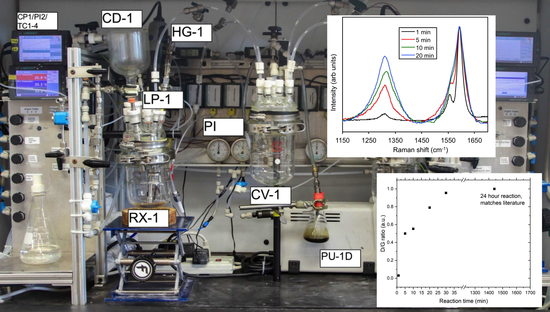
Apparatus for Scalable Functionalization of Single-Walled Carbon Nanotubes via the Billups-Birch Reduction
Abstract
:1. Introduction
2. Results and Discussion
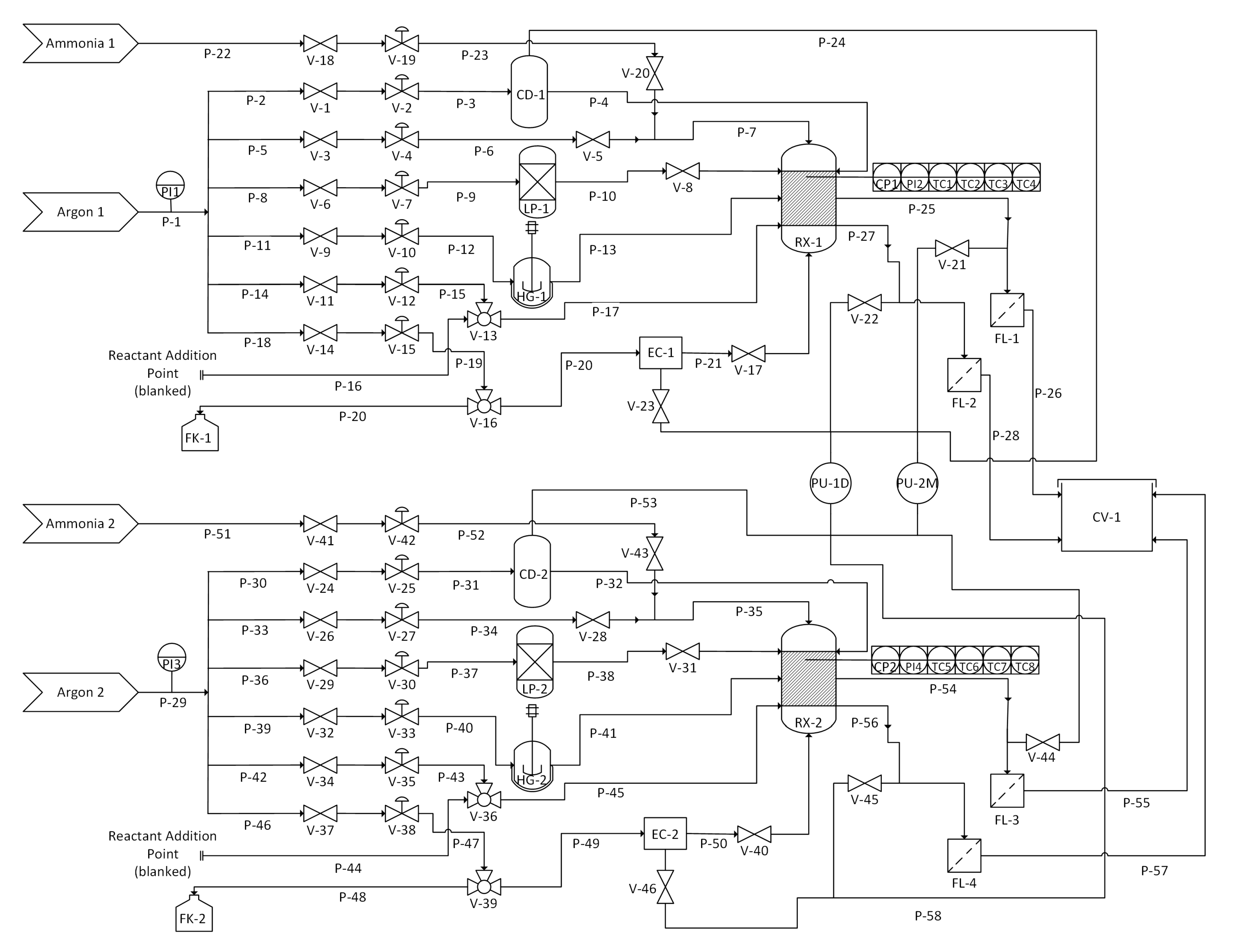
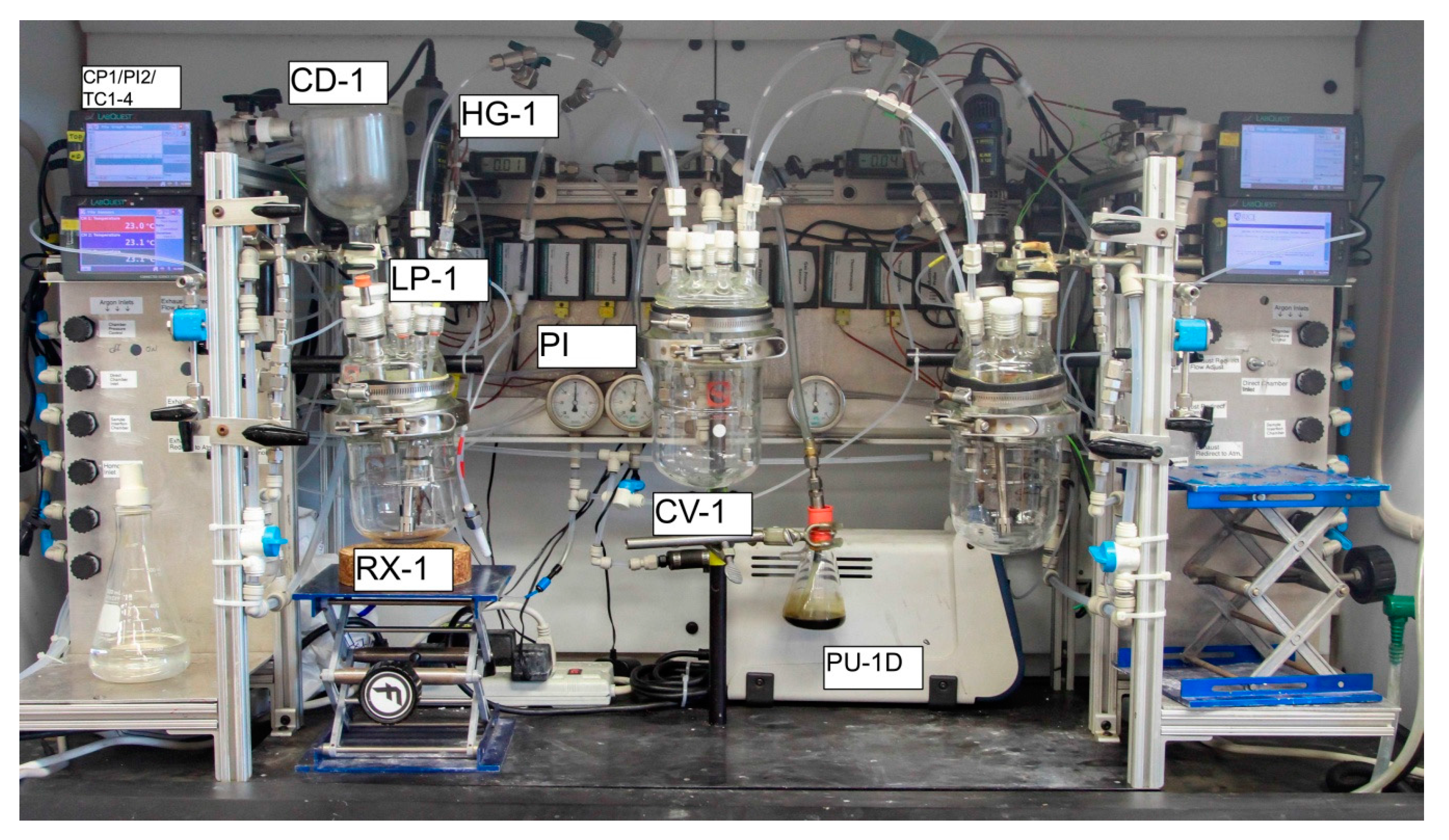
2.1. Apparatus Design and Operation
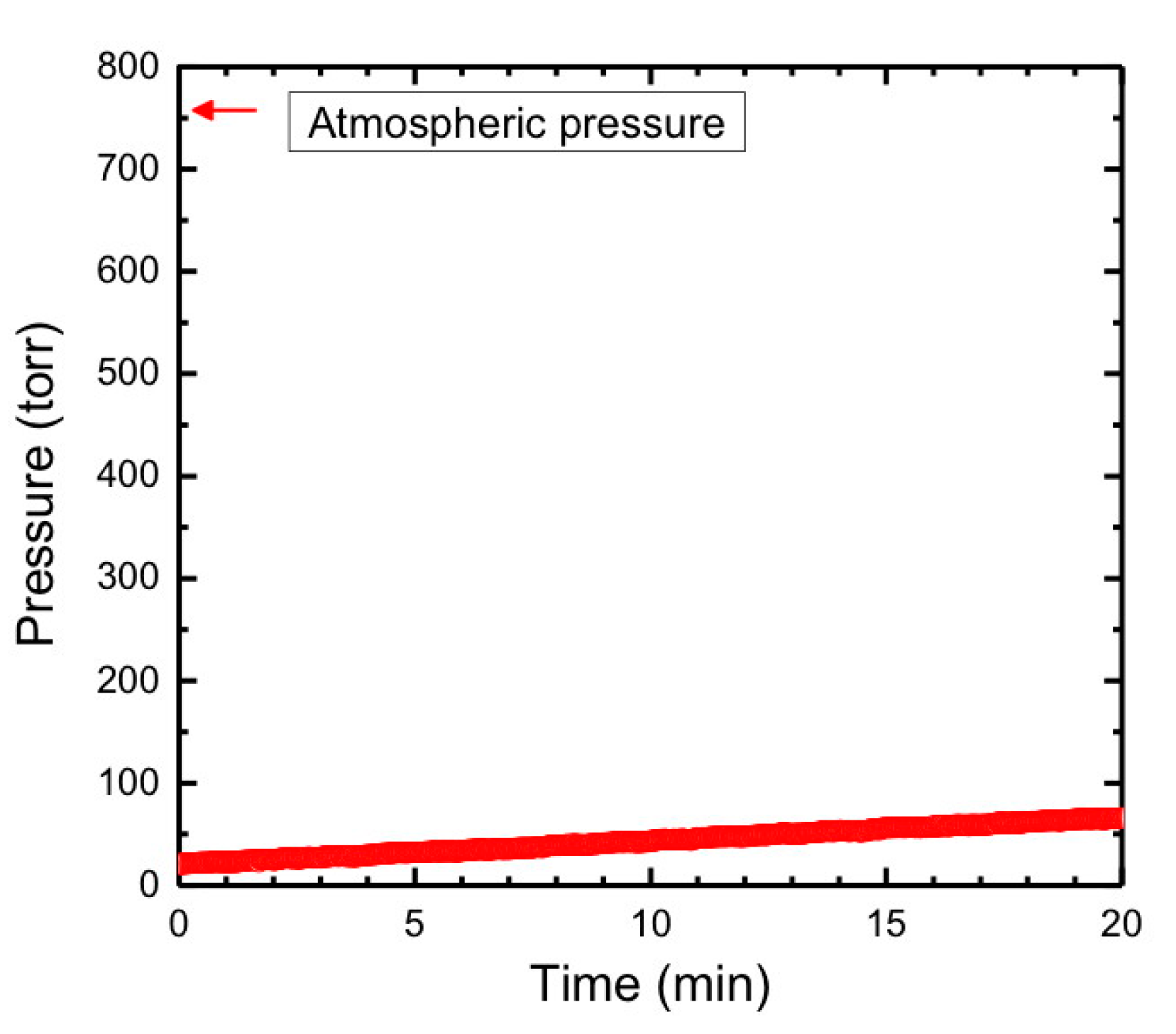
2.2. Hazards Associated with Lithium Metal and Ammonia
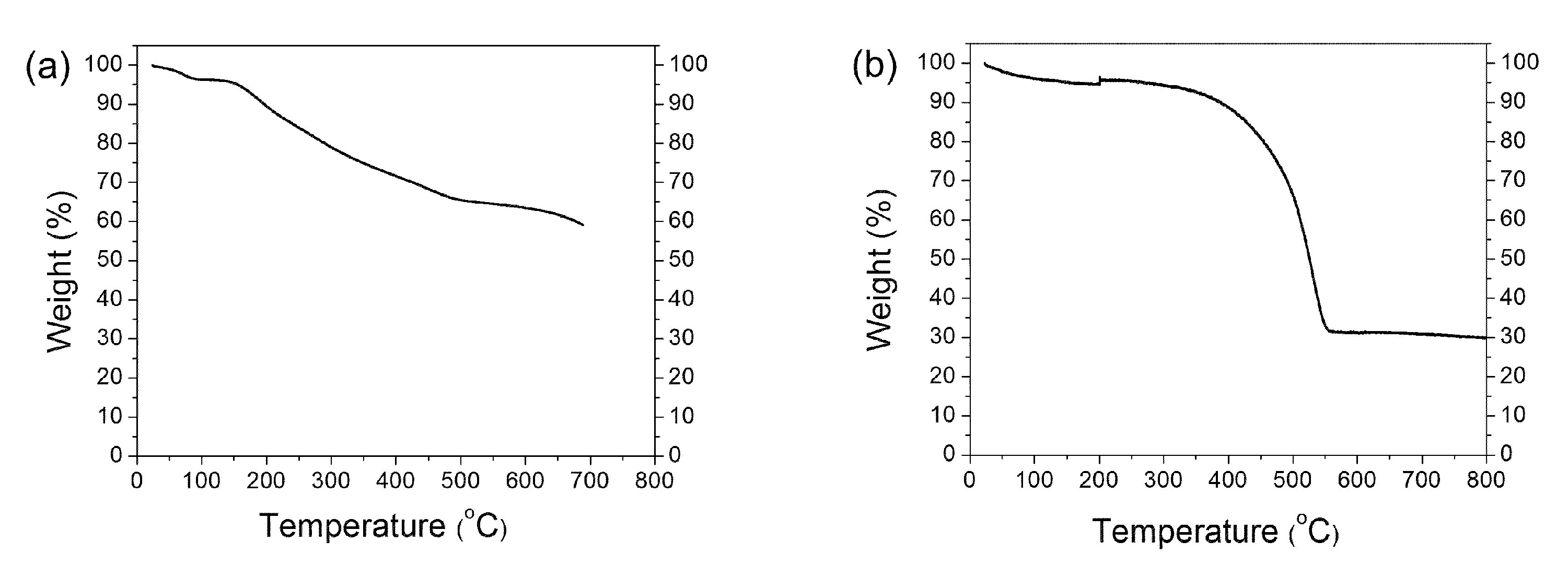
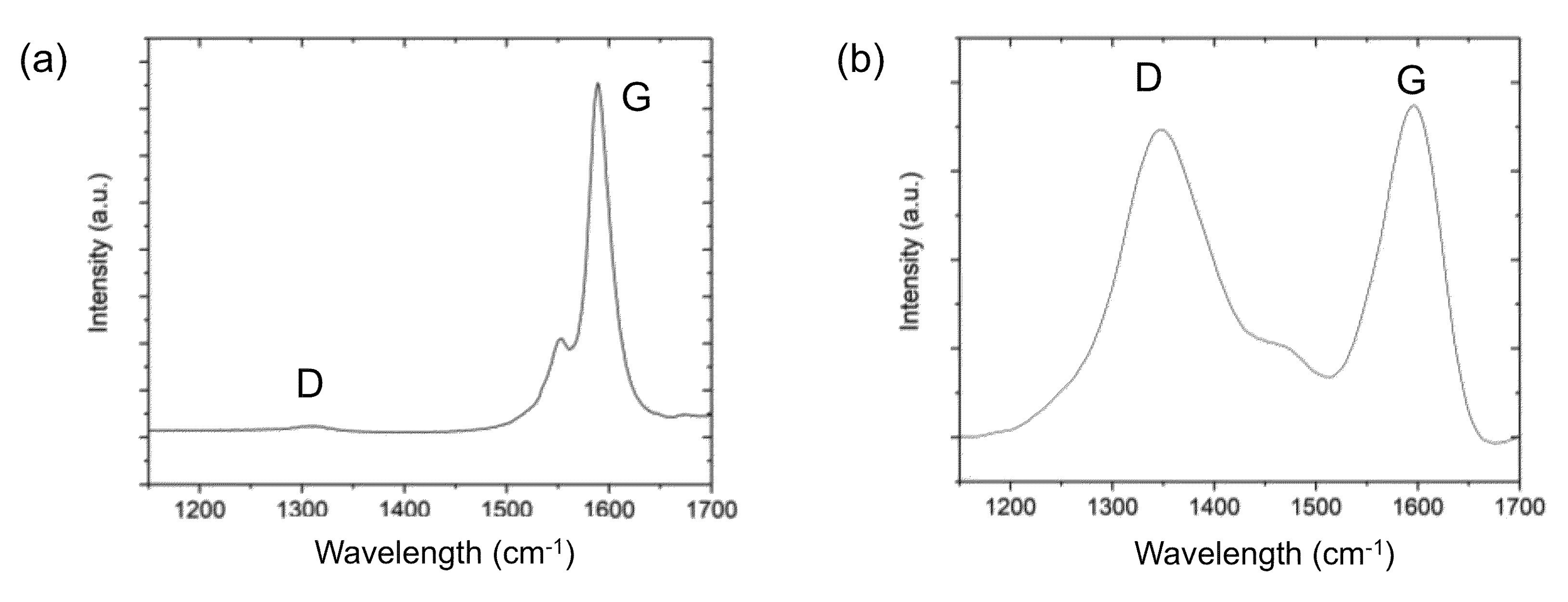
2.3. Example of SWCNT Functionalization
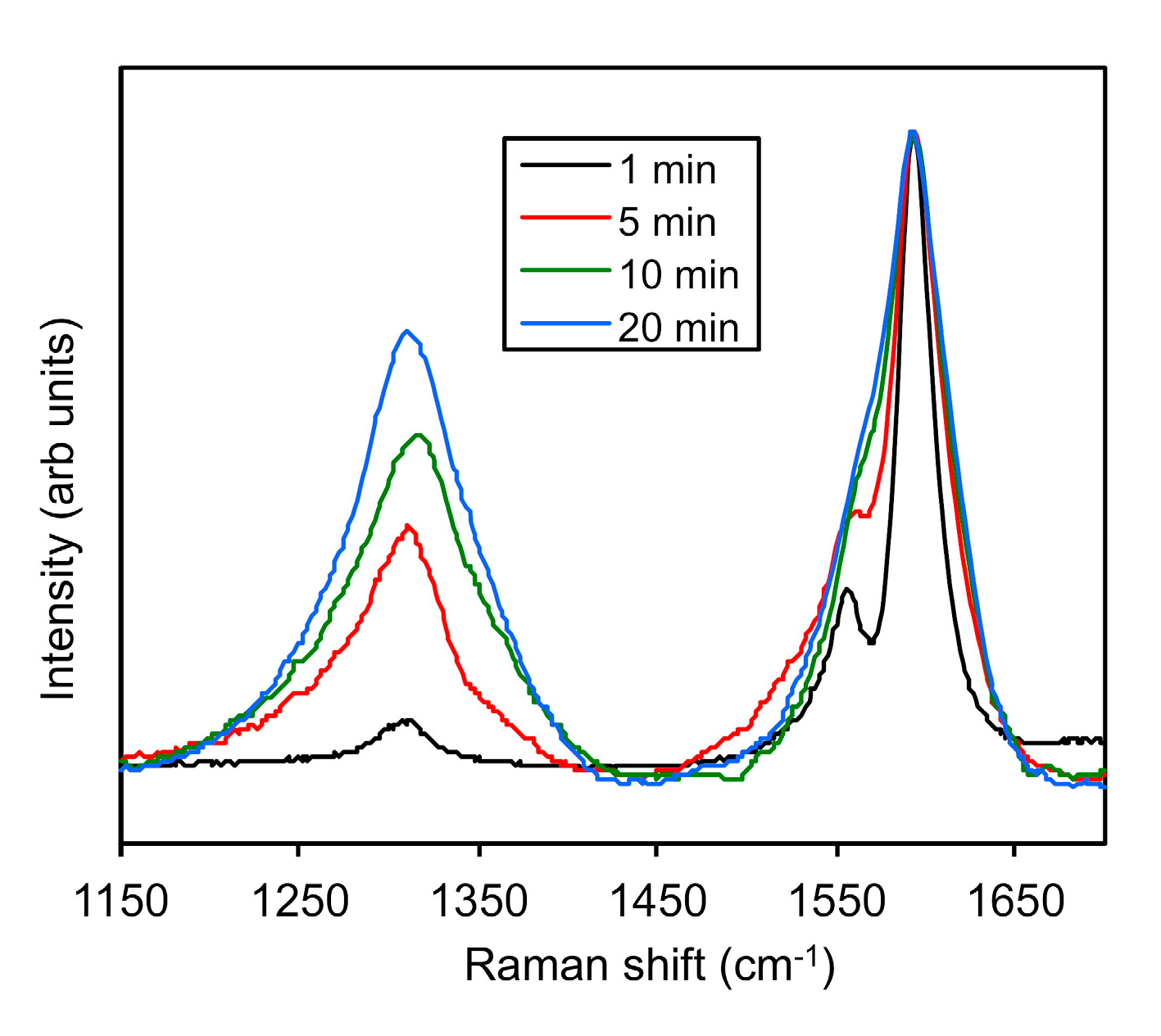
2.4. Monitoring the Alkylation Reaction
3. Experimental Section
3.1. Materials and Characterization
3.2. Apparatus
3.3. Alkylation of SWCNTs
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barron, A.R.; Khan, M.R. Carbon nanomaterials: Opportunities and challenges. Adv. Mater. Process. 2008, 166, 41–43. [Google Scholar]
- O’Connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef]
- Chen, J.; Hamon, M.A.; Hu, H.; Chen, Y.; Rao, A.M.; Eklund, P.C.; Haddon, R.C. Solution properties of single-walled carbon nanotubes. Science 1998, 282, 95–98. [Google Scholar] [CrossRef]
- Hamilton, C.E.; Barron, A.R. Phosphene functionalized single walled carbon nanotubes. Main Group Chem. 2009, 8, 275–281. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Fu, K.; Lin, Y.; Huang, W. Functionalized carbon nanotubes: Properties and applications. Acc. Chem. Res. 2002, 35, 1096–1104. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, L.; Barron, A.R. Tailoring aqueous solubility of functionalized single-wall carbon nanotubes over a wide pH range through substituent chain length. Nano Lett. 2005, 5, 2001–2004. [Google Scholar] [CrossRef]
- Peng, H.; Reverdy, P.; Khabashesku, V.N.; Margrave, J.L. Sidewall functionalization of single-walled carbon nanotubes with organic peroxides. Chem. Commun. 2003, 7, 362–363. [Google Scholar] [CrossRef]
- Zhang, X.; Sreekumar, T.V.; Liu, T.; Kumar, S. Properties and structure of nitric acid oxidized single wall carbon nanotube films. J. Phys. Chem. B 2004, 108, 16435–16440. [Google Scholar] [CrossRef]
- Bhakta, A.K.; Detriche, S.; Martis, P.; Mascarenhas, R.J.; Delhalle, J.; Mekhalif, Z. Decoration of tricarboxylic and monocarboxylic aryl diazonium functionalized multi-wall carbon nanotubes with iron nanoparticles. J. Mater. Sci. 2017. [Google Scholar] [CrossRef]
- Holzinger, M.; Vostrowsky, O.; Hirsch, A.; Hennrich, F.; Kappes, M.; Weiss, R.; Jellen, F. Sidewall functionalization of carbon nanotubes. Angew. Chem. Int. Ed. 2001, 40, 4002–4005. [Google Scholar] [CrossRef]
- Boul, P.J.; Liu, J.; Mickelson, E.T.; Huffman, C.B.; Ericson, L.M.; Chiang, I.W.; Smith, K.A.; Colbert, D.T.; Hauge, R.H.; Margrave, J.L.; et al. Reversible sidewall functionalization of buckytubes. Chem. Phys. Lett. 1999, 310, 367–372. [Google Scholar] [CrossRef]
- Dillon, E.P.; Andreoli, E.; Cullum, L.; Barron, A.R. Polyethyleneimine functionalized nanocarbons for the efficient absorption of carbon dioxide with a low temperature of regeneration. J. Exp. Nanosci. 2015, 10, 746–768. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.; Edwards, C.L.; Alemany, L.B.; Khabashesku, V.N.; Barron, A.R. Diels alder addition to fluorinated single walled carbon nanotubes. Chem. Commun. 2005, 14, 3265–3267. [Google Scholar] [CrossRef]
- Liang, F.; Sadana, A.K.; Peera, A.; Chattopadhyay, J.; Gu, Z.; Hauge, R.H.; Billups, W.E. A convenient route to functionalized carbon nanotubes. Nano Lett. 2004, 4, 1257–1260. [Google Scholar] [CrossRef]
- Chattopadhyay, J.; Sadana, A.K.; Liang, F.; Beach, J.M.; Xiao, Y.; Hauge, R.H.; Billups, W.E. Carbon nanotube salts. Arylation of single-wall carbon nanotubes. Org. Lett. 2005, 7, 4067–4069. [Google Scholar] [CrossRef]
- Anderson, R.E.; Barron, A.R. Solubilization of single-wall carbon nanotubes in organic solvents without sidewall functionalization. J. Nanosci. Nanotechnol. 2007, 7, 3436–3440. [Google Scholar] [CrossRef]
- Birch, A.J. Reduction by dissolving metals. Part I. J. Chem. Soc. 1944, 430–436. [Google Scholar] [CrossRef]
- Wilds, A.L.; Nelson, N.A. A superior method for reducing phenol ethers to dihydro derivatives and unsaturated ketones. J. Am. Chem. Soc. 1953, 75, 5360–5365. [Google Scholar] [CrossRef]
- Billups, W.E.; Sadana, A.K.; Liang, F.; Hauge, R.H. Reductive Functionalization of Carbon Nanotubes. U.S. Patent 7,758,841, 20 July 2010. [Google Scholar]
- Borondics, F.; Bokor, M.; Matus, P.; Tompa, K.; Pekker, S.; Jakab, E. Reductive functionalization of carbon nanotubes. Fuller. Nanotub. Carbon. Nan. 2007, 13, 375–382. [Google Scholar] [CrossRef]
- Joshi, D.K.; Sutton, J.W.; Carver, S.; Blanchard, J.P. Experiences with commercial production scale operation of dissolving metal reduction using lithium metal and liquid ammonia. Org. Process Res. Dev. 2005, 9, 997–1002. [Google Scholar] [CrossRef]
- Liang, F.; Alemany, L.B.; Beach, J.M.; Billups, E.W. Structure analyses of dodecylated single-walled carbon nanotubes. J. Am. Chem. Soc. 2005, 127, 13941–13948. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Schmandt, N.; Cratty, J.; Khabashesku, V.N.; Kelly, K.F.; Barron, A.R. AFM and STM characterization of thiol and thiophene functionalized SWNTs: Pitfalls in the use chemical markers to determine the extent of side-wall functionalization in SWNTs. Chem. Commun. 2005, 5429–5430. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Pimenta, M.A.; Ecklund, P.C.; Dresselhaus, G. Raman Scattering in Materials Science; Webber, W.H., Merlin, R., Eds.; Springer: Berlin, Germany, 2000. [Google Scholar]
- Gomez, V.; Irusta, S.; Lawal, O.B.; Adams, W.; Hauge, R.H.; Dunnill, C.W.; Barron, A.R. Enhanced purification of carbon nanotubes by microwave and chlorine cleaning procedures. RSC Adv. 2016, 6, 11895–11902. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, D.; Zhang, K.S.; Lawal, O.; Ghosh, S.; Gangoli, V.S.; Ainscough, T.J.; Kellogg, B.; Hauge, R.H.; Adams, W.W.; Barron, A.R. Apparatus for Scalable Functionalization of Single-Walled Carbon Nanotubes via the Billups-Birch Reduction. C 2017, 3, 19. https://doi.org/10.3390/c3020019
Pham D, Zhang KS, Lawal O, Ghosh S, Gangoli VS, Ainscough TJ, Kellogg B, Hauge RH, Adams WW, Barron AR. Apparatus for Scalable Functionalization of Single-Walled Carbon Nanotubes via the Billups-Birch Reduction. C. 2017; 3(2):19. https://doi.org/10.3390/c3020019
Chicago/Turabian StylePham, David, Kevin S. Zhang, Olawale Lawal, Saunab Ghosh, Varun Shenoy Gangoli, Thomas J. Ainscough, Bernie Kellogg, Robert H. Hauge, W. Wade Adams, and Andrew R. Barron. 2017. "Apparatus for Scalable Functionalization of Single-Walled Carbon Nanotubes via the Billups-Birch Reduction" C 3, no. 2: 19. https://doi.org/10.3390/c3020019