Long Non-Coding RNAs Regulating Immunity in Insects
Abstract
:1. Introduction
2. Identification and Classification of lncRNAs
3. lncRNAs in Mammalian Immune Response
4. lncRNAs in Insect Immunity
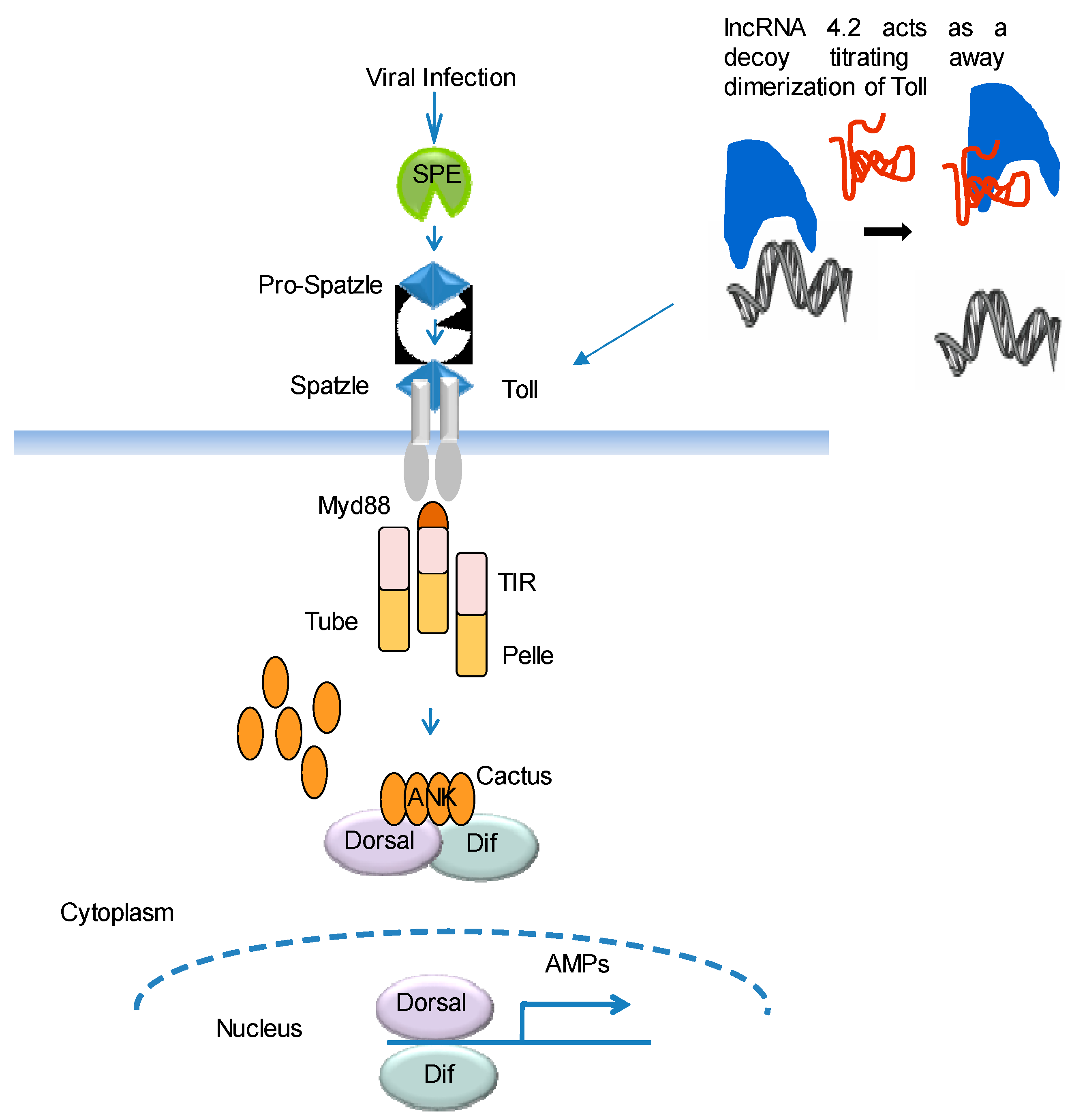
5. Mode of Action
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F.; et al. Landscape of transcription in human cells. Nature 2012, 488, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ulitsky, I.; Bartel, D.P. lincRNAs: Genomics, evolution, and mechanisms. Cell 2013, 154, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Fatica, A.; Bozzoni, I. Long non-coding RNAs: New players in cell differentiation and development. Nat. Rev. Genet. 2013, 15, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Gardini, A.; Shiekhattar, R. The many faces of long noncoding RNAs. FEBS J. 2015, 282, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Bonasio, R.; Shiekhattar, R. Regulation of transcription by long noncoding RNAs. Annu. Rev. Genet. 2014, 48, 433–455. [Google Scholar] [CrossRef] [PubMed]
- Kung, J.T.; Colognori, D.; Lee, J.T. Long noncoding RNAs: Past, present, and future. Genetics 2013, 193, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.D.; Wang, Z.; Kevin, V.; Morris, K.V. Long noncoding RNAs: A potent source of regulation in immunity and disease. Immunol. Cell Biol. 2015, 93, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.J.; Howard, Y. ChangUnique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Rinn, J.L.; Chang, H.Y. Genome Regulation by Long Noncoding RNAs. Annu. Rev. Biochem. 2012, 81, 1–822. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.B.; Boley, N.; Eisman, R.; May, G.E.; Stoiber, M.H.; Duff, M.O.; Booth, B.W.; Wen, J.; Park, S.; Suzuki, A.M.; et al. Diversity and dynamics of the Drosophila transcriptome. Nature 2014, 512, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.M.; Waterhouse, R.M.; Muskavitch, M.A. Long non-coding RNA discovery across the genus anopheles reveals conserved secondary structures within and beyond the Gambiae complex. BMC Genom. 2015, 16, 337. [Google Scholar] [CrossRef] [PubMed]
- Jayakodi, M.; Jung, J.W.; Park, D.; Ahn, Y.J.; Lee, S.C.; Shin, S.Y.; Shin, C.; Yang, T.J.; Kwon, H.W. Genome-wide characterization of long intergenic non-coding RNAs (lincRNAs) provides new insight into viral diseases in honey bees, Apiscerana and Apismellifera. BMC Genom. 2015, 16, 680. [Google Scholar] [CrossRef] [PubMed]
- Kiya, T.; Ugajin, A.; Kunieda, T.; Kubo, T. Identification of kakusei, a nuclear non-coding RNA, as an immediate early gene from the honeybee, and its application for neuroethological study. Int. J. Mol. Sci. 2012, 13, 15496–15509. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cheng, T.; Liu, C.; Liu, D.; Zhang, Q.; Long, R.; Zhao, P.; Xia, Q. Systematic identification and characterization of long non-coding RNAs in the silkworm, Bombyx mori. PLoS One 2016, 11, e0147147. [Google Scholar] [CrossRef] [PubMed]
- Heward, J.A.; Lindsay, M.A. Long non-coding RNAs in the regulation of the immune response. Trends Immunol. 2014, 35, 408–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krawczyk, M.; Emerson, B.M. p-50 associated Cox2 extragenic RNA (Pacer) activates Cox2 gene expression by occluding repressive NFκB complexes. eLife 2014, 3, e01776. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Xie, N.; Tan, Z.; Banerjee, S.; Thannickal, V.J.; Abraham, E.; Liu, G. The human long non coding RNA lnc-IL7R regulates the inflammatory response. Eur. J. Immunol. 2014, 44, 2085–2095. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Froberg, J.E.; Lee, J.T. Long non coding RNAs: Fresh perspectives into the RNA world. Trend Biochem. Sci. 2014, 39, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Garmire, L.X.; Garmire, D.G.; Huang, W.; Yao, J.; Glass, C.K.; Subramaniam, S. A global clustering algorithm to identify long intergenic non-coding RNA—With applications in mouse macrophages. PLoS ONE 2011, 6, e24051. [Google Scholar] [CrossRef] [PubMed]
- Lakhotia, S.C.; Mallik, M.; Singh, A.K.; Ray, M. The large noncoding hsromega-n transcripts are essential for thermotolerance and remobilization of hnRNPs, HP1 and RNA polymerase II during recovery from heat shock in Drosophila. Chromosoma 2012, 121, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Meller, V.H. Non-coding RNA in fly dosage compensation. Trends Biochem. Sci. 2006, 31, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Soshnev, A.A.; Ishimoto, H.; McAllister, B.F.; Li, X.; Wehling, M.D.; Kitamoto, T.; Geyer, P.K. A conserved long noncoding RNA affects sleep behavior in Drosophila. Genetics 2011, 189, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wen, S.; Guo, X.; Bai, B.; Gong, Z.; Liu, X.; Wang, Y.; Zhou, Y.; Chen, X.; Liu, L.; et al. The novel long non-coding RNA CRG regulates Drosophila locomotor behavior. Nucleic Acids Res. 2012, 40, 11714–11727. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dai, H.; Chen, S.; Zhang, L.; Long, M. Highly tissue specific expression of Sphinx supports its male courtship related role in Drosophila melanogaster. PLoS ONE 2011, 6, e18853. [Google Scholar] [CrossRef] [PubMed]
- Kiya, T.; Kunieda, T.; Kubo, T. Inducible- and constitutive-type transcript variants of kakusei, a novel non-coding immediate early gene, in the honeybee brain. Insect Mol. Biol. 2008, 17, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Sawata, M.; Takeuchi, H.; Kubo, T. Identification and analysis of the minimal promoter activity of a novel noncoding nuclear RNA gene, AncR-1, from the honeybee (Apismellifera L.). RNA 2004, 10, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Sawata, M.; Yoshino, D.; Takeuchi, H.; Kamikouchi, A.; Ohashi, K.; Kubo, T. Identification and punctate nuclear localization of a novel noncoding RNA, Ks-1, from the honeybee brain. RNA 2002, 8, 772–785. [Google Scholar] [CrossRef] [PubMed]
- Humann, F.C.; Tiberio, G.J.; Hartfelder, K. Sequence and expression characteristics of long noncoding RNAs in honey bee caste development-potential novel regulators for transgressive ovary size. PLoS ONE 2013, 8, e78915. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, X. Long noncoding RNAs in innate immunity. Cell. Mol. Immunol. 2016, 13, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Guttman, M.; Amit, I.; Garber, M.; French, C.; Lin, M.F.; Feldser, D.; Huarte, M.; Zuk, O.; Carey, B.W.; Cassady, J.P.; et al. Chromatin Signature reveals over a thousand conserved large non-coding RNs in mammals. Nature 2009, 458, 223–227. [Google Scholar] [CrossRef] [PubMed]
- IIott, N.E.; Heward, J.A.; Roux, B.; Tsitsiou, E.; Fenwick, P.S.; Lenzi, L.; Goodhead, I.; Hertz-Fowler, C.; Heger, A.; Hall, N.; et al. Long non coding RNAs and enhancer RNAs regulate the lipopolysaccharide- induced inflammatory response in human monocytes. Nat. Commun. 2014, 5, 3979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, G.; Tang, Q.; Sharma, S.; Yu, F.; Escobar, T.M.; Muljo, S.A.; Zhu, J.; Zhao, K. Expression and regulation of intergenic long non coding RNAs during T-cell development and differentiated. Nat. Immunol. 2014, 14, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chao, T.-C.; Chang, K.-Y.; Lin, N.; Patil, V.S.; Shimizu, C.; Head, S.R.; Burns, J.C.; Rana, T.M. The long noncoding RNA THRIL regulates TNFα expression through its interaction with hnRNPL. Proc. Natl. Acad. Sci. USA 2013, 111, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Gralinski, L.; Armour, C.D.; Ferris, M.T.; Thomas, M.J.; Proll, S.; Bradel-Tretheway, B.G.; Korth, M.J.; Castle, J.C.; Biery, M.C.; et al. Unique signatures of long non-coding RNAs expression in response to virus infection and altered innate immune signalling. mBio 2010, 1. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.; Aiello, D.; Atianand, M.K.; Ricci, E.P.; Gandhi, P.; Hall, L.L.; Byron, M.; Monks, B.; Henry-Bezy, M.; Lawrence, J.B.; et al. A long noncoding RNA mediates both activation and repression of immune response genes. Science 2013, 341, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Rapicavoli, N.A.; Qu, K.; Zhang, J.; Mikhail, M.; Laberge, R.; Chang, H.Y. A mammalian pseudogene lncRNA at the interface of inflammation & anti-inflammatory therapeutics. eLife 2013, 2, e00762. [Google Scholar] [PubMed]
- Medzhitov, R.; Horng, T. Transcriptional control of the inflammatory response. Nat. Rev. Immunol. 2009, 9, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Imamura, K.; Imamachi, N.; Akizuki, G.; Kumakura, M.; Kawaguchi, A.; Nagata, K.; Kato, A.; Kawaguchi, Y.; Sato, H.; Yoneda, M.; et al. Long noncoding RNA NEAT1-dependent SFPQ relocation from promoter region to paraspeckle mediates IL8 expression upon immune stimuli. Mol. Cell 2014, 53, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Song, L.; Fitzgerald, M.; Maurer, K.; Bagashev, A.; Sullivan, K.E. Noncoding RNAs and LRRFIP1 regulate TNF expression. J. Immunol. 2014, 192, 3057–3067. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xue, Y.; Han, Y.; Lin, L.; Wu, C.; Xu, S.; Jiang, Z.; Xu, J.; Liu, Q.; Cao, X. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science 2014, 344, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Maass, P.G.; Luft, F.C.; Bahring, S. Long non-coding RNA in health and disease. J. Mol. Med. 2014, 92, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S.; Coller, J. RNA in unexpected places: Long non-coding RNA functions in diverse cellular contexts. Nat. Rev. Mol. Cell Biol. 2013, 14, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Shippy, T.D.; Ronshaugen, M.; Cande, J.; He, J.; Beeman, R.W.; Levine, M.; Brown, S.J.; Denell, R.E. Analysis of the Tribolium homeotic complex: Insights into mechanisms constraining insect Hox clusters. Dev. Genes Evol. 2008, 218, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Akbari, O.S.; Antoshechkin, I.; Hay, B.A.; Ferree, P.M. Transcriptome profiling of Nasoniavitripennis testis reveals novel transcripts expressed from the selfish B chromosome, paternal sex ratio. G3 2013, 3, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, S.; Iwami, M.; Kiya, T. Identification and characterization of a novel nuclear noncoding RNA, Fben-1, which is preferentially expressed in the higher brain center of the female silkworm moth, Bombyx mori. Neurosci. Lett. 2011, 496, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-Z.; Zhang, B.; Yu, Q.-Y.; Zhang, Z. BmncRNAdb: A comprehensive database of non-coding RNAs in the silkworm, Bombyx mori. BMC Bioinform. 2016, 17, 370. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-D.; Liu, Z.-C.; Huang, L.; Jiang, Q.L.; Zhang, K.; Qiao, H.L.; Jiao, Z.J.; Yao, L.G.; Liu, R.Y.; Kan, Y.C. The expression analysis of silk gland-enriched intermediate-size non-coding RNAs in silkworm Bombyx mori. Insect Sci. 2014, 21, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Tadano, H.; Yamazaki, Y.; Takeuchi, H.; Kubo, T. Age- and division-of-labour-dependent differential expression of a novel non-coding RNA, Nb-1, in the brain of worker honeybees, Apismellifera L. Insect Mol. Biol. 2009, 18, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Lipshitz, H.D.; Peattie, D.A.; Hogness, D.S. Novel transcripts from the ultrabithorax domain of the bithorax complex. Genes Dev. 1987, 1, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Etebari, K.; Osei-Amo, S.; Blomberg, S.P.; Asgari, S. Dengue virus infection alters post-transcriptional modification of microRNAs in the mosquito vector Aedesaegypti. Sci. Rep. 2015, 5, 15968. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, K.M.; Park, D.; Wolf, A.R.; Van Tyne, D.; Sims, J.S.; Ribacke, U.; Volkman, S.; Duraisingh, M.; Wirth, D.; Sabeti, P.C.; et al. A global transcriptional analysis of Plasmodium falciparum malaria reveals a novel family of telomere-associated lncRNAs. Genome Biol. 2011, 12, R56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amit-Avraham, I.; Pozner, G.; Eshar, S.; Fastman, Y.; Kolevzon, N.; Yavin, E.; Dzikowski, R. Antisense long noncoding RNAs regulate var gene activation in the malaria parasite Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2015, 112, E982–E991. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, C.C.; Pari, G.S. PAN’s Labyrinth: Molecular biology of Kaposi’s sarcoma-associated herpesvirus (KSHV) PAN RNA, a multifunctional long noncoding RNA. Viruses 2014, 6, 4212–4226. [Google Scholar] [CrossRef] [PubMed]
- Stanojcic, S.; Gimenez, S.; Permal, E.; Cousserans, F.; Quesneville, H.; Fournier, P.; d’Alençon, E. Correlation of LNCR rasiRNAs expression with heterochromatin formation during development of the holocentric insect Spodopterafrugiperda. PLoS ONE 2011, 6, e24746. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R. Characterisation of long noncoding RNAs (lncRNAs) involved in immune response during baculoviral infection in Bombyx mori. Dissertation submitted towards completion of Summer Research Fellowship Program to Indian Academy of Sciences, Bangalore, India, May–June 2013. [Google Scholar]
| Species | Gene | Function | Reference |
|---|---|---|---|
| Anopheles gambiae | RNAseq | ||
| Apis mellifera | AncR-1 | Neural expression | [27] |
| Kakusei | RNA metabolism | [14] | |
| Ks-1 | Neural expression | [28] | |
| Lnccov1/2 | Autophagic cell death of ovarioles | [29] | |
| Nb-1 | Putative role in polyethism | [49] | |
| Bombyx mori | Fben-1 | Biosynthesis, translocation, and secretion of silk proteins | [46,47] |
| Drosophila melanogaster | bithora | Development of abdominal segments | [50] |
| hsr-w | Heat shock stress | [21] | |
| roX1/2 | Dosage compensation | [22] | |
| sphinx | Regulates sensory circuits | [25] | |
| yar | Regulator of yellow and achaete transcription | [23] | |
| Plutella xylostella | lincRNA | Detoxification and toxin related metabolism | [51] |
| Plasmodium falciparum | lncRNA-TARE | Plays a role intranscriptional regulation | [52] |
| Var | Regulates var gene activation | [53] | |
| PAN | Facilitates switch from latent to lytic infection | [54] | |
| Spodoptera frugiperda | LNCR | Formation of heterochromatin | [55] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satyavathi, V.; Ghosh, R.; Subramanian, S. Long Non-Coding RNAs Regulating Immunity in Insects. Non-Coding RNA 2017, 3, 14. https://doi.org/10.3390/ncrna3010014
Satyavathi V, Ghosh R, Subramanian S. Long Non-Coding RNAs Regulating Immunity in Insects. Non-Coding RNA. 2017; 3(1):14. https://doi.org/10.3390/ncrna3010014
Chicago/Turabian StyleSatyavathi, Valluri, Rupam Ghosh, and Srividya Subramanian. 2017. "Long Non-Coding RNAs Regulating Immunity in Insects" Non-Coding RNA 3, no. 1: 14. https://doi.org/10.3390/ncrna3010014





