Long Non-Coding RNA TUG1 Expression Is Associated with Different Subtypes in Human Breast Cancer
Abstract
:1. Introduction
2. Results
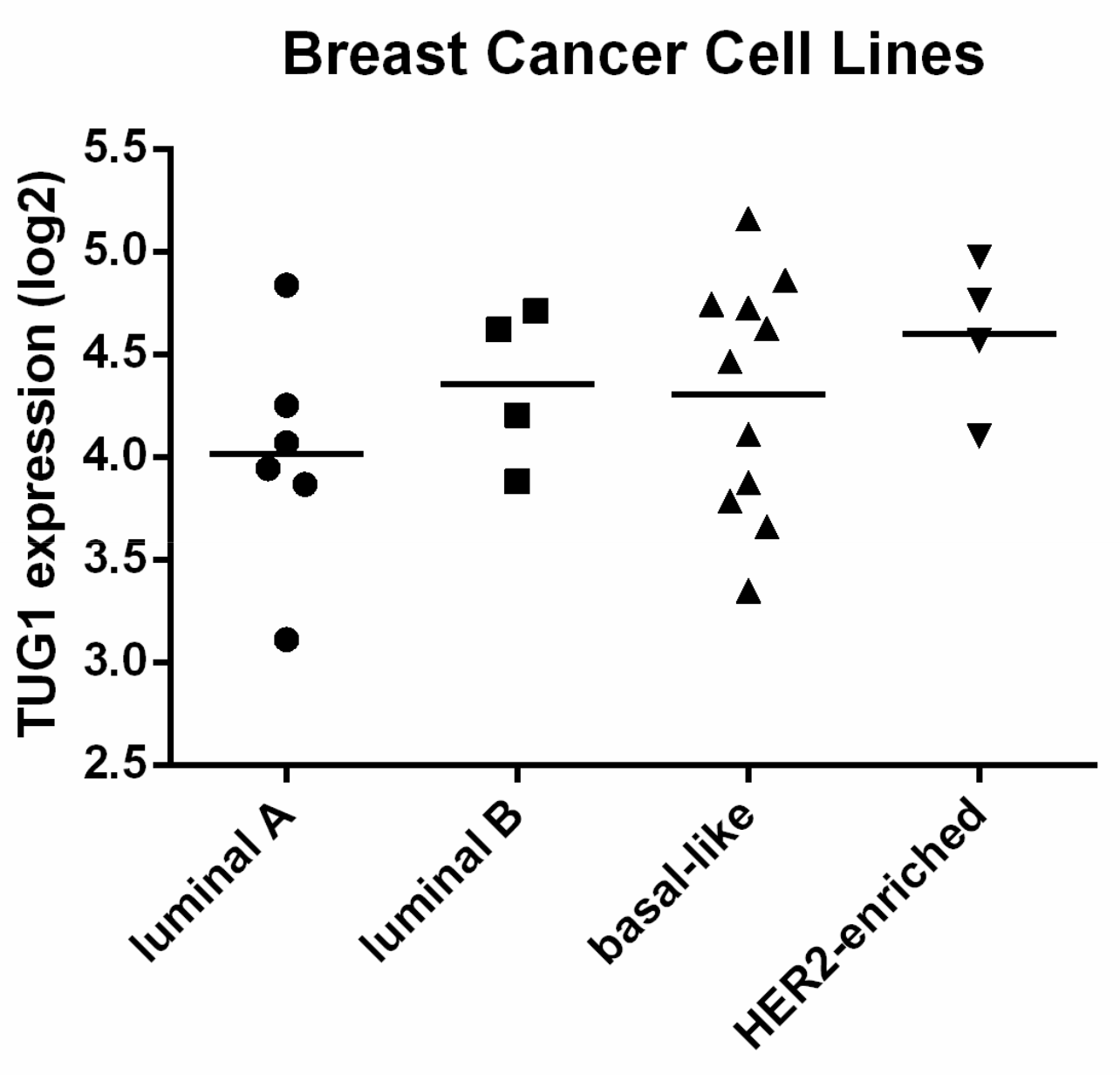
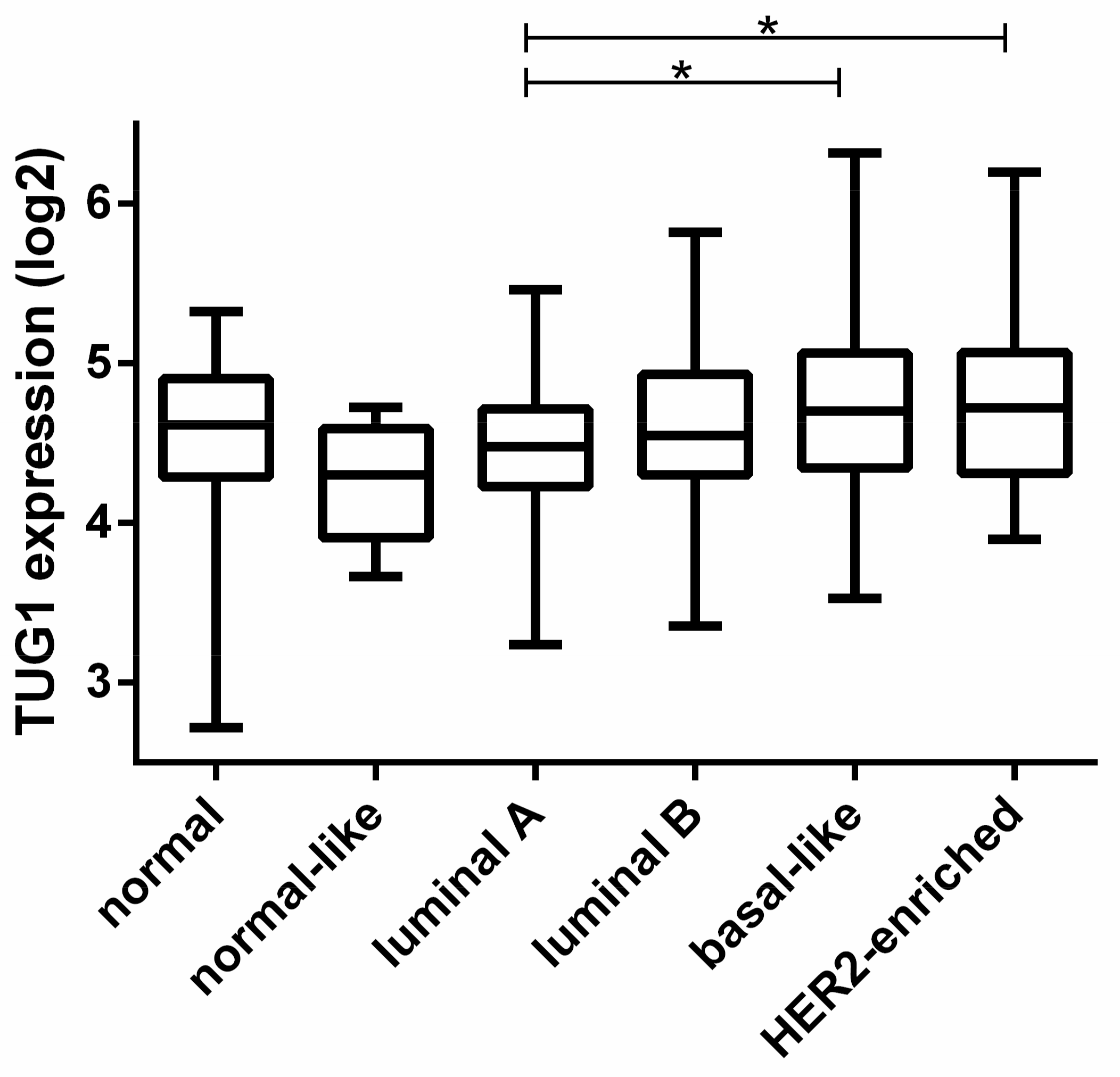
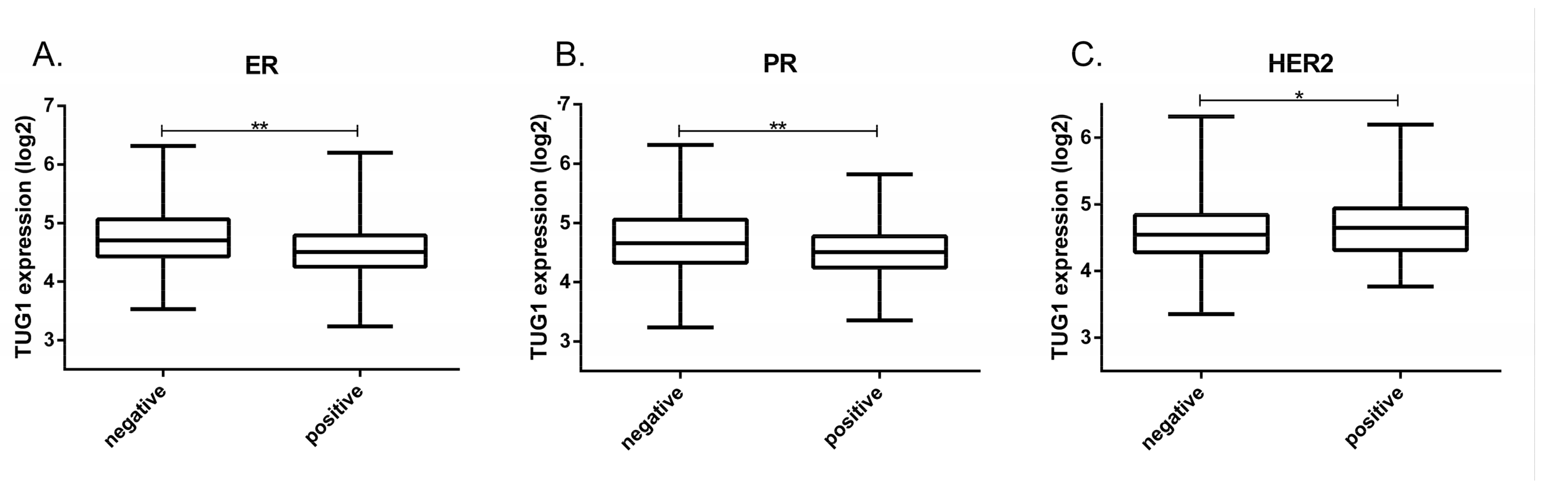
2.1. TUG1 Expression Is Increased in HER2-Enriched and Basal-Like Compared to Luminal A
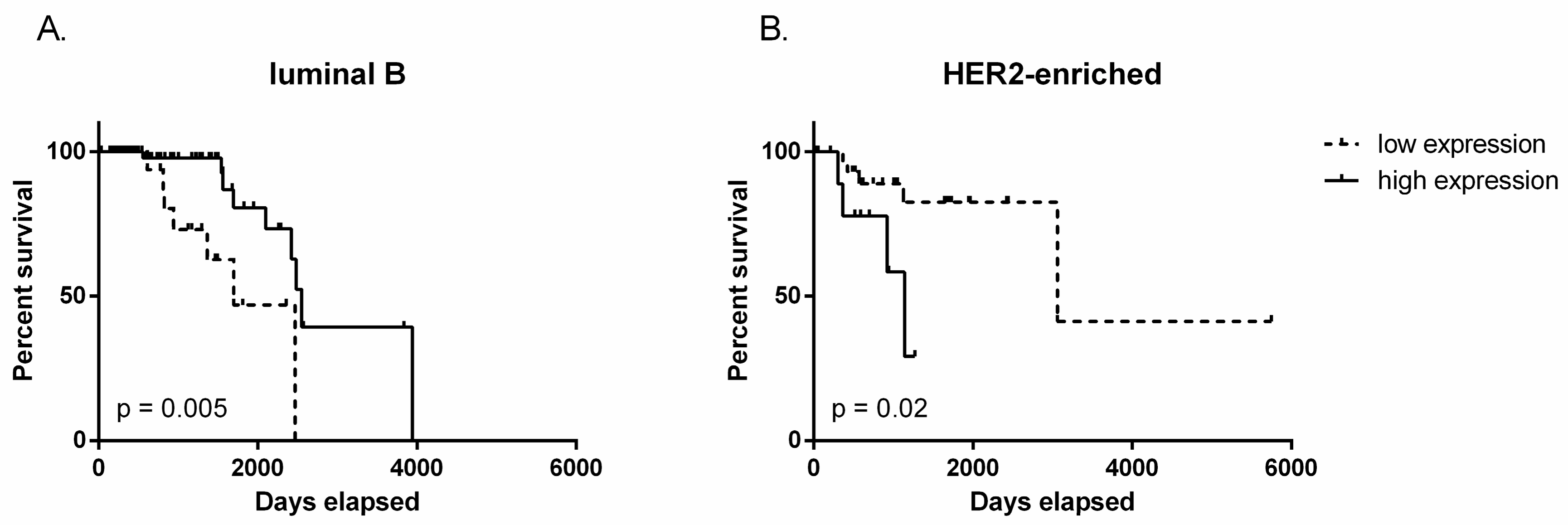
2.2. TUG1 Has a Different Impact on Survival Depending on Molecular Subtype
3. Discussion
4. Patients and Methods
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Rao, A.K.D.M.; Rajkumar, T.; Mani, S. Perspectives of long non-coding RNAs in cancer. Mol. Biol. Rep. 2017, 44, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Fatica, A.; Bozzoni, I. Long non-coding RNAs: New players in cell differentiation and development. Nat. Rev. Genet. 2014, 15, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Melissari, M.T.; Grote, P. Roles for long non-coding RNAs in physiology and disease. Pflug. Arch. 2016, 468, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Hon, C.C.; Ramilowski, J.A.; Harshbarger, J.; Bertin, N.; Rackham, O.J.; Gough, J.; Denisenko, E.; Schmeier, S.; Poulsen, T.M.; Severin, J.; et al. An atlas of human long non-coding rnas with accurate 5′ ends. Nature 2017, 543, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Dykes, I.M.; Emanueli, C. Transcriptional and post-transcriptional gene regulation by long non-coding RNA. Genom. Proteom. Bioinform. 2017, 15, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Young, T.L.; Matsuda, T.; Cepko, C.L. The noncoding RNA taurine upregulated gene 1 is required for differentiation of the murine retina. Curr. Biol. 2005, 15, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shen, J.; Chan, M.T.; Wu, W.K. TUG1: A pivotal oncogenic long non-coding RNA of human cancers. Cell Prolif. 2016, 49, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, Y.; Xiao, H.; Xu, G. Long non-coding RNA TUG1 promotes cell proliferation and metastasis in human breast cancer. Breast Cancer 2017, 24, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Sugita, B.; Gill, M.; Mahajan, A.; Duttargi, A.; Kirolikar, S.; Almeida, R.; Regis, K.; Oluwasanmi, O.L.; Marchi, F.; Marian, C.; et al. Differentially expressed miRNAs in triple negative breast cancer between african-american and non-hispanic white women. Oncotarget 2016, 7, 79274–79291. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.O.; Parker, J.S.; Leung, S.; Voduc, D.; Ebbert, M.; Vickery, T.; Davies, S.R.; Snider, J.; Stijleman, I.J.; Reed, J.; et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin. Cancer Res. 2010, 16, 5222–5232. [Google Scholar] [CrossRef] [PubMed]
- Singletary, S.E.; Connolly, J.L. Breast cancer staging: working with the sixth edition of the AJCC Cancer Staging Manual. CA Cancer J. Clin. 2006, 56, 37–47. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Connolly, J.L; Edge, S.B.; Mittendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D. L.; Winchester, D.J.; Hortobagyi, G.N. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.M.; Guttman, M.; Huarte, M.; Garber, M.; Raj, A.; Rivea Morales, D.; Thomas, K.; Presser, A.; Bernstein, B.E.; van Oudenaarden, A.; et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc. Natl. Acad. Sci. USA 2009, 106, 11667–11672. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Li, M.; Zhang, L.; Huang, M.; Lei, J.B.; Fu, G.H.; Liu, C.X.; Lai, Q.W.; Chen, Q.Q.; Wang, Y.L. Upregulation of long non-coding RNA TUG1 correlates with poor prognosis and disease status in osteosarcoma. Tumour Biol. 2016, 37, 4445–4455. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Liu, G.B.; Liu, B.H.; Chen, G.; Li, K.; Zheng, S.; Dong, K.R. Targeting long non-coding RNA-TUG1 inhibits tumor growth and angiogenesis in hepatoblastoma. Cell Death Dis. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.D.; Chen, W.M.; Qi, F.Z.; Sun, M.; Xu, T.P.; Ma, P.; Shu, Y.Q. Long non-coding RNA TUG1 is up-regulated in hepatocellular carcinoma and promotes cell growth and apoptosis by epigenetically silencing of KLF2. Mol. Cancer 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.B.; Yin, D.D.; Sun, M.; Kong, R.; Liu, X.H.; You, L.H.; Han, L.; Xia, R.; Wang, K.M.; Yang, J.S.; et al. P53-regulated long non-coding rna tug1 affects cell proliferation in human non-small cell lung cancer, partly through epigenetically regulating HOXB7 expression. Cell Death Dis. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; An, G.; Zhang, M.; Ma, Q. Long non-coding RNA TUG1 acts as a miR-26a sponge in human glioma cells. Biochem. Biophys. Res. Commun. 2016, 477, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Shi, K.; Kang, X.; Li, W. Prognostic value of long non-coding RNA TUG1 in various tumors. Oncotarget 2017, 8, 65659–65667. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lin, J.; Li, Y.; Zhang, Y.; Chen, X. Prognostic role of lncRNA TUG1 for cancer outcome: Evidence from 840 cancer patients. Oncotarget 2017, 8, 50051–50060. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Yang, Z.; Ke, Z.; Huang, K.; Liu, N.; Fang, X.; Wang, K. Downregulation of the long non-coding RNA TUG1 is associated with cell proliferation, migration, and invasion in breast cancer. Biomed. Pharm. 2017, 95, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Colleoni, M.; Munzone, E. Extended adjuvant chemotherapy in endocrine non-responsive disease. Breast 2013, 22 (Suppl. 2), S161–S164. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Qiu, K.; Li, M.; Liang, Y. Double-negative feedback loop between long non-coding RNA TUG1 and miR-145 promotes epithelial to mesenchymal transition and radioresistance in human bladder cancer cells. FEBS Lett. 2015, 589, 3175–3181. [Google Scholar] [CrossRef] [PubMed]
- Katsushima, K.; Natsume, A.; Ohka, F.; Shinjo, K.; Hatanaka, A.; Ichimura, N.; Sato, S.; Takahashi, S.; Kimura, H.; Totoki, Y.; et al. Targeting the Notch-regulated non-coding RNA TUG1 for glioma treatment. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Sun, T.; Hacisuleyman, E.; Fei, T.; Wang, X.; Brown, M.; Rinn, J.L.; Lee, M.G.; Chen, Y.; Kantoff, P.W.; et al. Integrative analyses reveal a long noncoding RNA-mediated sponge regulatory network in prostate cancer. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Network, C.G.A. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar]
- Parker, J.S.; Mullins, M.; Cheang, M.C.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, L.; Roebuck, P.; Diao, L.; Liu, L.; Yuan, Y.; Weinstein, J.N.; Liang, H. TANRIC: An Interactive Open Platform to Explore the Function of lncRNAs in Cancer. Cancer Res. 2015, 75, 3728–3737. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef] [PubMed]
- Neve, R.M.; Chin, K.; Fridlyand, J.; Yeh, J.; Baehner, F.L.; Fevr, T.; Clark, L.; Bayani, N.; Coppe, J.P.; Tong, F.; et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006, 10, 515–527. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. A Language and Environment for Statistical Computing 2017; R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
| Multivariate Cox Analysis | ||
|---|---|---|
| Covariate | Hazard Ratio (95% CI) | p-Value |
| Age at initial diagnosis | 1.0038 (0.9480–1.063) | 0.897 |
| TUG1 Expression | 0.5427 (0.1539–1.914) | 0.342 |
| Tumor Size (T1, n = 17; T2, n = 84; T3, n = 15; T4, n = 6) | 1.4296 (0.4925–4.150) | 0.511 |
| Node Metastasis (N0, n = 52; N1, n = 46; N2, n = 16; N3, n = 8) | 0.8022 (0.2955–2.178) | 0.665 |
| Distant Metastasis (M0, n = 114; M1, n = 5) | 3.3331 (0.2284–48.651) | 0.379 |
| Multivariate Cox Analysis | ||
|---|---|---|
| Covariate | Hazard Ratio (95% CI) | p-Value |
| Age at initial diagnosis | 1.130 (1.0110–1.264) | 0.0314 |
| TUG1 Expression | 37.262 (2.5634–541.647) | 0.00807 |
| Tumor Size (T1, n = 8; T2, n = 36; T3, n = 8; T4, n = 3) | 2.374 (0.5688–9.904) | 0.23564 |
| Node Metastasis (N0, n = 21; N1, n = 17; N2, n = 11; N3, n = 6) | 1.757 (0.6721–4.593) | 0.25039 |
| Distant Metastasis (M0, n = 53; M1, n = 2) | 27.257 (1.2025–617.812) | 0.03791 |
| Characteristics | ||
|---|---|---|
| Gender | Male (n = 8) | Female (n = 784) |
| Age at diagnosis | ≤35 (n = 27) | >35 (n = 765) |
| ER status | Negative (n = 173) | Positive (n = 582) |
| PR status | Negative (n = 247) | Positive (n = 506) |
| HER2 status | Negative (n = 628) | Positive (n = 113) |
| Tumor Size | ≤2cm (n = 202) | ≥2cm (n = 560) |
| Node Metastasis | Negative (n = 369) | Positive (n = 396) |
| Distant Metastasis | Negative (n = 745) | Positive (n = 14) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gradia, D.F.; Mathias, C.; Coutinho, R.; Cavalli, I.J.; Ribeiro, E.M.S.F.; De Oliveira, J.C. Long Non-Coding RNA TUG1 Expression Is Associated with Different Subtypes in Human Breast Cancer. Non-Coding RNA 2017, 3, 26. https://doi.org/10.3390/ncrna3040026
Gradia DF, Mathias C, Coutinho R, Cavalli IJ, Ribeiro EMSF, De Oliveira JC. Long Non-Coding RNA TUG1 Expression Is Associated with Different Subtypes in Human Breast Cancer. Non-Coding RNA. 2017; 3(4):26. https://doi.org/10.3390/ncrna3040026
Chicago/Turabian StyleGradia, Daniela F., Carolina Mathias, Rodrigo Coutinho, Iglenir J. Cavalli, Enilze M. S. F. Ribeiro, and Jaqueline C. De Oliveira. 2017. "Long Non-Coding RNA TUG1 Expression Is Associated with Different Subtypes in Human Breast Cancer" Non-Coding RNA 3, no. 4: 26. https://doi.org/10.3390/ncrna3040026





