Adsorption of Bovine Serum Albumin on Carbon-Based Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorption of Bovine Serum Albumin (BSA)
2.3. Porosity
2.4. Surface Characterization
3. Results and Discussion
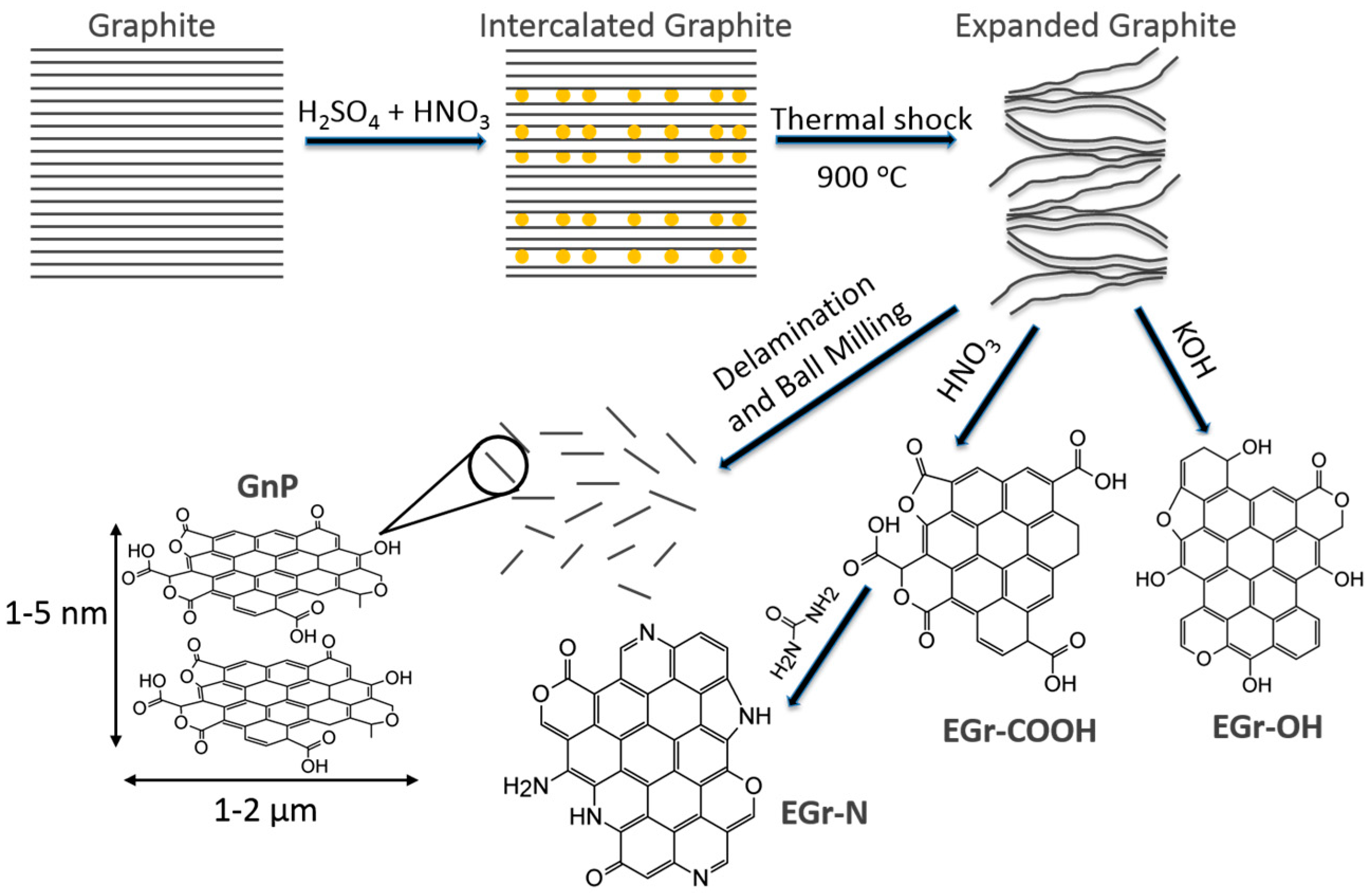
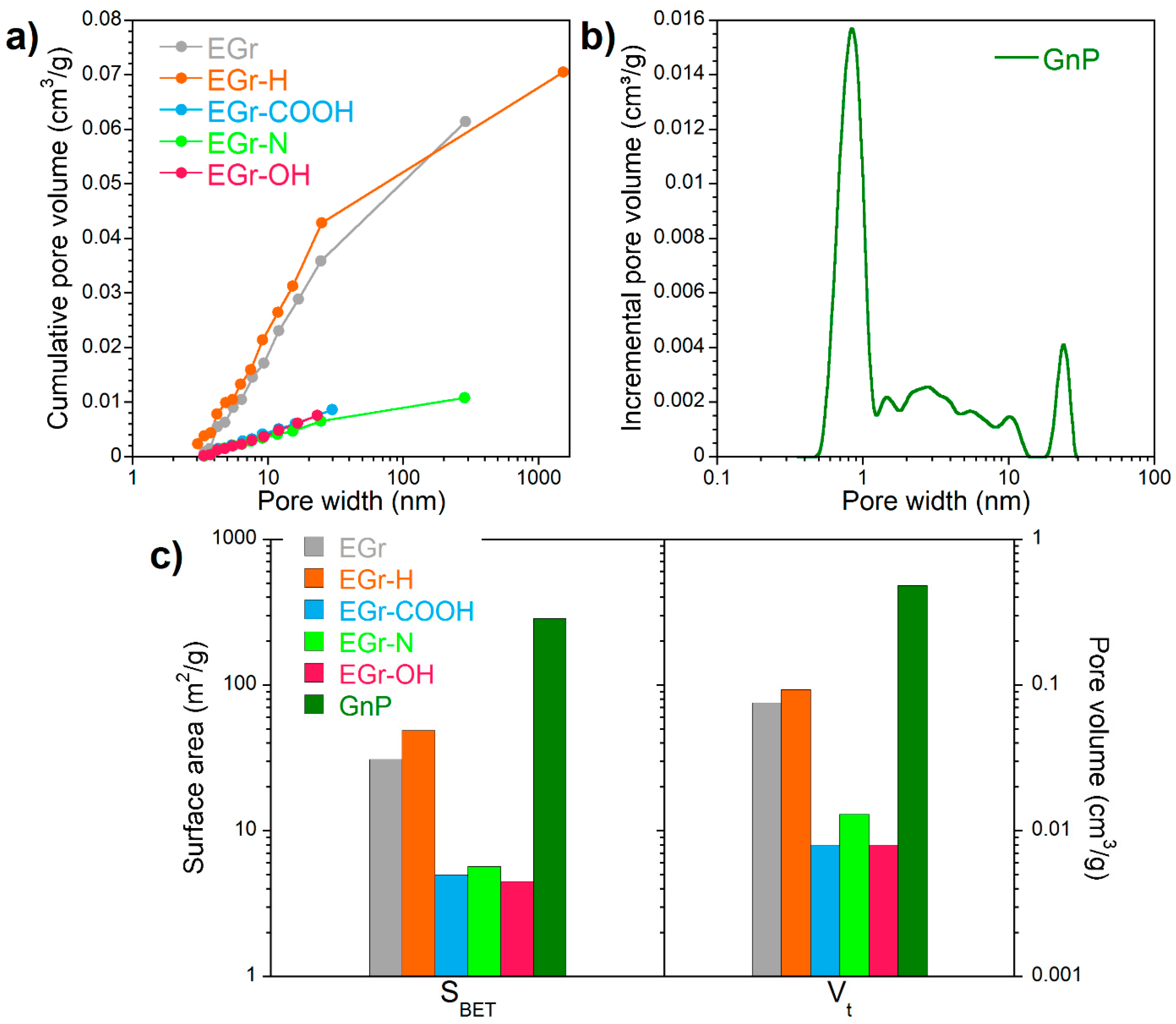
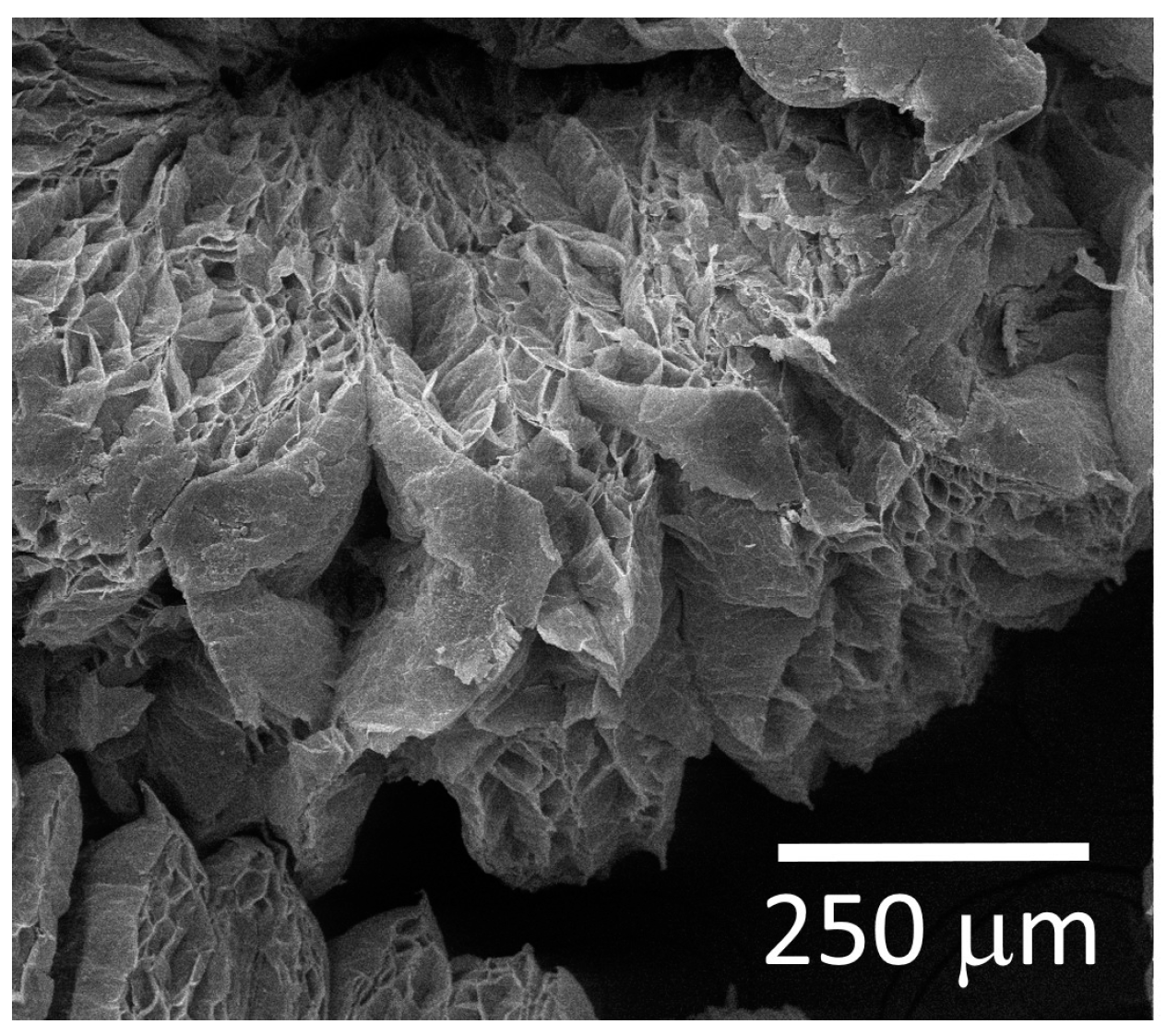
3.1. Porosity and Surface Chemistry of Carbon-Based Materials
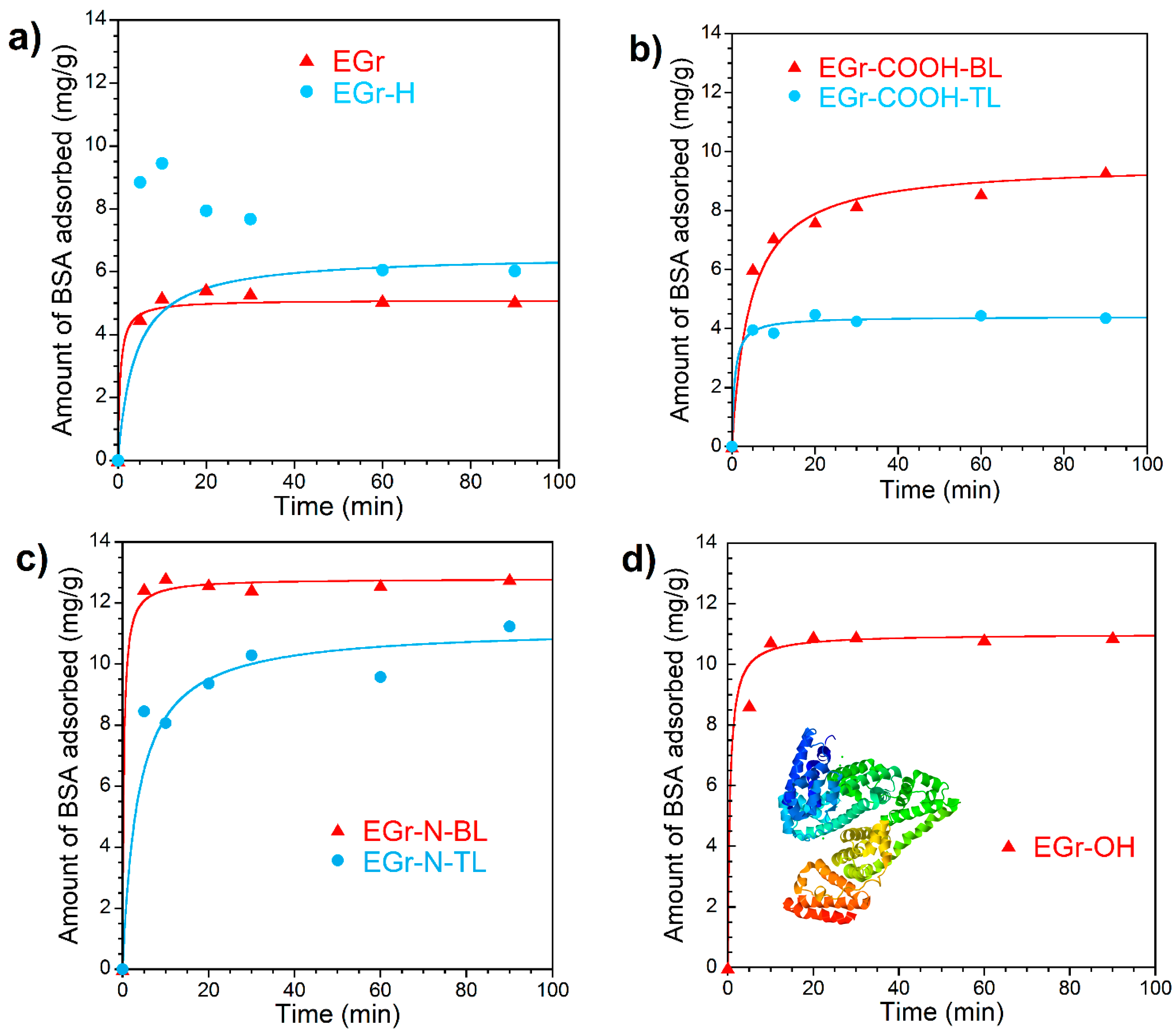
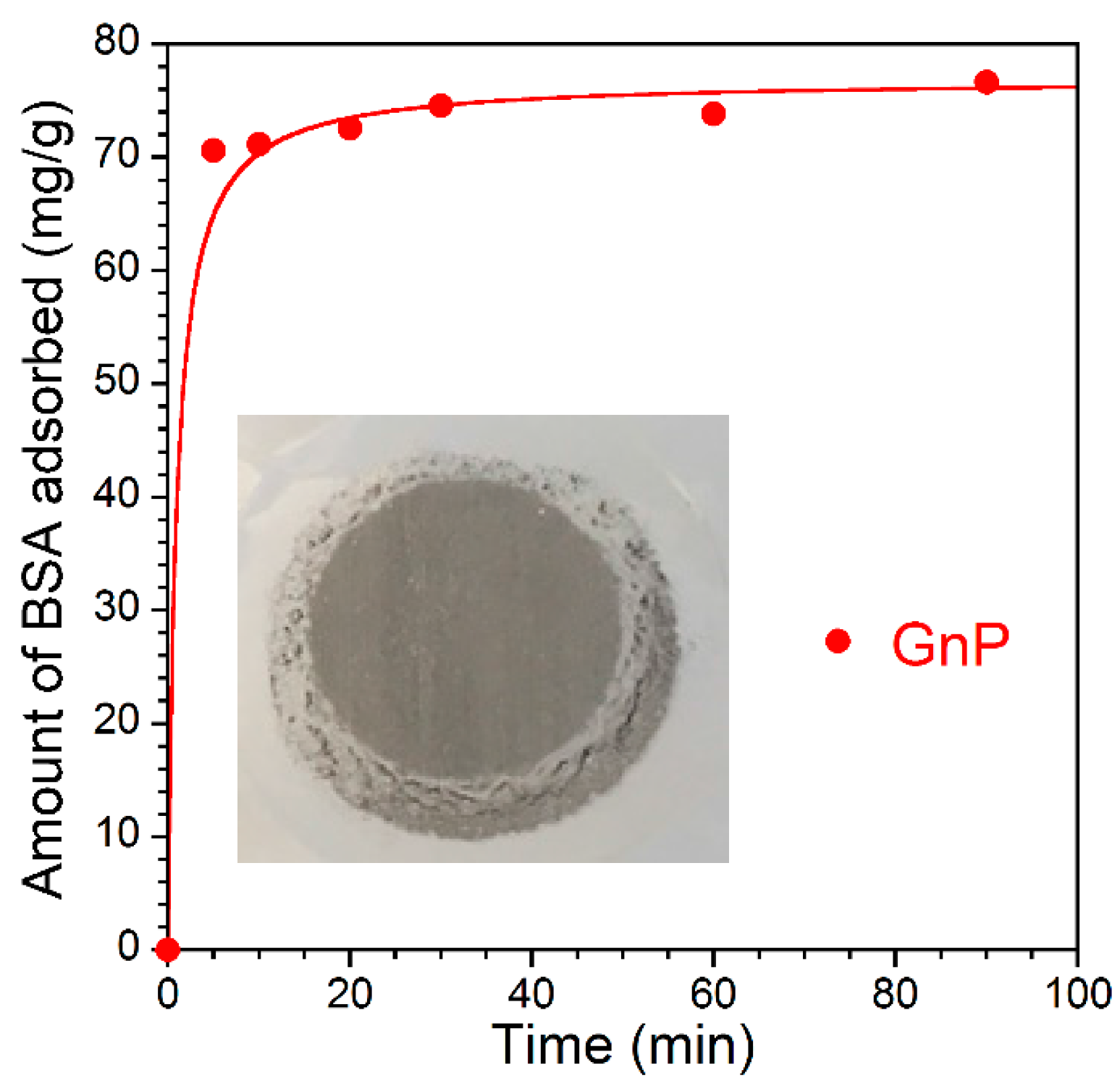
3.2. Adsorption Kinetics
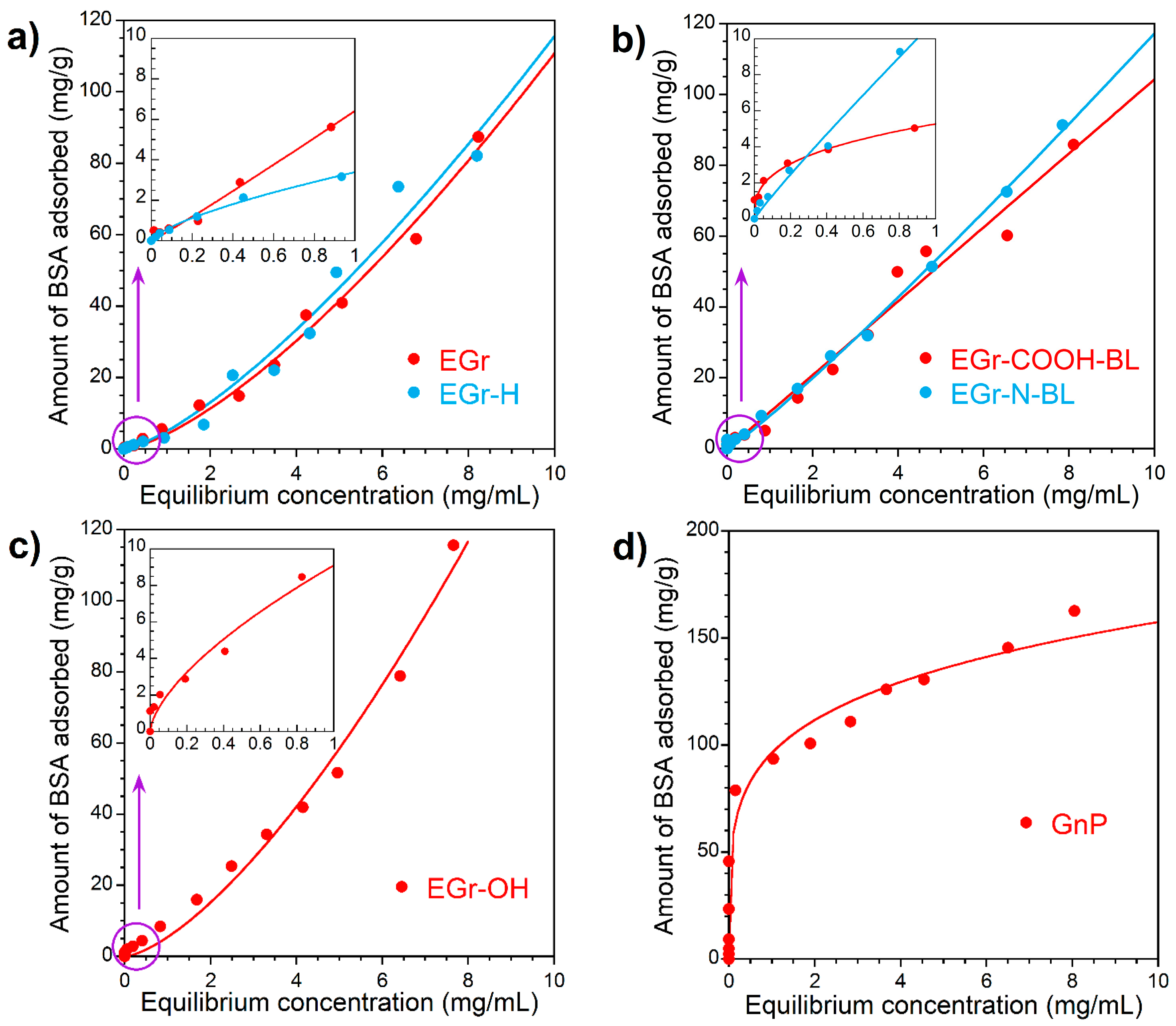
3.3. BSA Adsorption Isotherms
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bokros, J.C. Deposition, Structure and Properties of Pyrolytic Carbon. In Chemistry and Physics of Carbon; Walker, P.L., Ed.; Dekker: New York, NY, USA, 1969; Volume 5, pp. 1–118. [Google Scholar]
- Mikhalovsky, S.V.; Sandeman, S.R.; Howell, C.A.; Phillips, G.J.; Nikolaev, V.G. Biomedical Applications of Carbon Adsorbents. In Novel Carbon Adsorbents; Tascon, J.M.D., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; Chapter 21; pp. 639–669. [Google Scholar]
- Ray, S.C.; Jana, N.R. Carbon Nanomaterials for Biological and Medical Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–231. [Google Scholar]
- Kostarelos, K.; Novoselov, K.S. Graphene Devices for Life. Nat. Nanotechnol. 2014, 9, 744–745. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Denno, M.E.; Pyakurel, P.; Venton, B.J. Recent Trends in Carbon Nanomaterial-Based Electrochemical Sensors for Biomolecules: A Review. Anal. Chim. Acta 2015, 887, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Dee, K.C.; Puleo, D.A.; Bizios, R. An Introduction to Tissue-Biomaterial Interactions; Wiley-Liss, Inc.: Hoboken, NJ, USA, 2002; pp. 1–219. [Google Scholar]
- Tang, L.; Eaton, J.W. Natural Responses to Unnatural Materials: A Molecular Mechanism for Foreign Body Reactions. Mol. Med. 1999, 5, 351–358. [Google Scholar] [PubMed]
- Hu, W.; Peng, C.; Lv, M.; Li, X.; Zhang, Y.; Chen, N.; Fan, C.; Huang, Q. Protein Corona-Mediated Mitigation of Cytotoxicity of Graphene Oxide. ACS Nano 2011, 5, 3693–3700. [Google Scholar] [CrossRef] [PubMed]
- Krummel, T.; Hannedouche, T. Clinical Potentials of Adsorptive Dialysis Membranes. Blood Purif. 2013, 35 (Suppl. 2), 1–4. [Google Scholar] [CrossRef] [PubMed]
- Tengvall, P.; Lundstrom, I.; Liedberg, B. Protein Adsorption Studies on Model Organic Surfaces: An Ellipsometric and Infrared Spectroscopic Approach. Biomaterials 1998, 19, 407–422. [Google Scholar] [CrossRef]
- Yachamaneni, S.; Yushin, G.; Yeon, S.-H.; Gogotsi, Y.; Howell, C.; Sandeman, S.; Phillips, G.; Mikhalovsky, S. Mesoporous Carbide-Derived Carbon for Cytokine Removal from Blood Plasma. Biomaterials 2010, 31, 4789–4794. [Google Scholar] [CrossRef] [PubMed]
- Yushin, G.; Hoffman, E.N.; Barsoum, M.W.; Gogotsi, Y.; Howell, C.A.; Sandeman, S.R.; Phillips, G.J.; Lloyd, A.W.; Mikhalovsky, S.V. Mesoporous Carbide-Derived Carbon with Porosity Tuned for Efficient Adsorption of Cytokines. Biomaterials 2006, 27, 5755–5762. [Google Scholar] [CrossRef] [PubMed]
- Presser, V.; Yeon, S.-H.; Vakifahmetoglu, C.; Howell, C.A.; Sandeman, S.R.; Colombo, P.; Mikhalovsky, S.; Gogotsi, Y. Cytokine Removal: Hierarchical Porous Carbide-Derived Carbons for the Removal of Cytokines from Blood Plasma. Adv. Healthc. Mater. 2012, 1, 796–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, C.A.; Sandeman, S.R.; Phillips, G.J.; Lloyd, A.W.; Davies, J.G.; Mikhalovsky, S.V.; Tennison, S.R.; Rawlinson, A.P.; Kozynchenko, O.P.; Owen, H.L.H.; et al. The In Vitro Adsorption of Cytokines by Polymer-Pyrolysed Carbon. Biomaterials 2006, 27, 5286–5291. [Google Scholar] [CrossRef] [PubMed]
- Tripisciano, C.; Kozynchenko, O.P.; Linsberger, I.; Phillips, G.J.; Howell, C.A.; Sandeman, S.R.; Tennison, S.R.; Mikhalovsky, S.V.; Weber, V.; Falkenhagen, D. Activation-Dependent Adsorption of Cytokines and Toxins Related to Liver Failure to Carbon Beads. Biomacromolecules 2011, 12, 3733–3740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchigami, Y.; Nakashima, T. Fibrous Adsorbent for Hemoperfusion. U.S. Patent 4,248,736, 3 February 1981. [Google Scholar]
- Howell, C.A.; Sandeman, S.R.; Zheng, Y.; Mikhalovsky, S.V.; Nikolaev, V.G.; Sakhno, L.A.; Snezhkova, E.A. New Dextran Coated Activated Carbons for Medical Use. Carbon 2016, 97, 134–146. [Google Scholar] [CrossRef]
- Malmsten, M. Formation of Adsorbed Protein Layers. J. Colloid Interface Sci. 1998, 207, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Sakiyama, T.; Imamura, K. On the Adsorption of Proteins on Solid Surfaces, a Common but Very Complicated Phenomenon. J. Biosci. Bioeng. 2001, 91, 233–244. [Google Scholar] [CrossRef]
- Wanci, S.; Shizhu, W.; Naizhen, C.; Lu, Z.; Wei, Z.; Yingjie, L.; Jialin, G. Expanded Graphite. A New Kind of Biomedical Material. Carbon 1999, 37, 356–358. [Google Scholar] [CrossRef]
- Ma, C.-F.; Gao, Q.; Xia, K.-S.; Huang, Z.-Y.; Han, B.; Zhou, C.-G. Three-Dimensionally Porous Graphene: A High-Performance Adsorbent for Removal of Albumin-Bonded Bilirubin. Colloids Surf. B: Biointerfaces 2017, 149, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, B.; Gregory, W.E.; Zhu, J.; Dasetty, S.; Karakaya, M.; Brown, J.M.; Rao, A.M.; Barrows, J.K.; Sarupria, S.; Podila, R. Influence of Carbon Nanomaterial Defects on the Formation of Protein Corona. RSC Adv. 2015, 5, 82395–82402. [Google Scholar] [CrossRef] [PubMed]
- Yaroshenko, A.P.; Savos’kin, M.V.; Magazinskii, A.N.; Shologon, V.I.; Mysyk, R.D. Synthesis and Properties of Thermally Expandable Residual Graphite Hydrosulfite Obtained in the System HNO3-H2SO4. Russ. J. Appl. Chem. 2002, 75, 861–865. [Google Scholar] [CrossRef]
- Boehm, H.P. Some Aspects of the Surface Chemistry of Carbon Black and other Carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Li, X.; Biswas, S.; Drzal, L.T. High Temperature Vacuum Annealing and Hydrogenation Modification of Exfoliated Graphite Nanoplatelets. J. Eng. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Sorption of Dye from Aqueous Solution by Peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. Carbon Slit Pore Model Incorporating Surface Energetical Heterogeneity and Geometrical Corrugation. Adsorption 2013, 19, 777–783. [Google Scholar] [CrossRef]
- Chung, D.D.L. A Review of Exfoliated Graphite. J. Mater. Sci. 2016, 51, 554–568. [Google Scholar] [CrossRef]
- Zheng, Y.; Pescatore, N.; Gogotsi, Y.; Dyatkin, B.; Ingavle, G.; Mochalin, V.; Ozulumba, T.; Mikhalovsky, S.; Sandeman, S. Rapid Adsorption of Pro-inflammatory Cytokines by Graphene Nanoplatelets and Their Composites for Extracorporeal Detoxification. J. Nanomater. 2018, in press. [Google Scholar]
- Kharlamova, M.V.; Mochalin, V.N.; Lukatskaya, M.R.; Niu, J.; Presser, V.; Mikhalovsky, S.; Gogotsi, Y. Adsorption of Proteins in Channels of Carbon Nanotubes: Effect of Surface Chemistry. Mater. Express 2013, 3, 1–10. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Jaszczolt, K.; Michna, A.; Siwek, B.; Szyk-Warszynska, L.; Zembala, M. Irreversible Adsorption of Particles on Heterogeneous Surfaces. Adv. Colloid Interface Sci. 2005, 118, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Vroman, L.; Adams, A.L.; Fischer, G.C.; Munoz, P.C. Interaction of High Molecular Weight Kininogen, Factor XII and Fibrinogen in Plasma at Interfaces. Blood 1980, 55, 156–159. [Google Scholar] [PubMed]
- Kubiak-Ossowska, K.; Jachimska, B.; Mulheran, P.A. How Negatively Charged Proteins Adsorb to Negatively Charged Surfaces: A Molecular Dynamics Study of BSA Adsorption on Silica. J. Phys. Chem. B 2016, 120, 10463–10468. [Google Scholar] [CrossRef] [PubMed]
- Kubiak-Ossowska, K.; Tokarczyk, K.; Jachimska, B.; Mulheran, P.A. Bovine Serum Albumin Adsorption at a Silica Surface Explored by Simulation and Experiment. J. Phys. Chem. B 2017, 121, 3975–3986. [Google Scholar] [CrossRef] [PubMed]
- Majorek, K.A.; Porebski, P.J.; Dayal, A.; Zimmerman, M.D.; Jablonska, K.; Stewart, A.J.; Chruszcz, M.; Minor, W. Structural and Immunologic Characterization of Bovine, Horse and Rabbit Serum Albumins. Mol. Immunol. 2012, 52, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical Models of Sorption Kinetics Including a Surface Reaction Mechanism: A Review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Kuchlyan, J.; Kundu, N.; Banik, D.; Roy, A.; Sarkar, N. Spectroscopy and Fluorescence Lifetime Imaging Microscopy to Probe the Interaction of Bovine Serum Albumin with Graphene Oxide. Langmuir 2015, 31, 13793–13801. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.K.; Thompson, M.R. Hydrodynamic Structure of Bovine Serum Albumin Determined by Transient Electric Birefringence. Biophys. J. 1975, 15, 137–141. [Google Scholar] [CrossRef]
- Luik, A.I.; Lukianchuk, V.D. Serum Albumin and Biotransport of Poison; Medicina: Moscow, Russia, 1984; p. 224. [Google Scholar]
- Giles, C.; McEwan, T.; Nakhwa, S.; Smith, D.J. Studies in Adsorption. Part XI. A System of Classification of Solution Adsorption Isotherms and its Use in Diagnosis of Adsorption Mechanisms and in Measurement of Specific Surface Areas of Solids. J. Chem. Soc. 1960, 3973–3993. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die Adsorption in Lösungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
| Sample | Atomic Concentration (%) | ||
|---|---|---|---|
| C | O | N | |
| EGr | 97.2 | 2.8 | ND * |
| EGr-H | 97.3 | 2.7 | ND |
| EGr-COOH | 96.1 | 3.9 | ND |
| EGr-N | 96.8 | 2.0 | 1.2 |
| EGr-OH | 96.3 | 3.7 | ND |
| GnP | 92.1 | 7.9 | ND |
| Energy (eV) | Bond Assignment | EGr | EGr-H | EGr-COOH | EGr-N | EGr-OH | GnP |
|---|---|---|---|---|---|---|---|
| C 1s | |||||||
| 284.8 | C-C (sp2, graphitic carbon) | 75.3 | 75.8 | 74.3 | 74.6 | 72.6 | 70.7 |
| 285.5 | C-N (carbon–nitrogen structures) | 14.5 | |||||
| 285.9–286.2 | C-O (phenolic, alcoxy, ether) | 12.4 | 12.3 | 12.1 | 5.2 | 14.5 | 12.2 |
| 286.9–287.0 | C=O (carbonyl or quinone) | 4.4 | 4.6 | 4.8 | 2.5 | 4.8 | 3.7 |
| 288.4 | O-C=O (carboxyl or ester) | 1.6 | 1.6 | 1.8 | 1.4 | 2.8 | |
| 289.7–290.0 | Carbonate, occluded CO, π—electrons in aromatic ring | 1.6 | 1.2 | 1.2 | 1.5 | 1.6 | |
| 291.3 | π–π transition | 1.9 | 1.8 | 1.9 | 1.5 | 1.1 | |
| O 1s | |||||||
| 531.8–532.0 | O=C (in carboxyl/carbonyl) | 1.5 | 1.5 | 2.4 | 1.1 | 1.6 | 4.1 |
| 533.2–533.5 | O-C (in phenol/epoxy/ether) | 1.3 | 1.2 | 1.5 | 0.9 | 2.1 | 3.9 |
| N 1s | |||||||
| 398.6 | N-6 (in pyridine) | 0.59 | |||||
| 400.8 | N-5 (in pyrrole/amine/amide) | 0.61 | |||||
| Sample | Pseudo Second-Order | ||
|---|---|---|---|
| k2 (g mg−1 min−1) | qe (calc.) (mg/g) | R2 | |
| EGr | 0.39 | 5.1 | 0.9994 |
| EGr-H * | 0.040 | 6.5 | 0.9963 |
| EGr-COOH-BL | 0.024 | 9.6 | 0.9974 |
| EGr-COOH-TL | 0.35 | 4.4 | 0.9994 |
| EGr-N-BL | 0.26 | 12.8 | 0.9998 |
| EGr-N-TL | 0.025 | 11.2 | 0.9885 |
| EGr-OH | 0.18 | 11.0 | 0.9998 |
| GnP | 0.014 | 76.9 | 0.9995 |
| Sample | Langmuir-Freundlich Isotherm Constants | Freundlich Isotherm Constants | |||||
|---|---|---|---|---|---|---|---|
| qo (gBSA/g) | K (L/mg) | n | R2 | KF [(mg/g)(L/mg)1/n] | n | R2 | |
| EGr | 9.3 | 0.0045 | 1.42 | 0.9909 | 4.3 | 1.42 | 0.9909 |
| EGr-H | 11.0 | 0.0035 | 1.36 | 0.9745 | 5.1 | 1.36 | 0.9744 |
| EGr-COOH-BL | 8.7 | 0.0013 | 1.01 | 0.9762 | 10.4 | 1.00 | 0.9761 |
| EGr-N-BL | 12.6 | 0.0015 | 1.11 | 0.9975 | 9.3 | 1.10 | 0.9975 |
| EGr-OH | 22.2 | 0.0036 | 1.47 | 0.9876 | 5.5 | 1.47 | 0.9877 |
| GnP | 1.3 | 1.75 × 10−5 | 0.23 | 0.9243 | 96.4 | 0.21 | 0.9252 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seredych, M.; Mikhalovska, L.; Mikhalovsky, S.; Gogotsi, Y. Adsorption of Bovine Serum Albumin on Carbon-Based Materials. C 2018, 4, 3. https://doi.org/10.3390/c4010003
Seredych M, Mikhalovska L, Mikhalovsky S, Gogotsi Y. Adsorption of Bovine Serum Albumin on Carbon-Based Materials. C. 2018; 4(1):3. https://doi.org/10.3390/c4010003
Chicago/Turabian StyleSeredych, Mykola, Lyuba Mikhalovska, Sergey Mikhalovsky, and Yury Gogotsi. 2018. "Adsorption of Bovine Serum Albumin on Carbon-Based Materials" C 4, no. 1: 3. https://doi.org/10.3390/c4010003