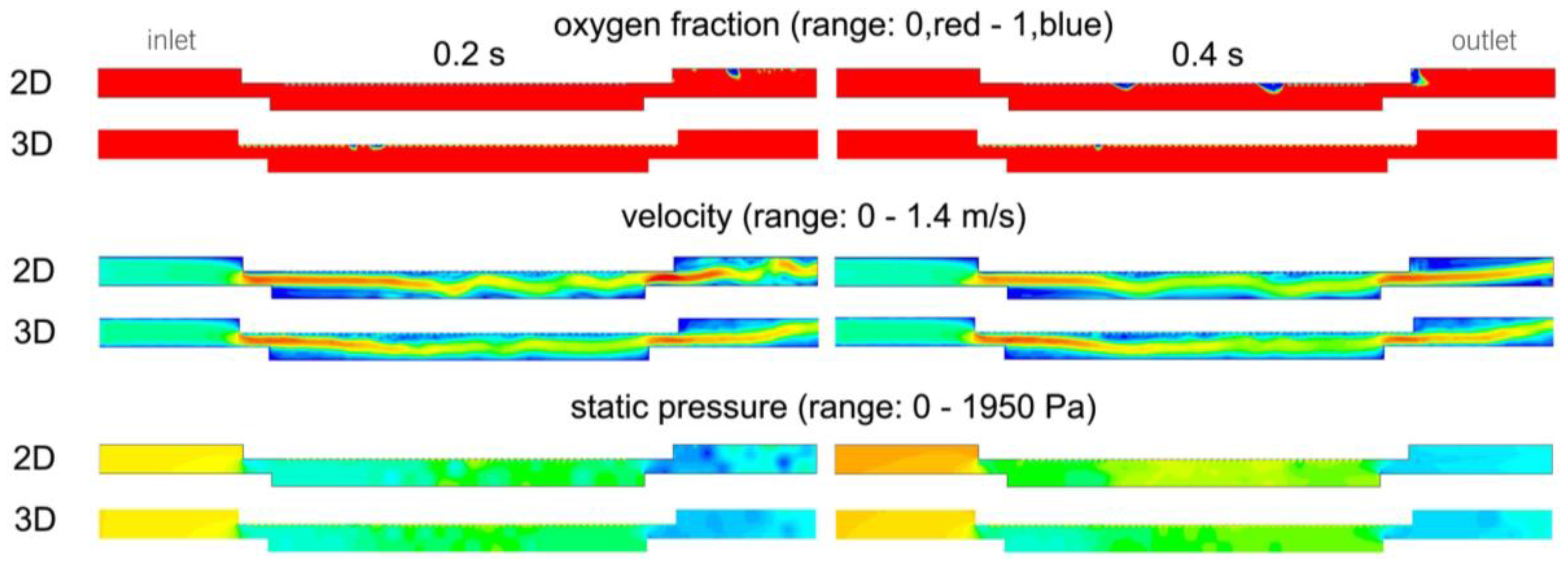
One 3D simulation was performed to check the 2D results when a relevant third dimension size, in agreement with the bubble size, was considered.
Table 7 shows some comparative results and
Figure 13 shows contour diagrams regarding the static pressure, velocity magnitude and oxygen fraction for both cases.
If the contour diagrams are examined, it can be seen that very similar profiles are obtained for the velocity and pressure. Differences regarding the position of the bubbles on the fraction contours emerge, but they are mainly due to the unsteady behavior of the flow. Nevertheless, the size of the bubbles and the coalescence level are very similar. This correspondence is also obtained for the numerical values. Because of the limited thickness of the 3D volume, the mean results pertaining to the catalytic surface are very close to the mean results pertaining to the whole volume.
3.2.5. Experimental Validation of the Simulation Results
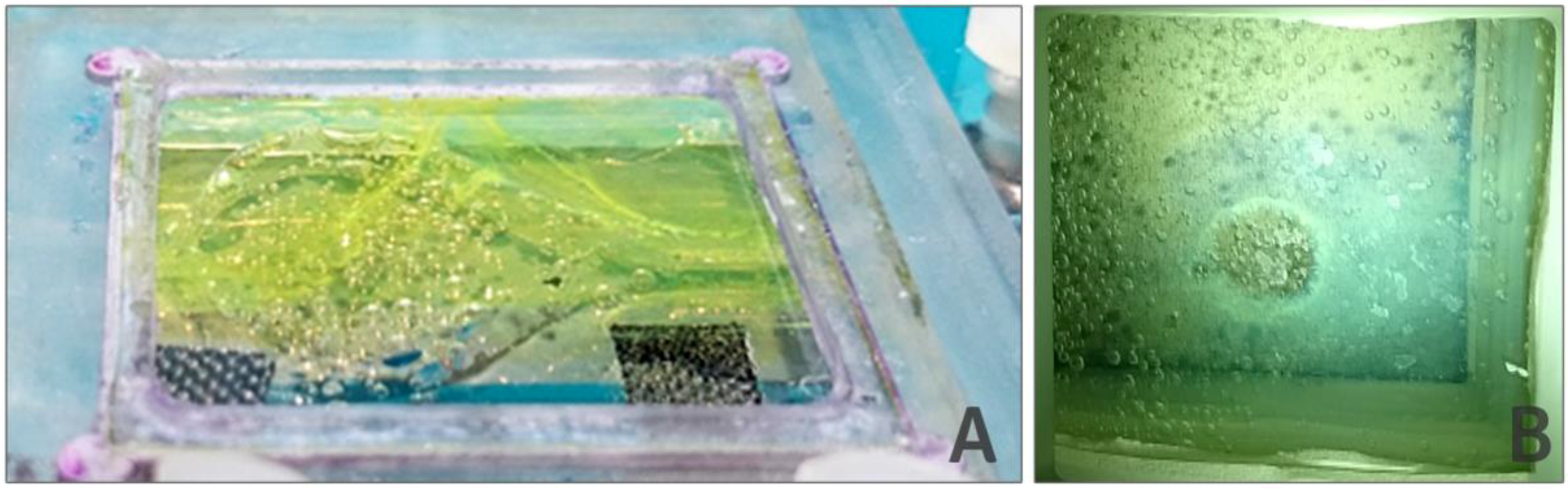
The results obtained in these simulations were compared with observations from several experiments. Videos were made in which bubbles and their movement inside the cell were recorded.
Figure 14 shows the obtained images for different liquid velocities, where different bubble sizes can be observed. Large bubbles were obtained for the stagnant liquid case. Two types of bubbles can be observed for the circulating liquid case (1 m/s): medium-sized bubbles attached to the surface (mean size of 0.57 mm and standard deviation of 0.14 mm) and small bubbles circulating in the bulk solution and moving toward the outlet (mean size of 0.21 mm and standard deviation of 0.01 mm). These results are in agreement with those obtained in the simulations. The results shown in
Figure 11B could fit better with simulations performed at a higher inlet liquid velocity than 2 m/s. However, these discrepancies were to be expected, considering the several limitations and hypothesis considered, such as the model used in the simulations (bubble generator), the difficulty of correctly assessing the exact gas inlet velocity in the experiments, the changes in the reaction yield due to surface coverage and the possible thermal effects due to light irradiance.
4. Conclusions
CFD simulations have been used to assess the process design and cell configuration of a photo-electrochemical (PEC) cell in which water is split into protons and oxygen on a catalytic surface.
From the hydrodynamic point of view, the purpose of a PEC cell is not to mix components but to facilitate the reaction on the catalyst surface. A regular distribution of the liquid phase over the catalyst surface is necessary, and stagnant zones should be avoided. Turbulence may also be a drawback, as it may lead to a non-regular fluid distribution. The shear stress caused by the motion of a liquid should allow oxygen bubbles to be dragged out of the cell. If the bubbles are not removed from the cell, they remain attached to the catalytic surface, thus preventing the reaction from occurring because the water is not able to wet the catalyst.
Channeled cells boost flow uniformity inside a cell, but the presence of channels can lead to a reduction in the active catalytic surface and can diminish the yield. Suitable initial distributors (i.e., two layered A/B geometry as previously explained) may be used to overcome these issues. Incrementing the thickness of the cell does not improve the hydrodynamics or the reaction yield, and the cell wall should be kept as thin as possible (no thicker than 3 mm) to avoid stagnant zones.
An inlet liquid velocity equal to or higher than 1.5 m/s may be used to avoid the coalescence of oxygen bubbles, and to drag them to the cell outlet. Gravity does not play a significant role at these velocities, and the orientation of the cell is not relevant. The magnitude of these velocities minimizes the effects of the preferred paths created by the initial distributors. Although the flow begins to show turbulent behavior, a reasonably regular liquid distribution inside the cell is maintained. In this way, the negative issues encountered in the hydrodynamic study can be avoided for the inlet liquid velocity. The here presented results can be used as guidelines for the optimum design of photocatalytic cells for the water splitting reaction for the production of solar fuels, such as H2 or other CO2 reduction products (i.e., CO, CH4, among others).