MoS2 Decorated Carbon Nanofibers as Efficient and Durable Electrocatalyst for Hydrogen Evolution Reaction
Abstract
:1. Introduction
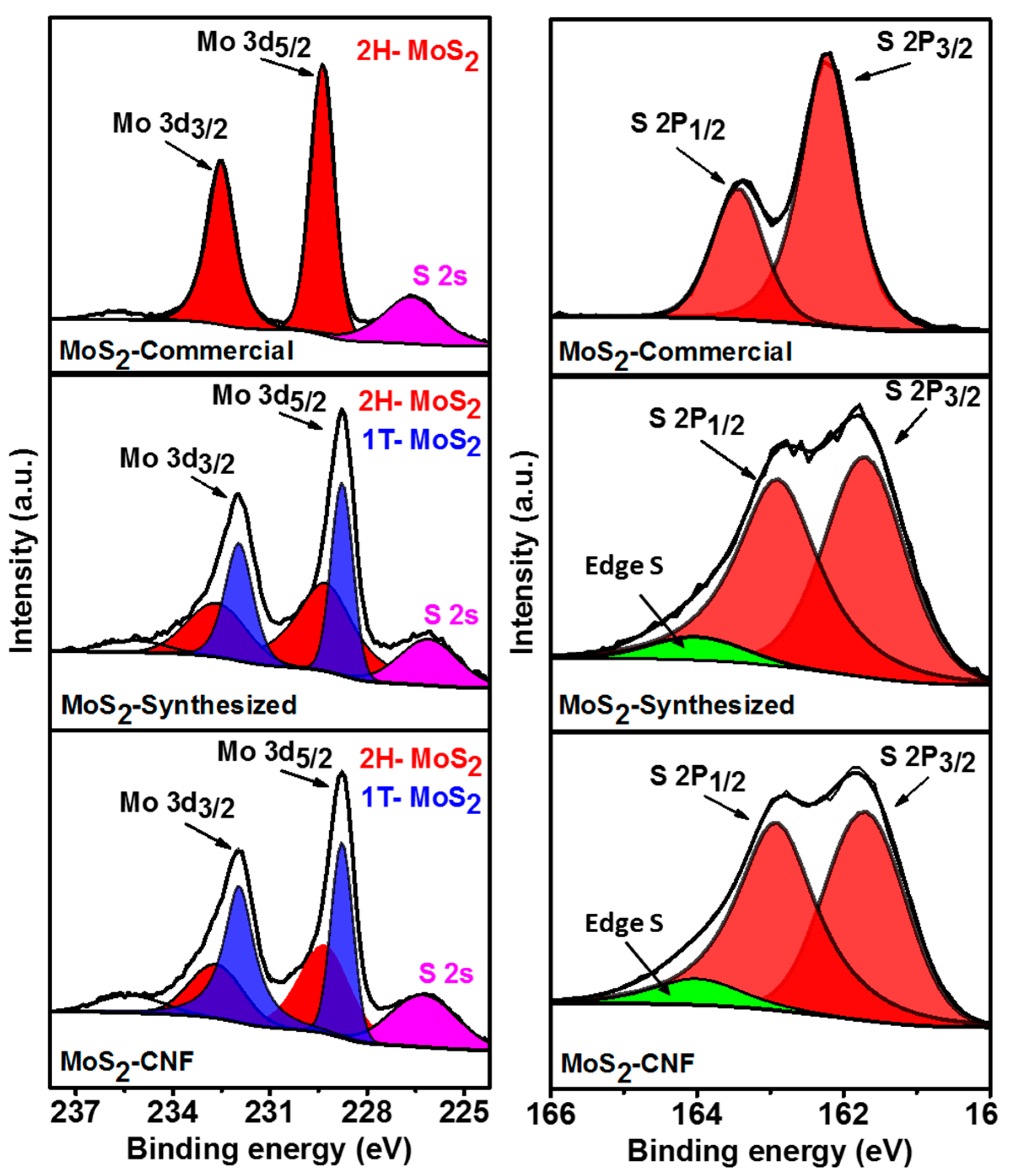
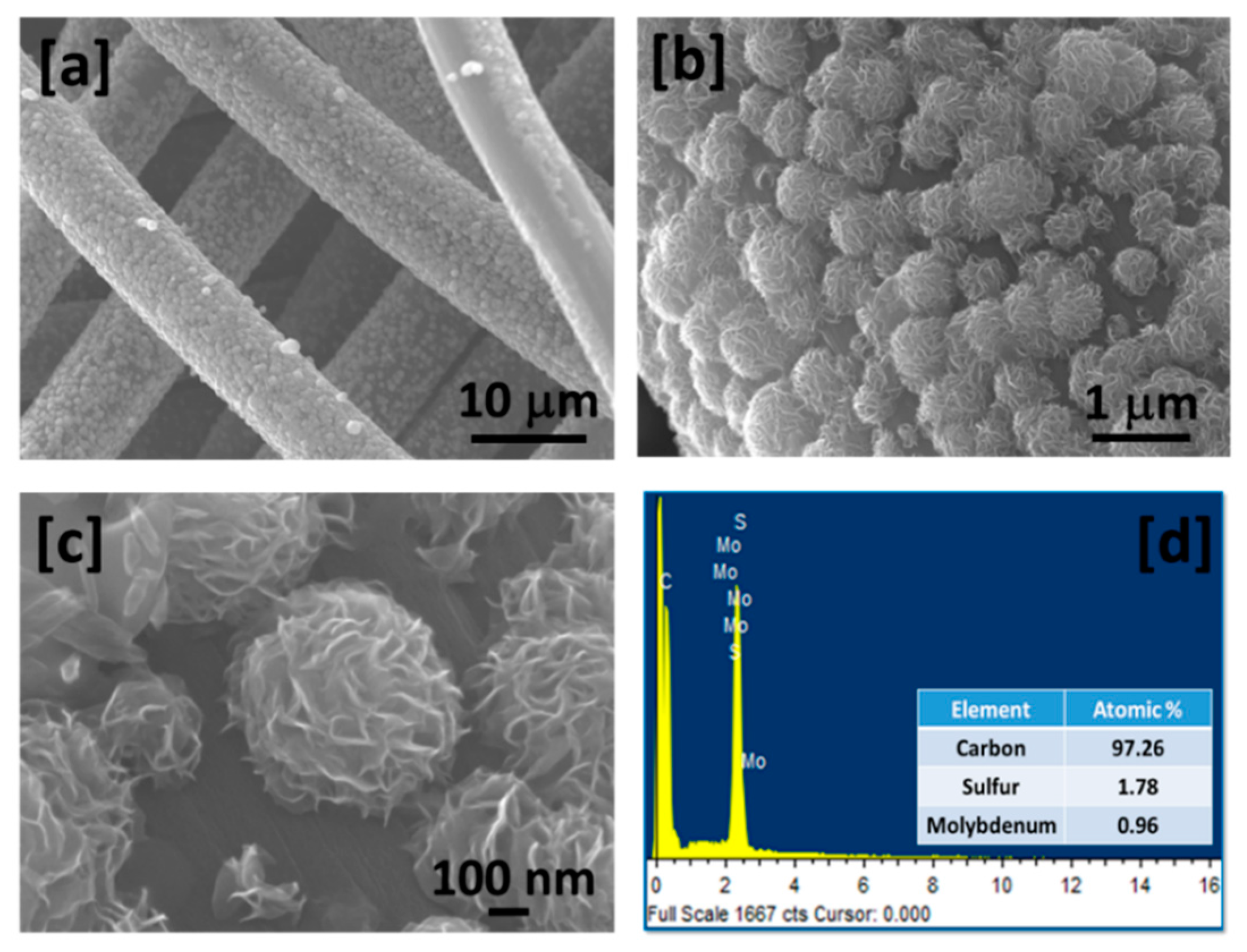
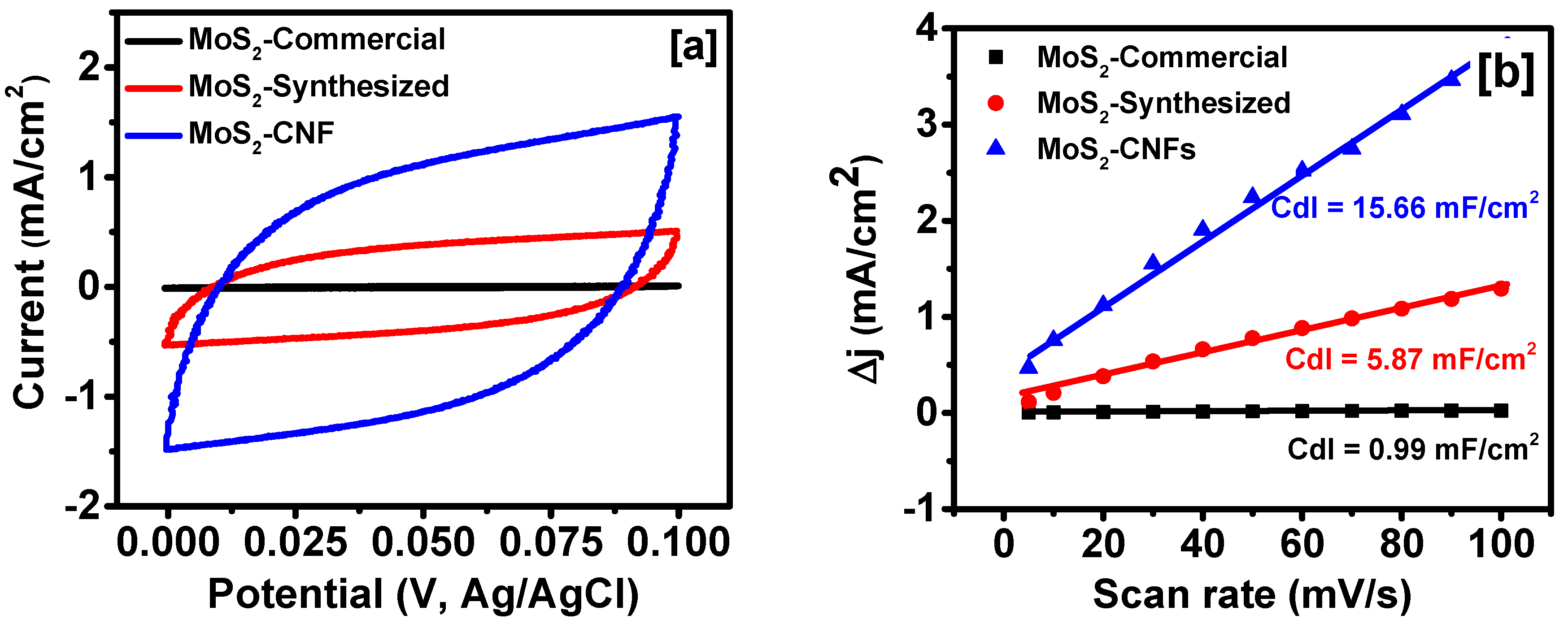
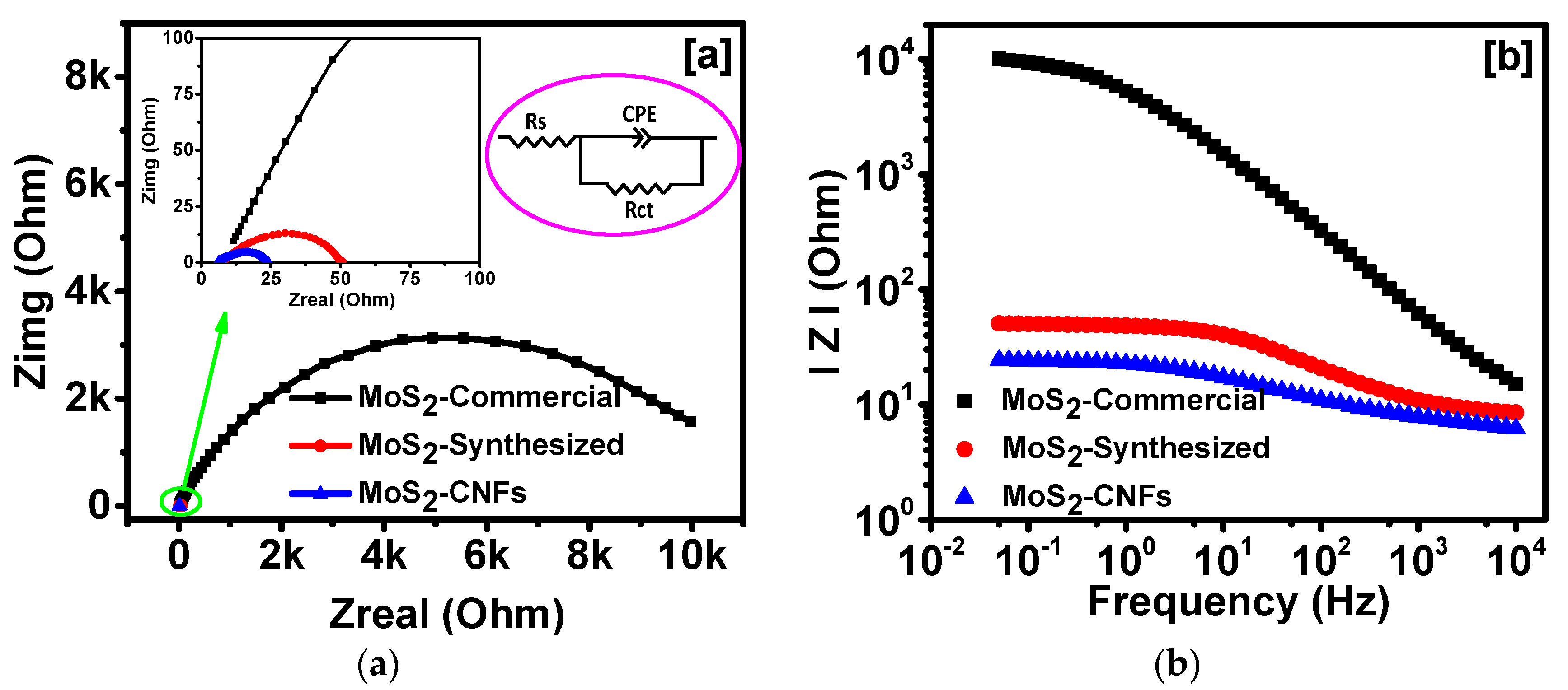
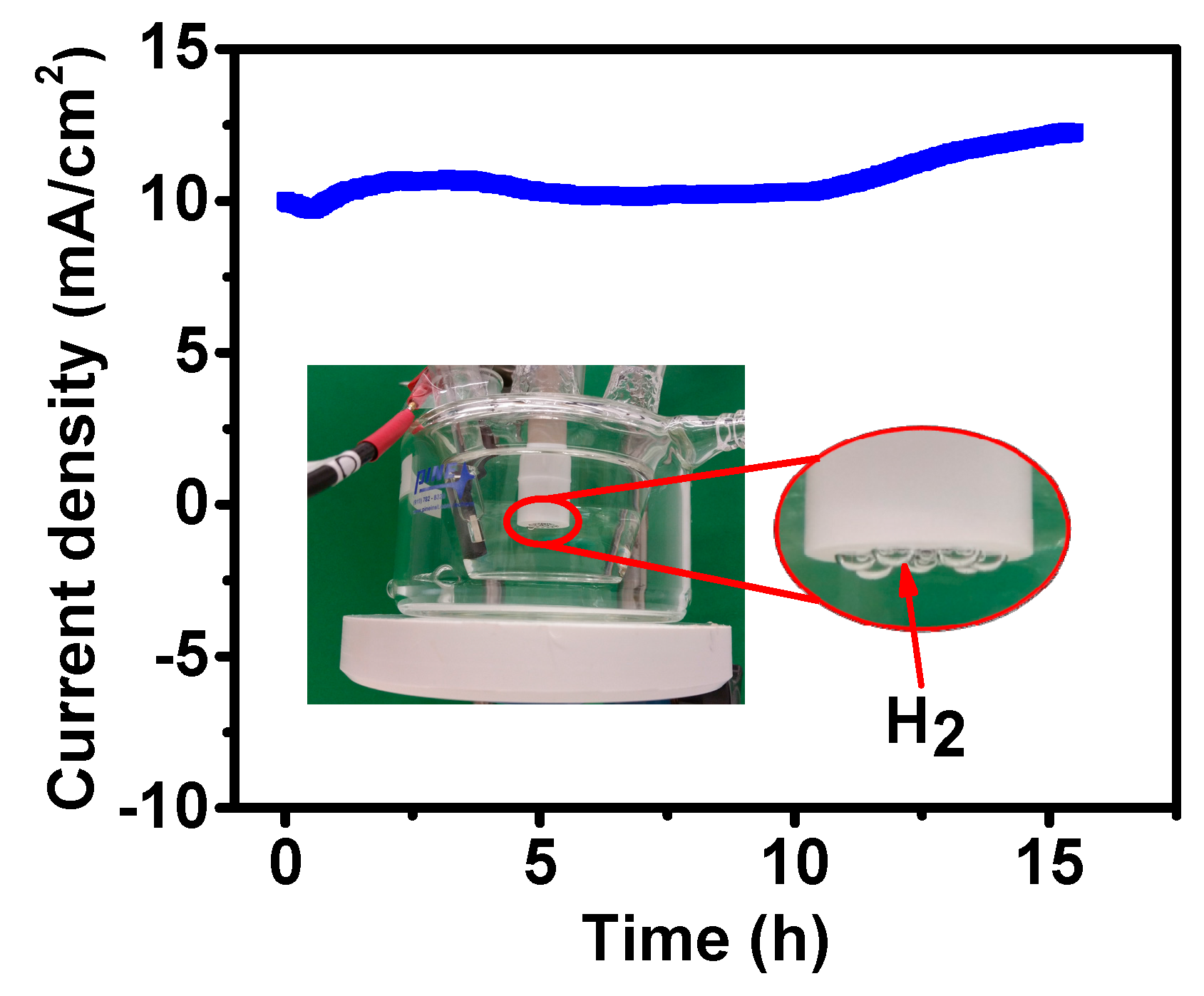
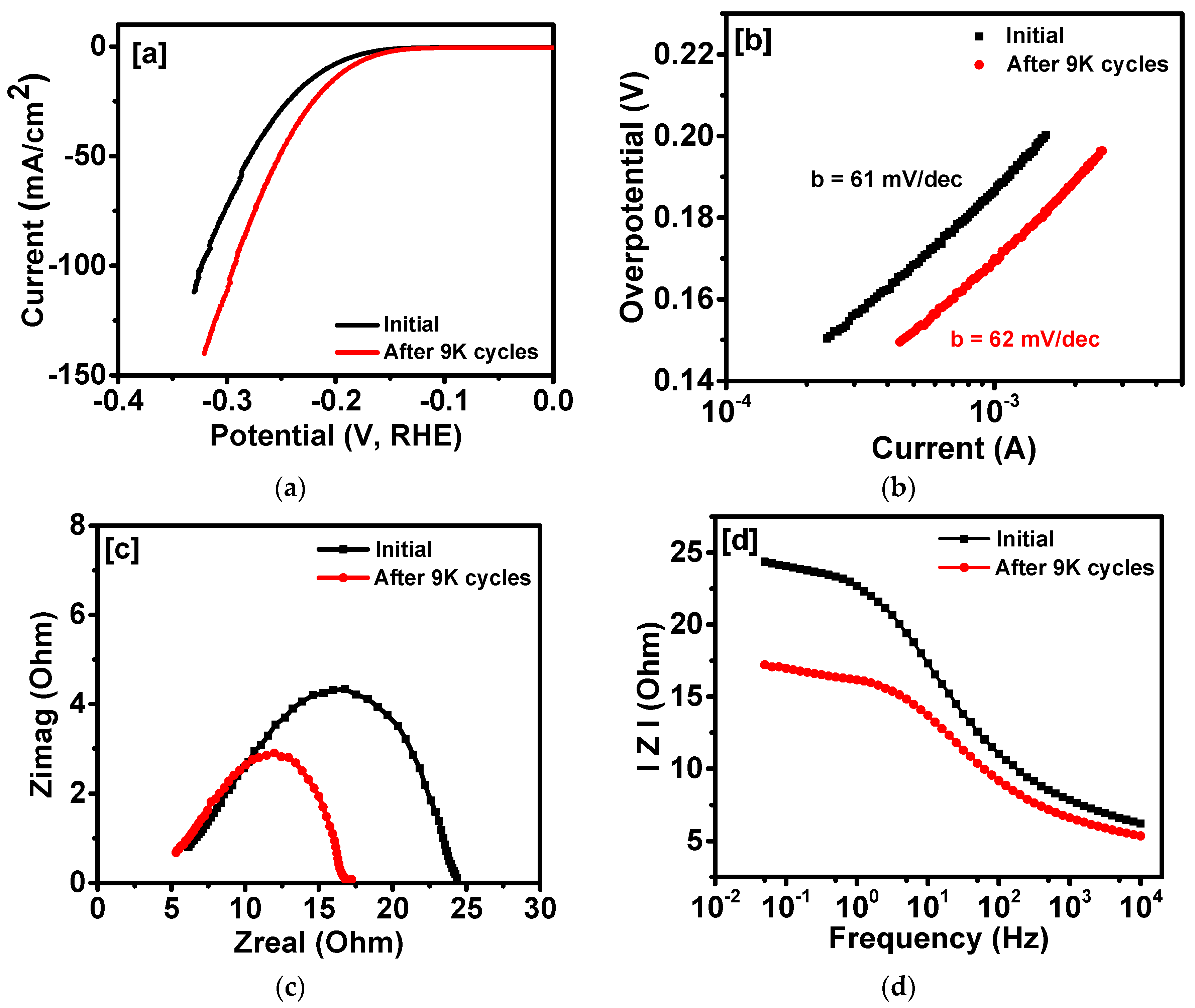
2. Results and Discussion
3. Experimental Details
3.1. Synthesis
3.2. Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gao, M.-R.; Liang, J.-X.; Zheng, Y.-R.; Xu, Y.-F.; Jiang, J.; Gao, Q.; Li, J.; Yu, S.-H. An Efficient Molybdenum Disulfide/cobalt Diselenide Hybrid Catalyst for Electrochemical Hydrogen Generation. Nat. Commun. 2015, 6, 5982. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6673. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lei, Z.; Wu, P. Facile Preparation of 3D MoS2/MoSe2 Nanosheet–graphene Networks as Efficient Electrocatalysts for the Hydrogen Evolution Reaction. J. Mater. Chem. A 2015, 3, 16337–16347. [Google Scholar] [CrossRef]
- Zhou, W.; Hou, D.; Sang, Y.; Yao, S.; Zhou, J.; Li, G.; Li, L.; Liu, H.; Chen, S. MoO2 Nanobelts@nitrogen Self-Doped MoS2 Nanosheets as Effective Electrocatalysts for Hydrogen Evolution Reaction. J. Mater. Chem. A 2014, 2, 11358–11364. [Google Scholar] [CrossRef]
- Yan, Y.; Ge, X.; Liu, Z.; Wang, J.-Y.; Lee, J.-M.; Wang, X. Facile Synthesis of Low Crystalline MoS2 Nanosheet-Coated CNTs for Enhanced Hydrogen Evolution Reaction. Nanoscale 2013, 5, 7768–7771. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Tang, C.; Liu, D.; Wang, J.; Asiri, A.M.; Sun, X. A Self-Standing Nanoporous MoP2 Nanosheet Array: An Advanced pH-Universal Catalytic Electrode for the Hydrogen Evolution Reaction. J. Mater. Chem. A 2016, 4, 7169–7173. [Google Scholar] [CrossRef]
- Jiang, N.; Bogoev, L.; Popova, M.; Gul, S.; Yano, J.; Sun, Y. Electrodeposited Nickel-Sulfide Films as Competent Hydrogen Evolution Catalysts in Neutral Water. J. Mater. Chem. A 2014, 2, 19407–19414. [Google Scholar] [CrossRef]
- Wu, H.B.; Xia, B.Y.; Yu, L.; Yu, X.-Y.; Lou, X.W. (David). Porous Molybdenum Carbide Nano-Octahedrons Synthesized via Confined Carburization in Metal-Organic Frameworks for Efficient Hydrogen Production. Nat. Commun. 2015, 6, 6512. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-F.; Wang, C.-H.; Sasaki, K.; Marinkovic, N.; Xu, W.; Muckerman, J.T.; Zhu, Y.; Adzic, R.R. Highly Active and Durable Nanostructured Molybdenum Carbide Electrocatalysts for Hydrogen Production. Energy Environ. Sci. 2013, 6, 943–951. [Google Scholar] [CrossRef]
- Pumera, M.; Sofer, Z.; Ambrosi, A. Layered Transition Metal Dichalcogenides for Electrochemical Energy Generation and Storage. J. Mater. Chem. A 2014, 2, 8981–8987. [Google Scholar] [CrossRef]
- Ranaweera, C.K.; Zhang, C.; Bhoyate, S.; Kahol, P.K.; Ghimire, M.; Mishra, S.R.; Perez, F.; Gupta, B.K.; Gupta, R.K. Flower-Shaped Cobalt Oxide Nano-Structures as an Efficient, Flexible and Stable Electrocatalyst for the Oxygen Evolution Reaction. Mater. Chem. Front. 2017, 1, 1580–1584. [Google Scholar] [CrossRef]
- Adhikari, H.; Neupane, D.; Ranaweera, C.K.; Candler, J.; Gupta, R.K.; Sapkota, S.; Shen, X.; Mishra, S.R. Template-Free Synthesis of Hierarchical Mixed-Metal Cobaltites: Electrocapacitive and Theoretical Study. Electrochim. Acta 2017, 225, 514–524. [Google Scholar] [CrossRef]
- Bhoyate, S.; Mensah-Darkwa, K.; Kahol, P.K.; Gupta, R.K. Recent Development on Nanocomposites of Graphene for Supercapacitor Applications. Curr. Graphene Sci. 2017, 1, 26–43. [Google Scholar] [CrossRef]
- Aloqayli, S.; Ranaweera, C.K; Wang, Z.; Siam, K.; Kahol, P.K.; Tripathi, P.; Srivastava, O.N.; Gupta, B.K.; Mishra, S.R.; Perez, P.; et al. Nanostructured Cobalt Oxide and Cobalt Sulfide for Flexible, High Performance and Durable Supercapacitors. Energy Storage Mater. 2017, 8, 68–76. [Google Scholar] [CrossRef]
- Alkhalaf, S.; Ranaweera, C.K.; Kahol, P.K.; Siam, K.; Adhikari, H.; Mishra, S.R.; Perez, F.; Gupta, B.K.; Ramasamy, K.; Gupta, R.K. Electrochemical Energy Storage Performance of Electrospun CoMn2O4 Nanofibers. J. Alloy. Compd. 2017, 692, 59–66. [Google Scholar] [CrossRef]
- Segev-Bar, M.; Haick, H. Flexible Sensors Based on Nanoparticles. ACS Nano 2013, 7, 8366–8378. [Google Scholar] [CrossRef] [PubMed]
- Naponiello, G.; Venditti, I.; Zardetto, V.; Saccone, D.; Di Carlo, A.; Fratoddi, I.; Barolo, C.; Dini, D. Photoelectrochemical Characterization of Squaraine-Sensitized Nickel Oxide Cathodes Deposited via Screen-Printing for p-type Dye-Sensitized Solar Cells. Appl. Surf. Sci. 2015, 356, 911–920. [Google Scholar] [CrossRef]
- Chung, D.Y.; Jun, S.W.; Yoon, G.; Kim, H.; Yoo, J.M.; Lee, K.S.; Kim, T.; Shin, H.; Sinha, A.K.; Kwon, S.G.; et al. Large-Scale Synthesis of Carbon-Shell-Coated FeP Nanoparticles for Robust Hydrogen Evolution Reaction Electrocatalyst. J. Am. Chem. Soc. 2017, 139, 6669–6674. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Jahromi, A.R.T; Ensafi, A.A. Ni-Co-Se Nanoparticles Modified Reduced Graphene Oxide Nanoflakes, An Advance Electrocatalyst for Highly Efficient Hydrogen Evolution Reaction. Electrochim. Acta 2016, 213, 423–431. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Hamb, D.J.; Thongtem, S.; Lee, J.S. Electrochemical Hydrogen Evolution Over MoO3 Nanowires Produced by Microwave-Assisted Hydrothermal Reaction. Electrochem. Commun. 2009, 11, 1740–1743. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, F.; Huang, Y.; Sun, J.; Zhu, Z.; Nielsen, R.J.; He, R.; Bao, J.; Goddard, W.A., III; Chen, S.; et al. Efficient Hydrogen Evolution by Ternary Molybdenum Sulfoselenide Particles on Self-Standing Porous Nickel Diselenide Foam. Nat. Commun. 2016, 7, 12765. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Z.; Kong, D.; Sun, J.; Hymel, T.M.; Cui, Y. Electrochemical Tuning of MoS2 Nanoparticles on Three-Dimensional Substrate for Efficient Hydrogen Evolution. ACS Nano 2014, 8, 4940–4947. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, T.F.; Jorgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS2 Nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T.F. Engineering the Surface Structure of MoS2 to Preferentially Expose Active Edge Sites for Electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Fullon, R.; Yang, J.; de Carvalho Castro e Silva, C.; Kappera, R.; Bozkurt, I.; Kaplan, D.; Lagos, M.J.; Batson, P.E.; Gupta, G.; et al. The Role of Electronic Coupling between Substrate and 2D MoS2 Nanosheets in Electrocatalytic Production of Hydrogen. Nat. Mater. 2016, 15, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhang, H.; Dong, S.; Liu, Y.; Tai Nai, C.; Suk Shin, H.; Young Jeong, H.; Liu, B.; Ping Loh, K. High Yield Exfoliation of Two-Dimensional Chalcogenides Using Sodium Naphthalenide. Nat. Commun. 2014, 5, 2995. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, D.; Damien, D.; Shaijumon, M.M. MoS2 Quantum Dot-Interspersed Exfoliated MoS2 Nanosheets. ACS Nano 2014, 8, 5297–5303. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gao, Q.; Li, Z. Effects of Additives on Morphology and Hydrogen Evolution Activities of Nickel Films Prepared by Electrodepositing. Int. J. Electrochem. Sci. 2015, 10, 8823–8833. [Google Scholar]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 Nanoparticles Grown on Graphene: An Advanced Catalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.; Wu, J.; Tao, L.; Shen, A.; Huo, J.; Wang, S. Carbon-Coated MoS2 Nanosheets as Highly Efficient Electrocatalysts for the Hydrogen Evolution Reaction. Nanotechnology 2016, 27, 045402. [Google Scholar] [CrossRef] [PubMed]
- Li, D.J.; Maiti, U.N.; Lim, J.; Choi, D.S.; Lee, W.J.; Oh, Y.; Lee, G.Y.; Kim, S.O. Molybdenum sulfide/N-Doped CNT Forest Hybrid Catalysts for High-Performance Hydrogen Evolution Reaction. Nano Lett. 2014, 14, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- You, B.; Jiang, N.; Sun, Y. Morphology–activity Correlation in Hydrogen Evolution Catalyzed by Cobalt Sulfides. Inorg. Chem. Front. 2016, 3, 279–285. [Google Scholar] [CrossRef]
- Cummins, D.R.; Martinez, U.; Sherehiy, A.; Kappera, R.; Martinez-Garcia, A.; Schulze, R.K.; Jasinski, J.; Zhang, J.; Gupta, R.K.; Lou, J.; et al. Efficient Hydrogen Evolution in Transition Metal Dichalcogenides via a Simple One-Step Hydrazine Reaction. Nat. Commun. 2016, 7, 11857. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.R.; Hu, W.H.; Li, X.; Dong, B.; Shang, X.; Han, G.Q.; Chai, Y.M.; Liu, Y.Q.; Liu, C.G. Facile One-Pot Synthesis of CoS2-MoS2/CNTs as Efficient Electrocatalyst for Hydrogen Evolution Reaction. Appl. Surf. Sci. 2016, 384, 51–57. [Google Scholar] [CrossRef]
- Faber, M.S.; Dziedzic, R.; Lukowski, M.A.; Kaiser, N.S.; Ding, Q.; Jin, S. High-Performance Electrocatalysis Using Metallic Cobalt Pyrite (CoS2) Micro- and Nanostructures. J. Am. Chem. Soc. 2014, 136, 10053–10061. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.S.; Lukowski, M.A.; Ding, Q.; Kaiser, N.S.; Jin, S. Earth-Abundant Metal Pyrites (FeS2, CoS2, NiS2, and Their Alloys) for Highly Efficient Hydrogen Evolution and Polysulfide Reduction Electrocatalysis. J. Phys. Chem. C 2014, 118, 21347–21356. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wang, H.; Lu, Z.; Cui, Y. CoSe2 Nanoparticles Grown on Carbon Fiber Paper: An Efficient and Stable Electrocatalyst for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2014, 136, 4897–4900. [Google Scholar] [CrossRef] [PubMed]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from Chemically Exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef] [PubMed]
- Acerce, M.; Voiry, D.; Chhowalla, M. Metallic 1T Phase MoS2 Nanosheets as Supercapacitor Electrode Materials. Nat. Nanotechnol. 2015, 10, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.; Smith, R.J.; McEvoy, N.; Berner, N.C.; McCloskey, D.; Nerl, H.C.; O’Neill, A.; King, P.J.; Higgins, T.; Hanlon, D.; et al. Edge and Confinement Effects Allow in Situ Measurement of Size and Thickness of Liquid-Exfoliated Nanosheets. Nat. Commun. 2014, 5, 4576. [Google Scholar] [CrossRef] [PubMed]
- Youn, D.H.; Han, S.; Kim, J.Y.; Kim, J.Y.; Park, H.; Choi, S.H.; Lee, J.S. Highly Active and Stable Hydrogen Evolution Electrocatalysts Based on Molybdenum Compounds on Carbon Nanotube-Graphene Hybrid Support. ACS Nano 2014, 8, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.; Xie, Y. Defect-Rich MoS2 Ultrathin Nanosheets with Additional Active Edge Sites for Enhanced Electrocatalytic Hydrogen Evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Gao, D.; Zhuo, J.; Zhu, Z.; Papakonstantinou, P.; Li, Y.; Li, M. Size-Dependent Enhancement of Electrocatalytic Oxygen-Reduction and Hydrogen-Evolution Performance of MoS2 Particles. Chem. A Eur. J. 2013, 19, 11939–11948. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cao, G.; Ding, F.; Li, X.; Zhen, S.; Xue, Y.; Yan, Y.; Liu, T.; Sun, K. A Bulky and Flexible Electrocatalyst for Efficient Hydrogen Evolution Based on the Growth of MoS2 Nanoparticles on Carbon Nanofiber Foam. J. Mater. Chem. A 2015, 3, 5041–5046. [Google Scholar] [CrossRef]
- Ren, X.; Pang, L.; Zhang, Y.; Ren, X.; Fan, H.; Liu, S. (Frank). One-Step Hydrothermal Synthesis of Monolayer MoS2 Quantum Dots for Highly Efficient Electrocatalytic Hydrogen Evolution. J. Mater. Chem. A 2015, 3, 10693–10697. [Google Scholar] [CrossRef]
- Bonde, J.; Moses, P.G.; Jaramillo, T.F.; Nørskov, J.K.; Chorkendorff, I. Hydrogen Evolution on Nano-Particulate Transition Metal Sulfides. Faraday Discuss. 2008, 140, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Laursen, A.B.; Kegnæs, S.; Dahl, S.; Chorkendorff, I. Molybdenum Sulfides—efficient and Viable Materials for Electro—And Photoelectrocatalytic Hydrogen Evolution. Energy Environ. Sci. 2012, 5, 5577–5591. [Google Scholar] [CrossRef]
- Xu, K.; Wang, F.; Wang, Z.; Zhan, X.; Wang, Q.; Cheng, Z.; Safdar, M.; He, J. Component-Controllable WS2(1−x)Se2x Nanotubes for Efficient Hydrogen Evolution Reaction. ACS Nano 2014, 8, 8468–8476. [Google Scholar] [CrossRef] [PubMed]
- Benck, J.D.; Chen, Z.; Kuritzky, L.Y.; Forman, A.J.; Jaramillo, T.F. Amorphous Molybdenum Sulfide Catalysts for Electrochemical Hydrogen Production: Insights into the Origin of Their Catalytic Activity. ACS Catal. 2012, 2, 1916–1923. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wang, Z.; Bhoyate, S.; Morey, T.; Neria, B.L.; Vasiraju, V.; Gupta, G.; Palchoudhury, S.; Kahol, P.K.; Mishra, S.R.; et al. MoS2 Decorated Carbon Nanofibers as Efficient and Durable Electrocatalyst for Hydrogen Evolution Reaction. C 2017, 3, 33. https://doi.org/10.3390/c3040033
Zhang C, Wang Z, Bhoyate S, Morey T, Neria BL, Vasiraju V, Gupta G, Palchoudhury S, Kahol PK, Mishra SR, et al. MoS2 Decorated Carbon Nanofibers as Efficient and Durable Electrocatalyst for Hydrogen Evolution Reaction. C. 2017; 3(4):33. https://doi.org/10.3390/c3040033
Chicago/Turabian StyleZhang, C., Z. Wang, S. Bhoyate, T. Morey, Brooks L. Neria, Venkata Vasiraju, Gautam Gupta, Soubantika Palchoudhury, P. K. Kahol, S. R. Mishra, and et al. 2017. "MoS2 Decorated Carbon Nanofibers as Efficient and Durable Electrocatalyst for Hydrogen Evolution Reaction" C 3, no. 4: 33. https://doi.org/10.3390/c3040033




