1. Introduction
Presently, more research and development are being directed towards graphene based nano-composites. In the last few years, the momentum in research and development of graphene/noble metal composites has attracted the attention of scientists and technologists, for practical applications. Recently, it has been observed that the graphene-based composites have wider applications than pristine or doped graphene. The chemical interactions between graphene and transition metals, including the noble metals, have improved mechanical, thermal, chemical, electrical and optical properties, to a large extent. Specifically, noble metal decorated graphene-based hybrid materials have been found to be highly sensitive and selective for gas-sensing, particularly hydrogen-sensing, owing to the synergistic effect of the compound configuration [
1].
Different solid state, liquid state and electrochemical methods have been developed to produce graphene nano-composites. The correlation of atomic bonding between metal and graphene has also been highlighted. The chemical interaction between graphene and metal matrix at the interface of the composite is an interesting phenomenon, which is a valuable mechanism for interpreting the reinforcement of graphene–metal nano-composites.
In this communication, we would like to highlight the nature of graphene nano-composites with different noble metals and their alloys. The modifications of mechanical and electrical properties are of prime importance in view of the present applications of graphene/metal nano-composites for gas sensors. Nowadays, chemical gas sensors are almost unavoidable for realizing and trying to contain uncontrolled pollutions around us, because of the modernization of the human society using advanced technology. Therefore, we need to devise appliances to monitor and control the pollutants around us. Gas sensors can make us alert and save us from airborne pollutions, like toxic and inflammable gases, mainly coming out of auto emissions, but also from other sources. For the development of efficient gas sensors, nano-structured composite materials are most effective. Although novel nano-composite structures involving graphene and metals, polymers and ceramics that have been developed for various applications show superior performances, we shall confine specifically to the utility of graphene/noble metal nano-composites for hydrogen sensors. Hydrogen is one of the most important gases in the industry, due to its versatile applications, but it is the most dangerous gas, as we all know, because of its spontaneous and severe affinity for combustion in the presence of air/oxygen. Therefore, the knowledge and understanding to instantaneously sense the presence of hydrogen in the atmosphere below the lowest explosion limit (LEL) can save lives and property in most cases. In this article, an attempt has been made to collect the views of researchers around the globe, to understand the electronic mechanisms behind hydrogen sensing, with special emphasis on electron transfer and intercalation effects at the graphene/noble metal nano-structure interface.
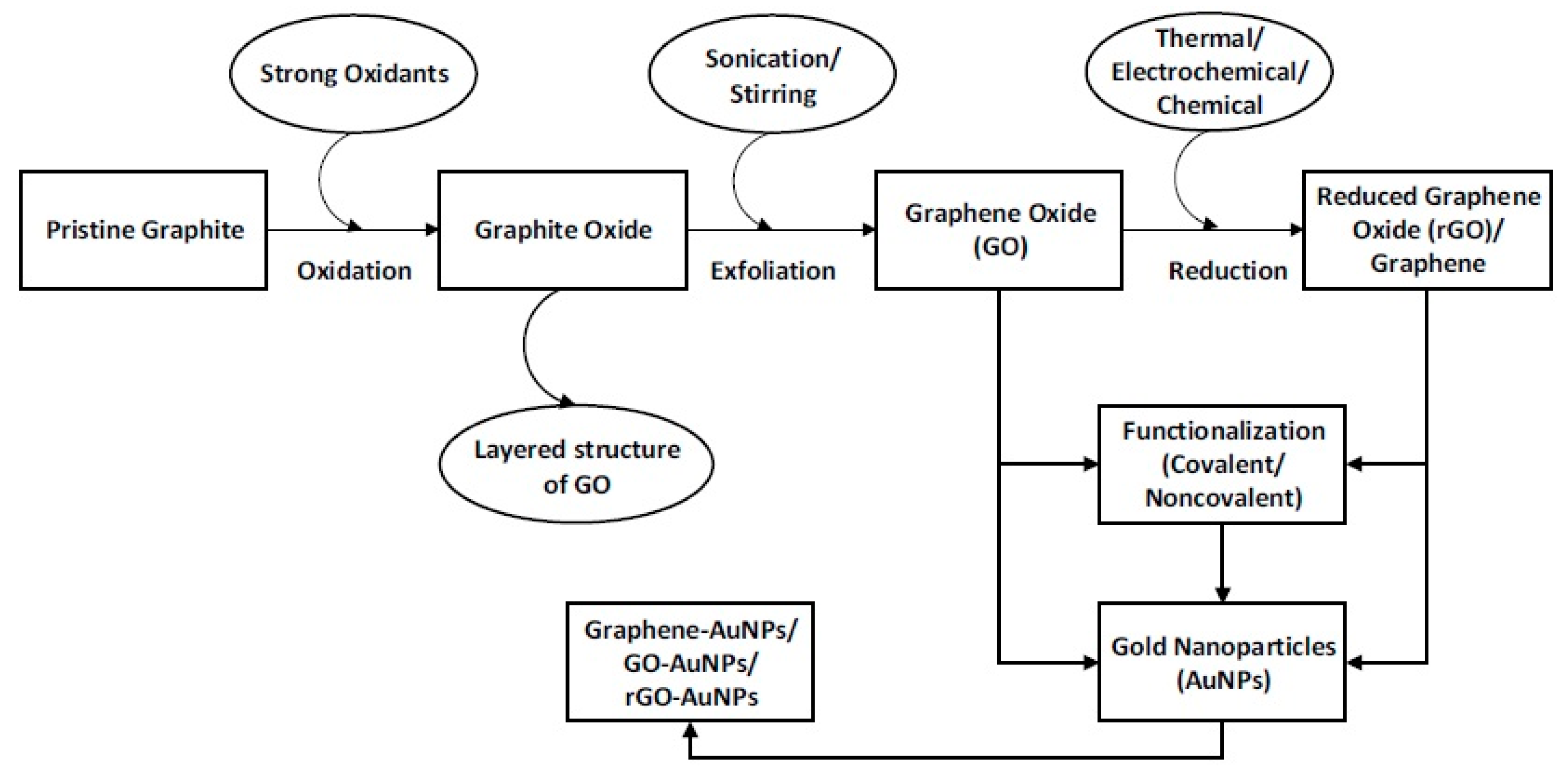
2. Preparation of Graphene–Metal Nano-Composites
Although Chemical Vapor Deposition (CVD) is a standard method for large scale production of graphene, the solution methods are mostly followed for the preparation of graphene–metal nano-composites. The most convenient technique for synthesizing graphene–metal nano-composites is the use of graphene oxide (GO) or reduced graphene oxide (rGO) and metal nano-particles in the solution phase.
Figure 1 schematically presents various steps to prepare graphene–gold nano-composites (Gr–AuNPs) in the solution phase.
W. Tremel and co-workers [
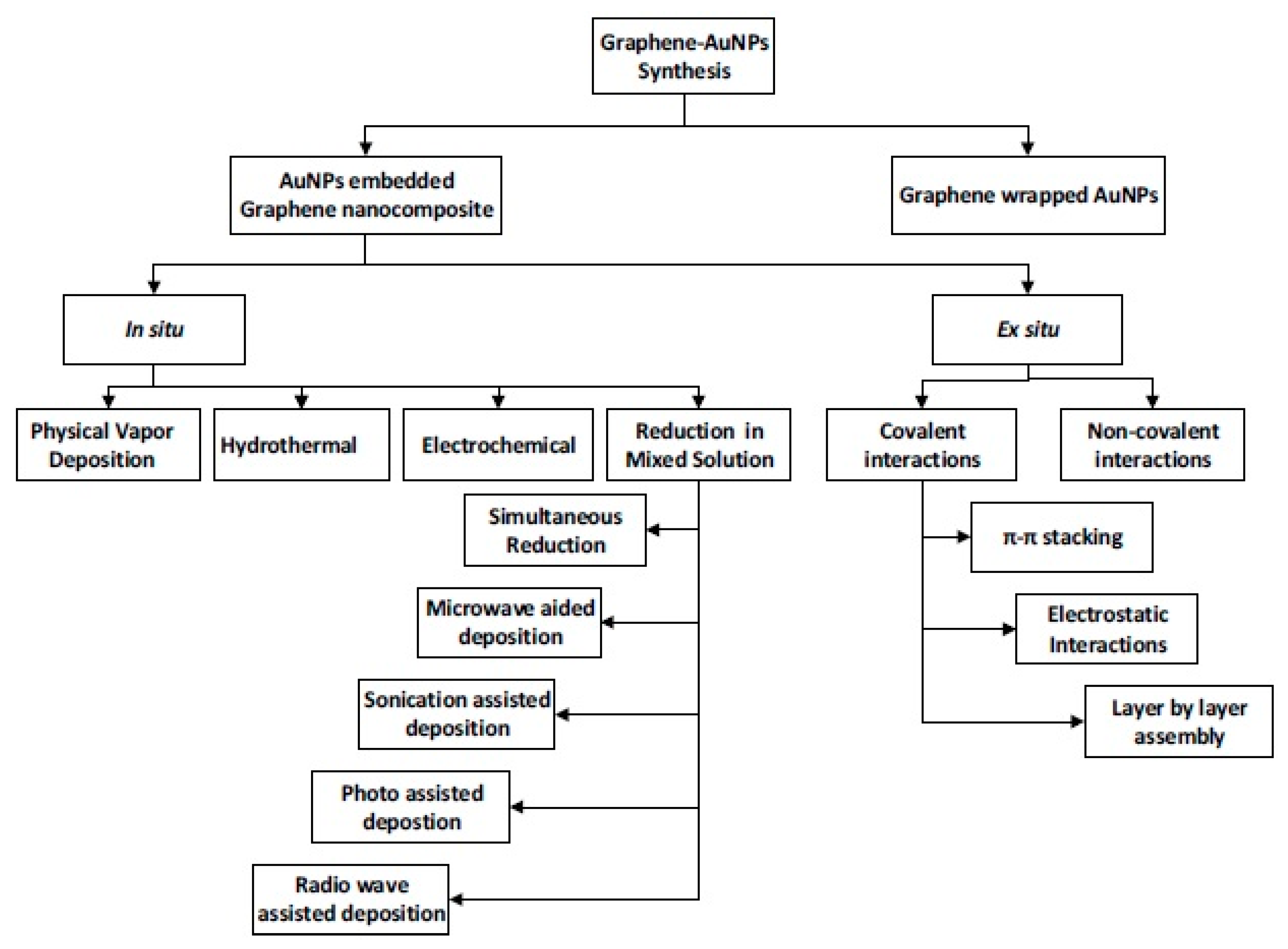
3] have published a review article giving details of different steps to be followed for preparing graphene–metal nano-composites. Generally, there are two methods for loading metal nano-particles on graphene, e.g., in situ crystallization and ex situ hybridization (
Figure 2). In the in situ crystallization method, which is relatively new, graphene–metal nano-composites are synthesized by simultaneous reduction of graphene oxides (GO or rGO) and metal salts. In the ex situ hybridization method, (also known as post immobilization method) nano-composites are prepared by mixing separate solutions of graphene nano-sheets and pre-synthesized metal nano-particles. The graphene nano-sheets can be functionalized through C–C coupling reactions, which significantly increase the solubility and thus facilitate handling during processing. However, one disadvantage of the ex situ method is the formation of non-uniform nano-structures in the composite because there is a lack of the uniform coverage, due to the non-uniformity of graphene sheets. Metal and graphene nano-composites are normally prepared by in situ chemical reductions of metal precursors like HAuCl
4, AgNO
3, K
2PtCl
4, and H
2PdCl
6 with reducing agents like hydrazine hydrate, amines, and NaBH
4. For example, the synthesis of Au–graphene or Ag–graphene nano-composite is accomplished by reducing HAuCl
4 or AgNO
3 using the reducing agent NaBH
4. Similarly, bimetallic graphene–metal nano-composites, like rGO/Pt/Pd can be synthesized using two metal precursors being reduced by HCOOH and ascorbic acid. The hydrothermal and solvothermal solution methods are quite convenient and popular for preparing graphene–metal and graphene–metal oxide nano-composites. For example, rGO/TiO
2 nano-composites were first prepared in an ethanol–water mixture under hydrothermal conditions. Glucose, which is more eco-friendly and has a greater reducing ability compared to hydrazine hydrate, is a good reducing agent for the reduction of graphene oxide.
Following the top down approach for the composite formation of graphene sheets, prepared by ex situ methods, the unique features of nano-structures may be utilized as convenient nano-scale building blocks for exploring a new method for preparing graphene–metal nano-composites [
4]. It can act as a supporting platform for dispersing metal nano-particles. For example, the preparation of graphene–metal nano-composites by solution methods can be undertaken in a water–ethylene glycol system, using graphene oxide and noble metal nano-particles (e.g., Pd, Pt, Au etc.) [
5]. The adsorbed metal nano-particles of graphene oxide can help as catalysts for the reduction of graphene oxide in ethylene glycol to graphene and subsequently the formation of graphene–metal particle nano-composites (
Figure 2). The graphene–Pt composite prepared by this method has been proven by cyclic voltammogram to be useful for methanol fuel cells.
The bimetallic noble metals and graphene nano-composites have recently become a research interest [
6,
7] because of their improved catalytic, optical and electronic properties [
8]. The bimetallic noble metal nano-species, like Au–Pt, Pt–Pd, Pt–Ru, Au–Pd, Pt–Ag, Pd–Ag and Pd–Ru etc., are reported to have been deposited on the graphene surface [
9]. These bimetallic nano-structures were prepared by one step in situ reduction of chloride salts and uniformly distributed on the graphene surface. In a recent report, graphene nano-sheets, decorated with Pd–Ag nano-rings were prepared by the galvanic displacement reaction between the pre-synthesized Ag NPs and Pd ions. Similarly, Au–Pd nano-particles were prepared by the in situ reduction method. Both Ag–Pd and Au–Pd nano-particles were dispersed on graphene nano-sheets.
A top down approach is quite effective for the preparation of graphene–metal nano-composites with improved structural and other properties. Also, cheaper materials, like graphite, may be used to prepare graphene and graphene–metal nano-composites for large scale production. Moreover, graphene obtained by this method can be easily processed. Nonetheless, a bottom up approach is also quite useful and advantageous in some cases. Despite certain positive aspects of CVD methods, solution methods can be employed for large scale productions of graphene–metal nano-particle composites, using a cheap and simple procedure.
3. Properties of Graphene–Noble Metal Nano-Composites
The π conjugated electrons over the graphene sheets are indeed a mobile cloud of electrons, but they also protect individual carbon atoms from chemical interactions. However, topological defects in graphene-like point defects, large vacancies, line defects and grain boundaries can break this barrier to expose reactive carbon atoms, which are responsible for superior chemical properties and even sharing the mechanical stability of graphene nano-composites, where dangling atoms or functional groups can make strong bonds with the second component. Therefore, the electrical conductivity of graphene can be increased by increasing defects—contrary to common understanding. It is already recognized by this time that graphene has exceptionally superior mechanical, electrical, thermal and chemical properties, which contribute to graphene’s categorization as a wonder material. It is interesting to know further, that metal reinforced graphene composites possess even better performances. Its two-dimensional structure and outstanding physical and mechanical properties make graphene an ideal reinforcement material for composites. Therefore, metal–graphene composites can produce structural materials with high specific strength, and functional materials with superior thermal and electrical properties. Since graphene has a zero band gap, defects or functional groups are used to open a band gap and induce semiconductor behavior [
10]. By implanting platelets or few-layer sheets of graphene in metallic matrices, one can produce graphene–metal nano-composites with superior mechanical properties, like strength and hardness. It is worth mentioning here that metal–graphene nano-composites can exhibit spectacular improvements in strength and hardness, through the inclusion of even low volume or weight fractions of graphene. For example, Al–graphene nano-composites, with 0.3 wt % graphene nano-sheets, showed a tensile strength ~62% higher than the strength of pure Al. Similar studies on the improvement in mechanical stabilities of Cu–graphene and Ni–graphene composites have been reported. The hardness of Ni–graphene nano-composite is four times higher than that of electrodeposited Ni metal.
The reduction of lattice dislocation slips by graphene nano-sheets in metal–graphene nanocomposites is confirmed by computer simulations of deformation behavior exhibited by Ni–graphene nanocomposites [
11]. The other strengthening mechanism in metal–graphene nano-composites is the stress transfer. The effect of this mechanism on both strength and elastic properties of nano-composites is sensitive to chemical binding between graphene and a metallic matrix. Other factors, like large aspect ratios, large lengths and width of graphene sheets enhance the stress transfer in metal–graphene nano-composites. Therefore, graphene nano-inclusions in metallic matrices create large contact areas and thus provide improved mechanical properties. More importantly, graphene nano-sheets restrict grain boundary migration and thus obstruct grain growth in metal–graphene nano-composites. As a result, metal–graphene nano-composites have lower grain sizes than that of the pure metal matrix. The increased strength of graphene–noble metal nano-composites offers enhanced stability of the hydrogen gas sensors. Electrical transport properties like mobility, charge transfer and device barriers of metal–graphene nano-composites are improved significantly compared to the corresponding individual components, like graphene and metals.
4. Bonding between Graphene and Noble Metals in the Nano-Composite Structure
In metal–graphene nano-composites, the properties of graphene are strongly influenced by the overlapping of valence states of graphene and metal at the interface. As a result, the electronic states of graphene are modified, such as shift of the Fermi level, doping of graphene, disturbance of the Dirac cone due to hybridization of graphene and metal and the band gap opening in graphene. These changes strongly affect both the individual properties of graphene and the transport phenomena at the interface of graphene and metal. Y.S. Dedkov and E.N. Voloshina [
12] suggested tailoring the properties of graphene–metal interfaces to overcome such problems. They tried to clarify how to understand the influence of a metallic substrate on the electronic properties of a graphene overlayer and how its properties can be modified in a controlled way. From the large collections of theoretical and experimental data, they made an attempt to explain the phenomena logically.
To clarify the chemical interactions between metal and graphene in the metal–graphene nano-composites, one has to primarily understand the atomic bonding that is created due to hybridization at the interface. Wang et al. [
13] provided direct experimental evidence for the bonding between metal nanostructures and graphene. Their evidence corresponded well with DFT calculations. They have shown an example of a chemical interaction of Cr metal with graphene and demonstrated no electron-induced interactions between Cr and pristine graphene, despite the strong reactivity of Cr with graphene. They envisaged a future application for “metal-decorated-graphene” in carbon-based electronics. K.N. Novoselov and his co-workers [
14] studied the distribution of metal nanoparticles in graphene–metal nano-composites by using High Resolution Transmission Electron Microscope (HRTEM). They interestingly showed the differences between metal interfaces with graphene at different sites. They observed that the defective sites of graphene are favored compared to clean graphene surfaces, for the dispersion of metal nanoparticles. This is the reason for the non-uniform distribution of metal particles in graphene. Thus, it was difficult to obtain a continuous metal film and instead, a cluster of nanocrystals was obtained, causing a weak interaction between metal and graphene. However, it was suggested that this difficulty could be partially overcome by modification of graphene surface, either by hydrogenation or by vacuum annealing. A sort of “nanomanipulation” could be possible by metal-mediated etching under high vacuum and 60 kV electron acceleration for all metals, except gold.
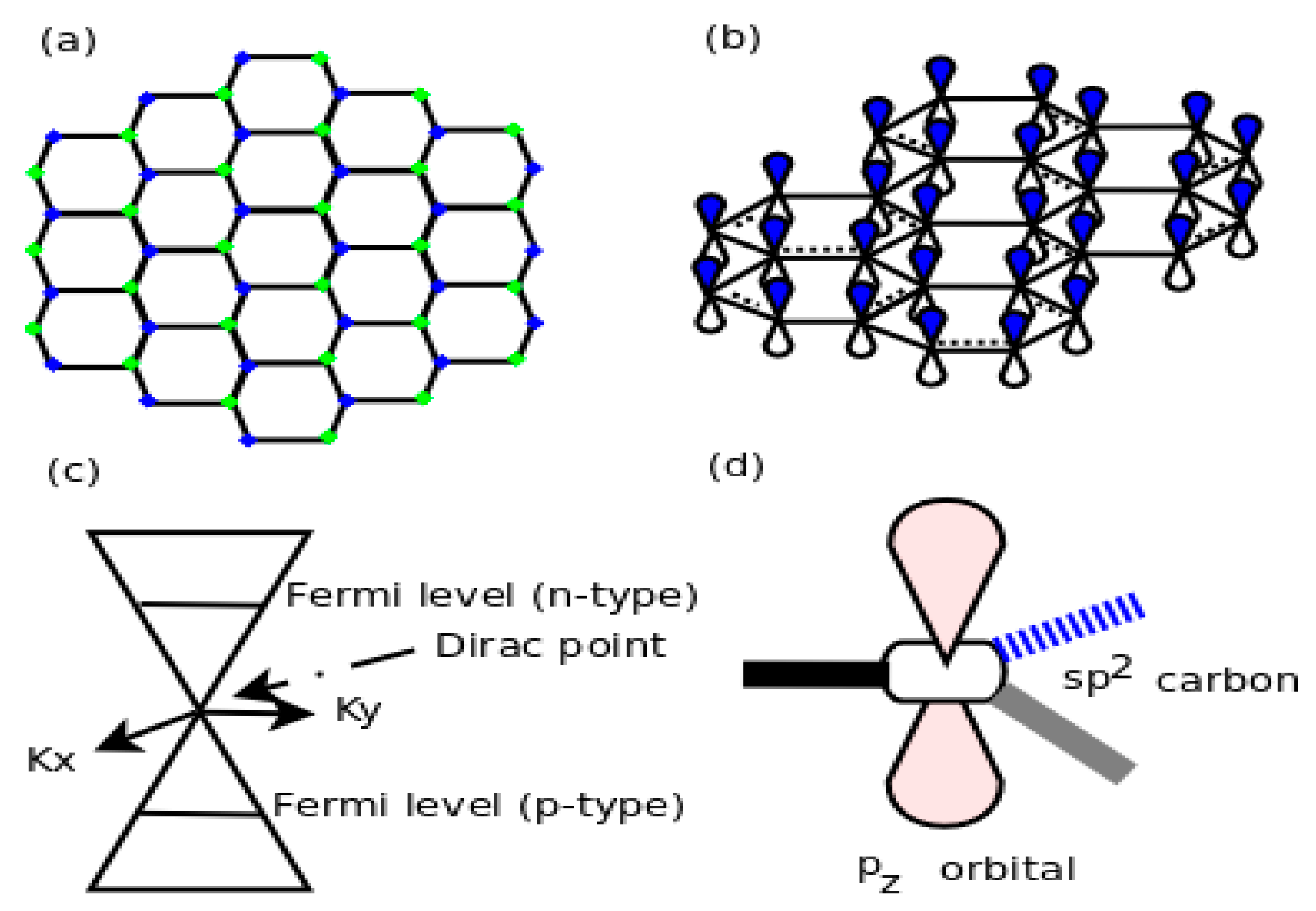
The interaction of graphene with transition metals is especially useful for practical applications. As usual, an attempt was made to understand such interactions theoretically but complications arose due to the interactions between van der Waals (vdW) forces and hybridization in the transition metals. Ambrosetti and co-workers [
15] put forth a concept of semi-local Density Functional Theory via a combined DFT/vdW method. They derived a reliable model that speaks of metal screening effects that happen due to contributions of both p- and s-like quasi free electrons and the localized d-states of the transition metals. Thus, the complex binding mechanism explained by them was in fairly good agreement with the experimental data (
Figure 3).
P. A. Khomyakov et al. [
16] studied theoretically the adsorption of graphene on metal substrates by DFT calculations. They observed that the bonding of graphene to metals like Al, Ag, Cu, Au and Pt (111) surfaces was very weak. The interaction between graphene and these metals takes place via a charge transfer band structure. As a result, for a metal with a work function of approximately 5.4 eV (larger than the work function (4.5 eV) of a free-standing grapheme) a transition from p-type to n-type doping occurs. Thus, Khomyakov and his co-researchers conceived and developed a naive analytical model that shows that the Fermi level in graphene shifts relative to the metal substrate work function. On the other hand, graphene interacts and binds more strongly with transition metals, like Co, Ni, Pd and Ti. The hybridization between d states of transition metals with p
z states of graphene takes place, which is responsible for a band gap opening in graphene and reduction of the work function. Therefore, graphene deposited on the metal substrates behaves as n-type, due to work function lowering, which shifts the Fermi level upward by 0.5 eV with respect to the conical point of graphene (
Figure 3). The graphene supported on the substrate is effectively n-type doped because in “current-in-plane device geometry” electrons are transferred to the unsupported part of the graphene sheet by the work function lowering.
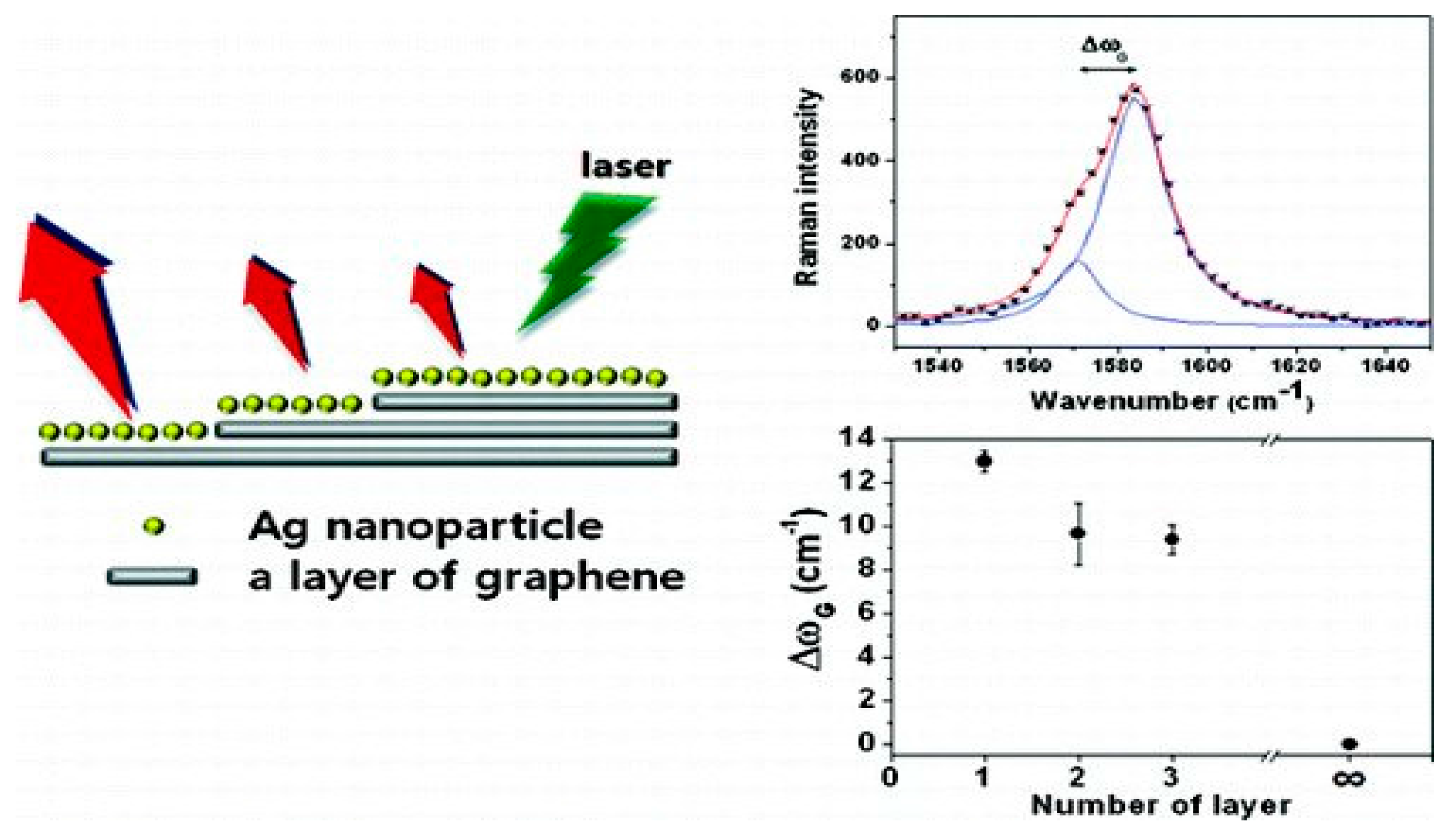
Figure 4 shows that graphene may be formed as single layer or few layer films, depending on the growth conditions. The interaction between metal and graphene is dependent on the number of layers of graphene. Therefore, the metal–graphene hybrid materials show their physical and chemical properties according to the number of graphene layers involved in the composite formation. Surface Enhanced Raman Scattering (SERS) of single layer (SLG), bi-layer (BLG) and tri-layer (TLG) graphene was investigated by Lee, Novoselov and Shin [
17]. A detailed study of the Ag–graphene nano-composite revealed that the interactions between Ag and SLG, BLG, TLG and MLG (multilayer graphene) are different and this was confirmed by the extent of G-band splitting in Raman Spectra.
A descending order of the G-band splitting was reported, for example, 13.0 cm
−1 for single-layer, 9.6 cm
−1 for bi-layer, and 9.4 cm
−1 for tri-layer graphene, whereas multilayer graphene, which is thicker, did not show any splitting of the G-band. Correspondingly, the SERS enhancement factor of the G-band was ~24 for single-layer, ~15 for bi-layer, and ~10 for tri-layer graphene. This is a convincing indication for a correlation between G-band splitting and SERS enhancement factors. Therefore, it is apparent that single layer graphene strongly interacts with Ag. It was also found from Raman Spectrum that the intensity ratio,
I2D/
IG, decreased after the deposition of Ag metal on graphene. From the changes of the positions of G and two-dimensional (2D) bands, it can be inferred that metal deposition induces doping of graphene. It was experimentally verified that Ag deposition produces n-doped graphene and Au deposition produces p-doped graphene. Actually, the reaction between metal and graphene takes place at an elevated temperature and a thin layer of metal–graphene nano-composite is formed at the interface. However, electronic structure, charge neutrality point and electron–phonon coupling of graphene remain nearly intact on gold depositions. The charge carrier density at gold–graphene contact is not pinned and can be tuned by an electrostatic gate [
18].
Since the property of 2D layered materials, like graphene, strongly exhibits dependence on the substrate, the adhesion energy of synthesized nano-materials with substrates needs intense research to understand the bonding characteristics, to explain and interpret the growth mechanism, properties and transfer processes. For the last several years investigations have been in the process of revealing the importance of graphene, a sp2 bonded allotrope of carbon, in two dimensions. Its exceptional electronic properties, like the quantum Hall effect, ballistic charge transport, high charge carrier density, tunable band, and inversion in types of semi-conductivity, make graphene a potential material for future technologies. The adhesion energies of graphene deposited on Cu and Ni substrates were measured to be 12.8 and 72.7 Jm2, respectively. The Density Functional Theory (DFT) inferred that the Ni/graphene interface has stronger covalent bonding than Cu/graphene, due to its partial ionic characteristics. This is further supported by the higher adhesion energy and high thermal conductivity of Ni/graphene. This kind of study is necessary for providing important information on the stability of the graphene–metal nano-composites for their reliable technological applications, including gas sensors.
5. Intercalation of Metals and Non-Metals in Graphene
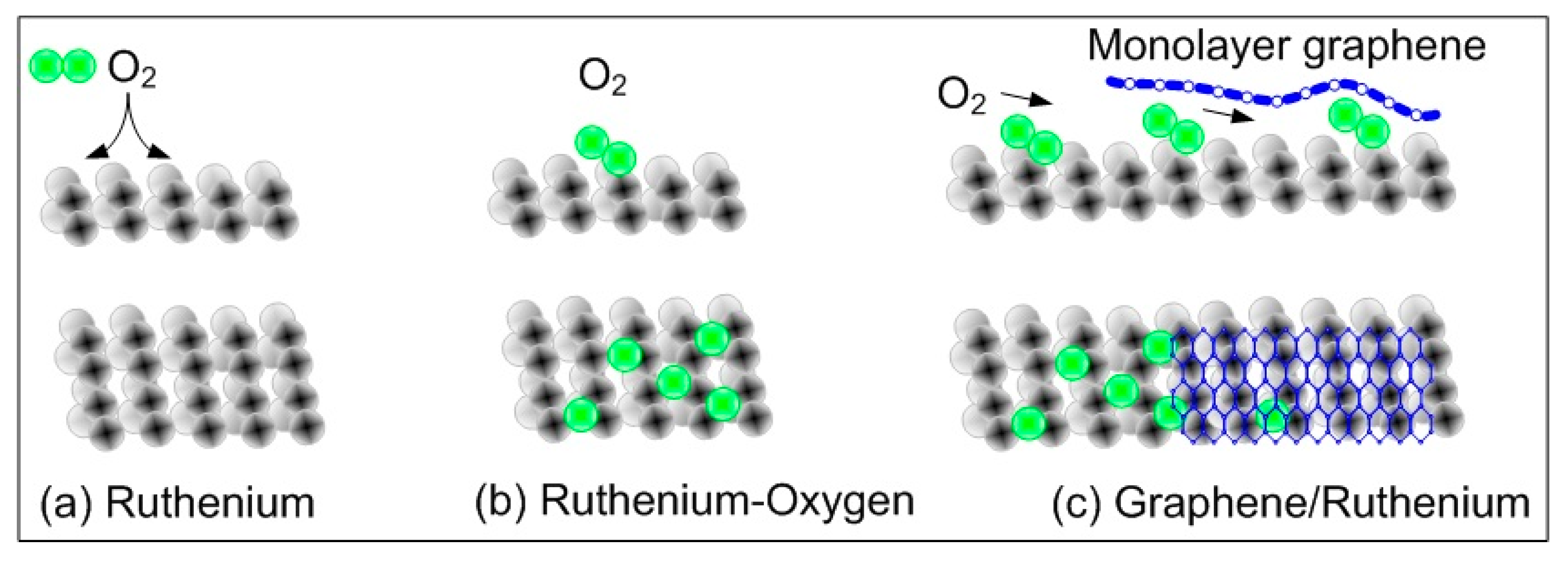
The intercalation of metal atoms is another important factor in determining a reliable route for the coupling of graphene to a substrate. Reactive species, like oxygen or hydrogen, can be helpful for revealing a wide spectrum of interfacial chemistry. The controlled modification of a macroscopic graphene–metal interface by oxygen intercalation was demonstrated by P.W. Sutter et al. [
19]. They reported that the oxidation of ruthenium surfaces under the deposited graphene film removes the strong metal–carbon coupling and recovers the characteristic Dirac cones of an isolated graphene monolayer.
Figure 5 demonstrates the sequence of oxygen and graphene depositions. Their experiments suggest that small molecules can populate the space between graphene and metals, and the adsorbate–metal interaction is appreciably modified by graphene. Actually, such findings are beneficial for the processing of graphene for devices (especially for gas sensors) and for chemical interactions between a metal surface and a graphene sheet to produce a graphene–metal nano-composite. Hydrogen has also been studied as the intercalating agent in multilayer graphene (MLG), deposited on SiO
2/Si by CVD, to fabricate a hydrogen sensor [
20].
Florian Banhart and co-researchers [
21] discussed structural defects in graphene in detail. Graphene is one of the most promising materials in modern nano-technology. The electrical, mechanical and chemical properties of graphene samples with high perfection of the atomic lattice are remarkable and may be usefully utilized to design and fabricate sophisticated electronic or mechanical devices. However, their performance may be deteriorated due to structural defects that may appear during growth or processing. However, such defective graphene can be useful in some important applications, like chemical gas sensors, where it is possible to tailor the properties of graphene to produce “new functionalities”. While graphene samples with precise perfection of the atomic lattice are useful for efficient performance of mechanical and electronic devices, structurally defective graphene is useful in chemical gas sensor applications. The defects are normally generated during the growth and processing of graphene. There may be intrinsic defects as well as extrinsic defects. The defects may make it possible to tailor the properties of graphene locally, to create new functionalities. Graphene has a special capacity to reconstruct its lattice around intrinsic defects, giving rise to some specific effects. However, extrinsic defects in graphene, like foreign atoms, are important for designing graphene-based devices with tailor made properties.
Epitaxial growth of graphene on transition metal surfaces has been proposed as one of the most convenient methods for large scale preparation of superior quality graphene. However, the intrinsic electronic structure of graphene may be affected significantly after contact with the substrate. However, grown graphene may be decoupled from the substrate by the intercalation method. Taking the graphene/Ni (1 1 1) surface as an example, W. Zhang and C. Chen [
22] suggested, by Density Functional Theory calculation, that oxygen may be a suitable intercalation element to facilitate the dispersion of graphene. They considered different possible intercalations and different oxygen coverage in a detailed analysis of the geometrical configuration and also the electronic structure. Their results indicate that the binding between graphene and O/Ni (1 1 1) substrates become stronger with higher oxygen coverages, compared to pure Ni (1 1 1) substrates. It is interesting that the electronic structure of pristine graphene was almost recovered after oxygen intercalation, and the Dirac points moved towards the high-energy region relative to the Fermi level. A graphene/oxygen/Ni (1 1 1) system is thus suggested to be a p-type doped, strongly bound Dirac system. Detailed analyses have indicated that the intercalated oxygen atoms strongly react with Ni (1 1 1) surfaces, block the strong interactions between Ni and graphene and passivate oxygen 2p states. This intercalation mechanism of Zhang and Chen may be helpful for understanding and clarifying other complex intercalation systems. In
Figure 5 we have illustrated the case of deposition of oxygen and graphene on Ru surfaces. Deposited oxygen helps in the de-intercalation of graphene layers from the Ru substrate.
R.K. Biroju and P.K. Giri [
23] reported on the role of defects in graphene on the physical functionalization of graphene grown by the chemical vapor deposition technique (
Figure 6). The effect of intrinsic defects in graphene on the ultra-thin gold layer, deposited on different layers (SLG, BLG, TLG etc.) of graphene was studied by the authors, using resonance Raman spectroscopy and also by high resolution transmission electron microscopy. They observed a large enhancement in the intensity of D and D’ bands, but much less enhancement of G and 2D bands in graphene after the deposition of gold thin films, by sputtering. They explained this phenomenon as being due to strong interactions between Au and graphene following the charge transfer from metal to graphene.
Figure 6 schematically demonstrates the effect. It was further confirmed by X-ray photoelectron spectroscopy (XPS) analysis and HRTEM imaging showing the bonding of gold to the graphene layer. The authors revealed the real defect density from the Raman spectral line shape analysis and the inter defect distance from the theoretical calculations, respectively. It was observed that gold functionalization depends on the number of layers in graphene. This also signifies a possibility for physical functionalization of graphene with foreign atoms, following the principles of defect engineering.
6. Graphene/Noble Metal Hybrid Hydrogen Sensors
Recently, graphene hybrid devices have been used to manufacture gas sensors with enhanced performances, like high percentage responses, fast detection, quick recovery, repeatability and selectivity. There are already a large number of reports on the use of only the noble metals as hydrogen gas sensors, because of their high catalytic activity with flammable gases and they mostly operate at higher temperatures. However, it was recently observed that the graphene–noble metal hybrid sensors operate even at room temperature, mainly due to the combined effects of the catalytic activities of noble metals and the ability of graphene to promptly transfer electrons to the metal electrodes to accelerate the catalytic process [
24,
25]. Some examples of the performance of graphene–noble metal hybrid H
2 sensors are shown in
Table 1 below.
The electrical conductivity of graphene is a critical parameter for gas detection because the electrons from catalyzed decomposition reactions of the sensing gases are transferred to the electrodes via graphene. Although perfect graphene may be useful for other applications, defective graphene is more reactive chemically and therefore it is appropriate for gas sensor applications.
Figure 7c shows different types of defects in graphene. These defects are mainly responsible for the high chemical reactivity. These defects help in forming nano-composites with noble metals by the process of hybridization. Pak et al. [
5] used graphene nanoribbons and palladium for the fabrication of hydrogen gas sensors and Dutta et al. [
26] studied multilayer graphene films and Pd for hydrogen sensing. The thickness of the graphene film and that of the catalytic metal layer are the prime factors for the efficiency of such gas sensors. Also, the nanostructure of catalytic noble metals increases the efficiency of gas sensing. Therefore, the morphology and the particle size of the noble metals are also important parameters for improving the sensing activity of the hybrid nanosensors. Phan et al. [
25] reported the improvement of sensitivity by controlling the particle sizes and morphologies of noble metals. Moreover, the oxygen-containing functional groups on the graphene surface are very much effective for the adsorption of sensing gases and make the sensor more efficient at sensing much lower concentrations of gases [
27]. The significance of these studies was to demonstrate accurate and fast sensing of oxidizing and reducing gases, like NO
2 and NH
3, respectively, with a higher selectivity.
J. Hong et al. [
28] reported a method to prepare graphene–Pd nanoparticle hybrid sensors for hydrogen detection. This method consists of four steps: CVD growth of single layer graphene on Cu foil; depositions of Pd nanoparticles on single layer graphene using a galvanic displacement reaction; transfer of Pd/SLG hybrid on an electrode-patterned glass substrate; and spin coating of polymethyl methacrylate (PMMA). Hydrogen sensing follows the conventional mechanisms of adsorption and dissociation of hydrogen molecules to hydrogen atoms by the catalytic Pd nanoparticles on graphene. They explained the decrease of the catalytic process with continued sensing as being due to the formation of Pd hydride (PdHx), which increases the resistance and decreases the gas response of the PMMA/Pd NP/SLG sensor structure.
There is a rapidly growing interest in graphene nanoribbons (GNRs). The GNR structure has better chemical reactivity than flat graphene, due to the presence of potentially reactive edge atoms in GNRs. Thus, GNRs are promising materials for hydrogen sensors. Because of the mechanical instability of bigger stand-alone sheets of graphene, they form smaller ribbons by breaking the 2D structure within the solution [
29].
Pak et al. [
5] reported on the graphene nanoribbon (GNR) hydrogen gas sensors. In this report a Pd-decorated GNR (Pd–GNR) sensor structure was used to increase electrical conductivity and Pd Nanoparticle (NP) was used to catalyze the hydrogen sensing operation. In this work, a periodically aligned GNR array was synthesized by introducing a chromium interlayer under the photo resist, using interference lithography, followed by incorporating it in the Pd decorated hydrogen sensors. They observed an encouraging hydrogen response for the response time and recovery time in the order of 60 s and 90 s, respectively. The mechanism of H
2 detection by SLG/noble metal nanostructures can be traditionally explained by adsorption, dissolution in Pd and dissociation of H
2 molecules into atomic hydrogen in the presence of Pd NPs deposited on graphene. With increasing H atom concentrations, Pd-hydride (PdHx) is formed. PdHx increases the resistance of sensor structures. As a result, conduction pathways are directed from graphene to Pd film and thus decrease the gas response
Phan et al. [
30] fabricated a hydrogen sensor, based on a graphene and Pt/Pd bimetallic catalyst core shell hybrid. A uniform colloidal solution of Pd nanocubes was used as the core and then Pt was coated on Pd cubes. In the next step, graphene was decorated with the Pt/Pd core shells to synthesize a Pt/Pd core shell/graphene hybrid. The sensor response towards 1% H
2 in air was 36% at room temperature, the detectable range of the Pt/Pd core-shell hybrid was 1–40,000 ppm, and the response/recovery time was 3/1.2 min, which was inferior to the Pd-GNR hybrid sensors. Pt coated multilayer graphene was also reported as a hydrogen sensor [
31]. D.T. Phan and G.S. Chung [
32] studied the effect of the size of Pd nanocubes on the hydrogen sensing characteristics of graphene–Pd hybrid nanostructures. The Pd cube–graphene hybrid resistive sensor showed good responses and linearity, in the detectable range, from 10,000 to 10 ppm at room temperature. They reported that the bigger Pd nanocubes showed better responses to H
2 gas even at higher temperatures. The improved hydrogen sensing with Pd nanocubes of different sizes was explained by the spillover mechanism as well as the effect of the Pd size of the graphene–Pd nanocube hybrid nanostructures.
The hydrothermal microwave exfoliation method was deployed by Martinez-Orozco et al. [
33] to prepare palladium–graphene nanostructures from high-quality graphene layers and well monodispersed palladium nanoparticles. The structural and morphological characteristics of palladium–graphene hybrids indicate that this method allows the reduction of metal precursors and anchoring on exfoliated graphene layers. The synthesized palladium–graphene nanostructures were deposited as the active layers on a suitable substrate, which efficiently sensed hydrogen gas (H
2). This sensor structure performed reproducibly with a fairly fast response time of (~30 s) at room temperature in the hydrogen concentration range between 0.01 and 5 volume % in air. In this study, the impedance response measurement proved a highly feasible sensor technique. The authors have claimed that this method is feasible for obtaining a reliable and reproducible gas sensor measurement technique to determine the gas sensing parameters more accurately. By means of a simple and cost-effective preparation method, the authors made it feasible to obtain an efficient H
2-sensor with reliable and reproducible sensing properties in the actual atmospheric conditions.
Chu et al. [
34] used modified graphene nanoribbons, and palladium and platinum, respectively, for the preparation of hydrogen gas sensors. They commented that the quality of graphene film, the thickness and the catalytic ability of deposited nanostructure noble metal layers are the key factors for efficient performance of a gas sensor. Phan et al. [
25] also reported that sensor response was improved considerably by controlling particle size as well as morphology of the noble metal. Noburu Yamazoe and co-workers have reported the effect of grain size on gas sensor efficiency. A model for the grain-size effects has beenproposed, in which the transducer function is operated by a mechanism of grain control, neck control or grain–boundary control, depending on the crystallite size [
35]. Such sensors become more effective if the functional groups associated with oxygen are introduced onto graphene surface. The noble metals also act as a fast conduction route for electrons and improve the gas response time.
Research on graphene/nanostructure hybrid materials has been gaining momentum in recent years, with wide-ranging applications in gas sensing. Specifically, noble metal decorated graphene-based novel structures were found to be extremely sensitive and selective, owing to the synergistic effect of the compound configuration.
The recent developments in graphene/noble metal nanostructure hybrids and their promising potential in gas sensing applications has been highlighted by A.V. Singhal and coworkers [
36]. More significantly, an understanding of the electronic mechanisms of gas sensing was presented, with specific emphasis on electron transfer and junction effects at the graphene/nanostructure interfaces. Finally, future research prospects for the application of bi-metallic and tri-metallic nanostructure/graphene-based hybrids and the challenges in this new and rapidly growing domain were discussed.
Most of the graphene–noble metal based hybrid sensors are effective, even at room temperature, in contrast to sensors based on only noble metals, which perform at high temperatures. Moreover, graphene hybrid/noble metal sensors have high catalytic activities and graphene is able to quickly transfer electrons into metal electrodes, which can enhance the kinetics of the catalyst process.
Table 1 demonstrates the hydrogen gas detection performances of various noble metal–graphene hybrids. The operating principle of most sensors is the catalyzed decomposition and adsorption of the sensing gas on the surface of noble metals and the electrons from the catalyzed reactions are transferred to the electrodes via graphene. This means that the electrical conductivity of graphene is a critical parameter in the process of gas detection. Therefore, the quality of coated graphene and the thickness of deposited noble metal layers are the key factors for the efficiency of gas sensors. The catalytic activity of noble metals is another factor affecting the efficiency of the sensor, Therefore, noble metal nanostructures that are active at crystal regions have high crystalline activity, resulting in increased sensitivity. The oxygen-containing functional group on the graphene surface increases gas adsorption, making the sensor more sensitive. In addition to the catalytic properties, noble metals can also act as conducting paths for charge carriers. As already mentioned, the hybridization of the valence band states of metal and graphene, and/or band gap openings in graphene on the “metals electronic spectrum” of graphene states takes place due to violation of the “sub lattice symmetry” in graphene.
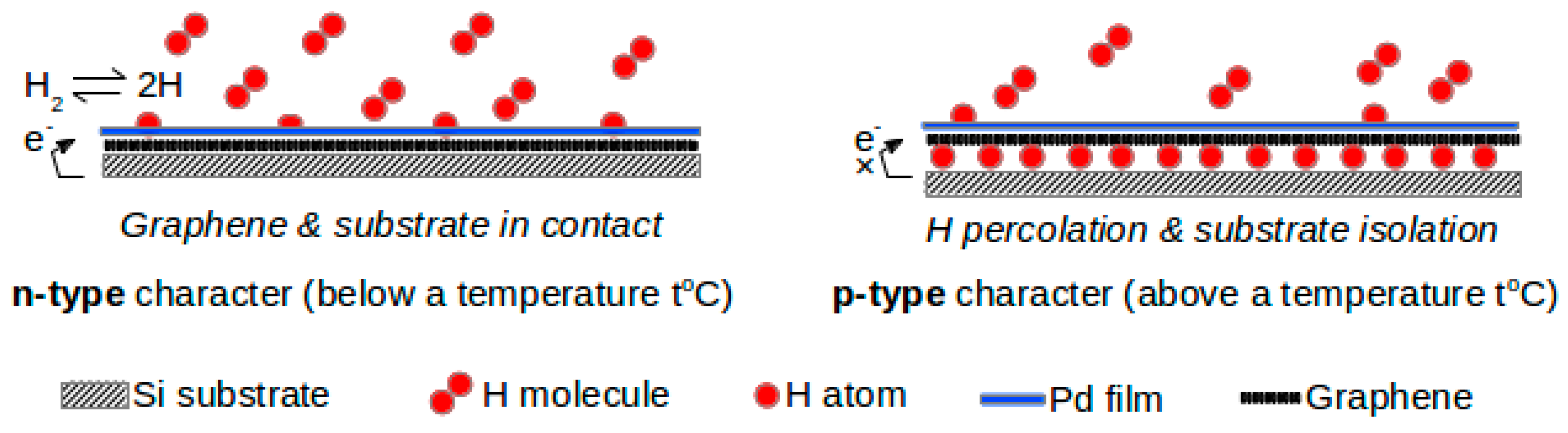
Ulrich Lange and co-workers [
37] developed a novel nanocomposite chemosensitive material, consisting of chemically-derived graphene and palladium nanoparticles. First, graphene oxide was synthesized by a modified Hummers method [
38] and subsequently graphene was obtained by the reduction of graphene oxide. The nano-composite was prepared via layer-by-layer (LbL) deposition of graphene and Pd nanoparticles on gold electrodes. Since this material is catalytically active, it can be conveniently used to detect hydrogen. The highlight of this work was the effect of the number of layers on hydrogen-sensing performance. The reason for the decrease in the electrical resistance of this sensor in the presence of hydrogen was probably due to the dissolution and dissociation of hydrogen at the PdNPs. This phenomenon, in all probability, may have decreased the particle’s work function and transferred electrons to graphene. They observed that the graphene–Pd composite with seven layers registered a stronger response to hydrogen than ten layers of the same composite. However, graphene without Pd nanoparticles displayed a small response to hydrogen. This is a clear indication of the role of palladium nanoparticles acting as the catalyst in n-doping of graphene by molecular hydrogen. The graphene/PdNP composite may be considered an economic substitute for bulk palladium-based hydrogen sensors. Basu et al. [
39] also observed a similar intercalation effect with multilayer (<10 layers) graphene with n-type conductivity at room temperature but it changed to p-type conductivity at higher temperatures.