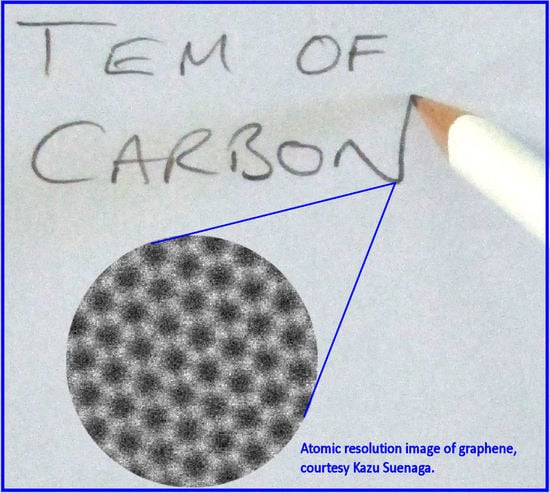
Transmission Electron Microscopy of Carbon: A Brief History
Abstract
:1. Introduction
2. The Development of Transmission Electron Microscopy
3. Landmarks in the Application of TEM to Carbon
3.1. 1940s and 1950s
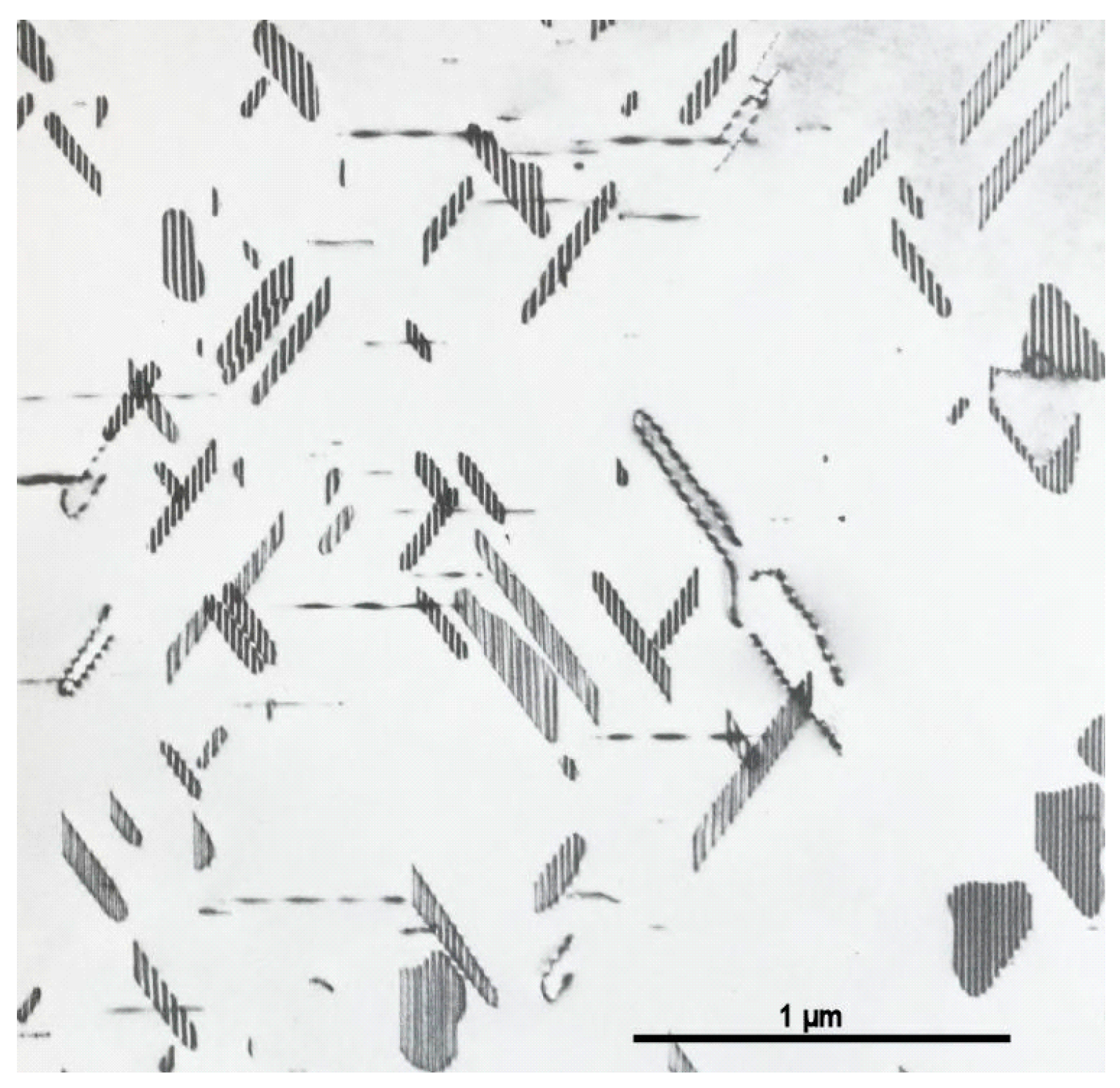
3.2. 1960s and 1970s
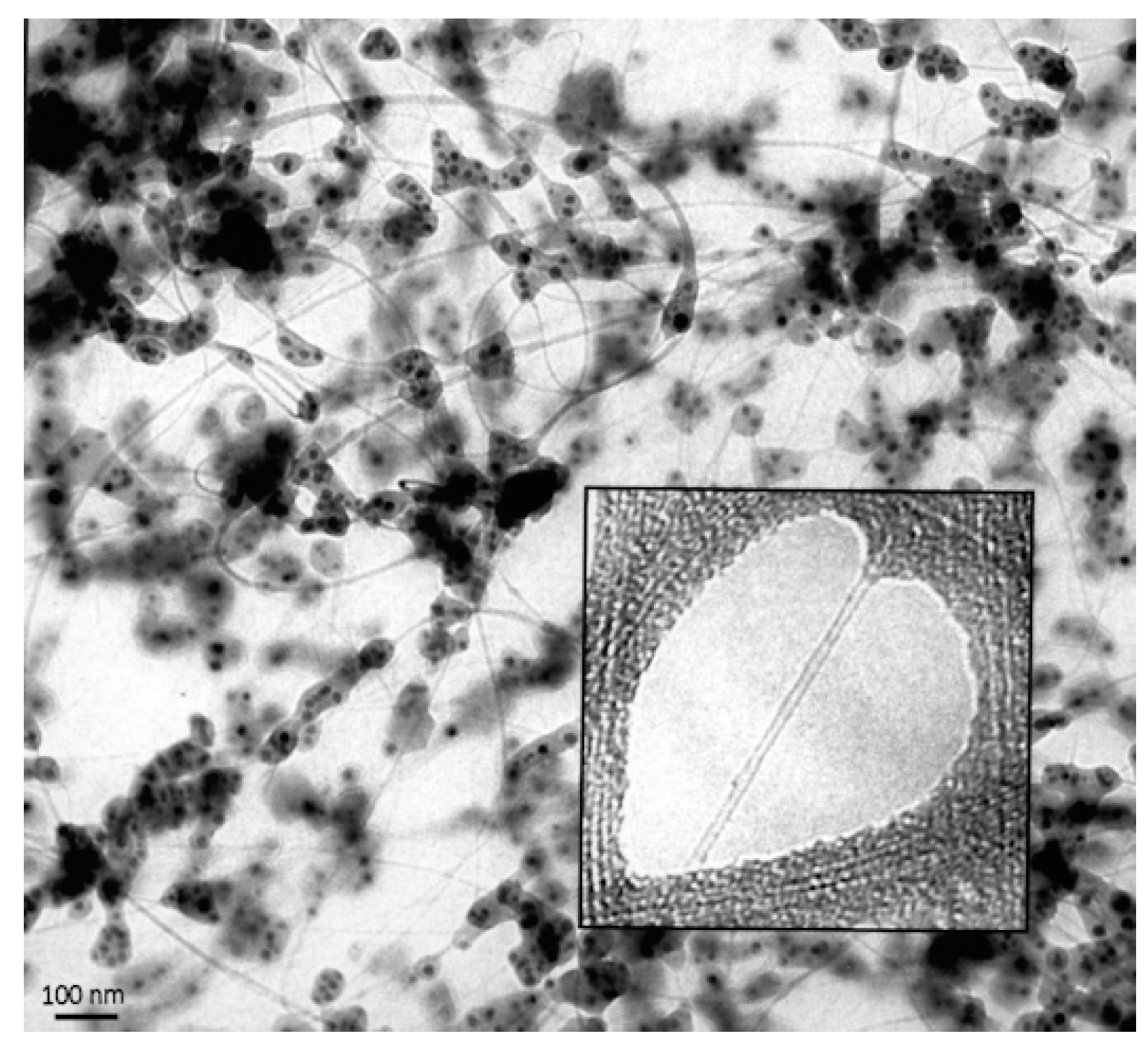
3.3. 1980s and 1990s
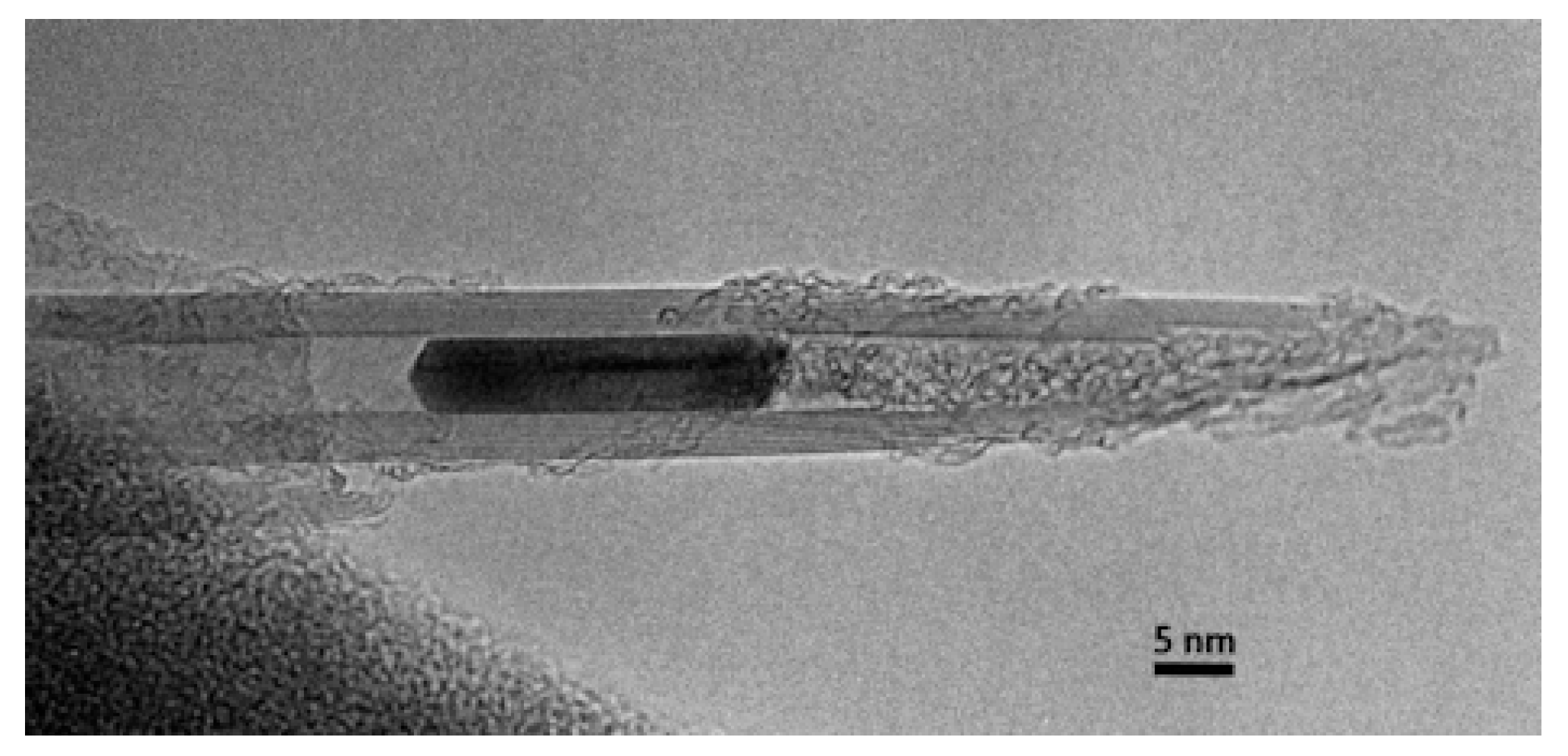
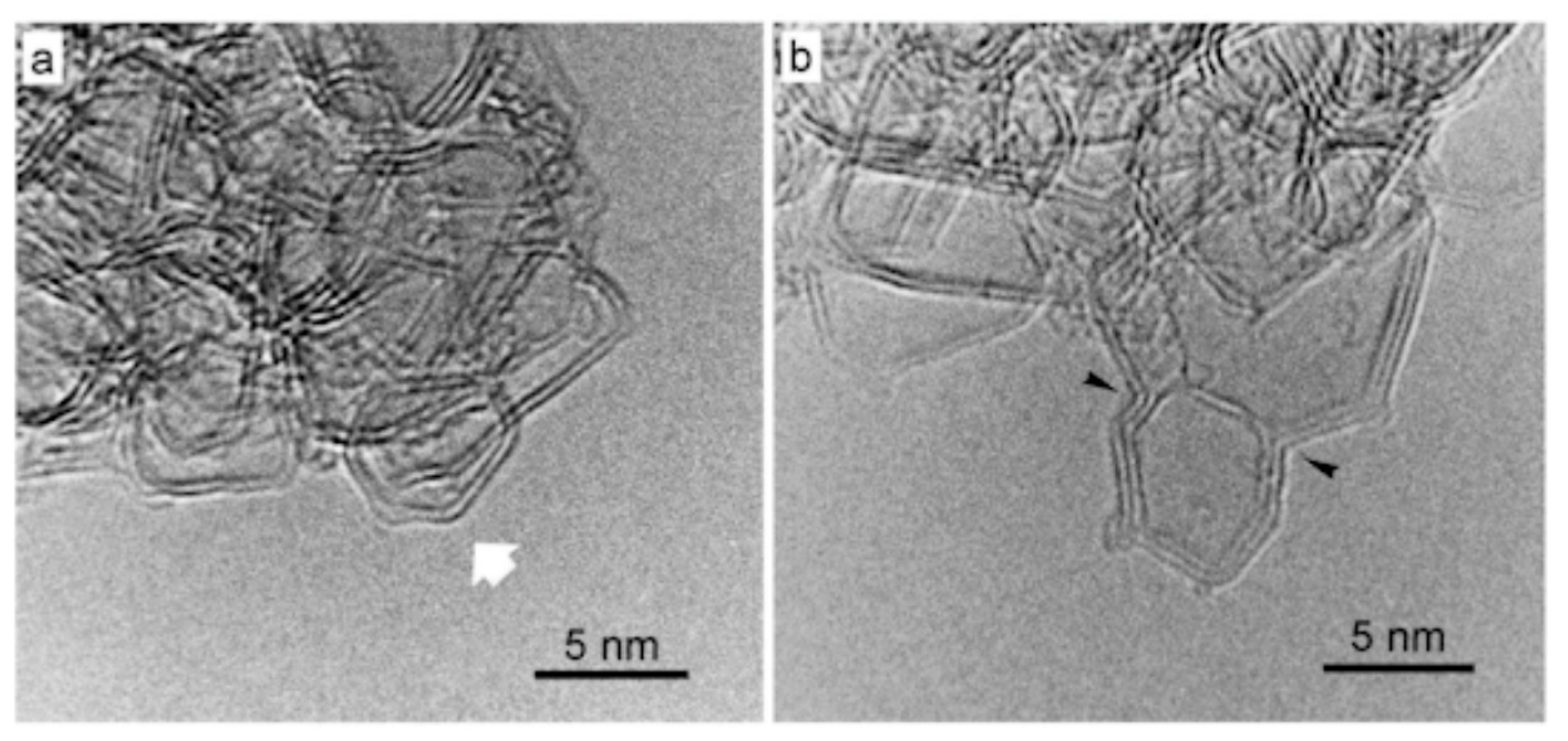
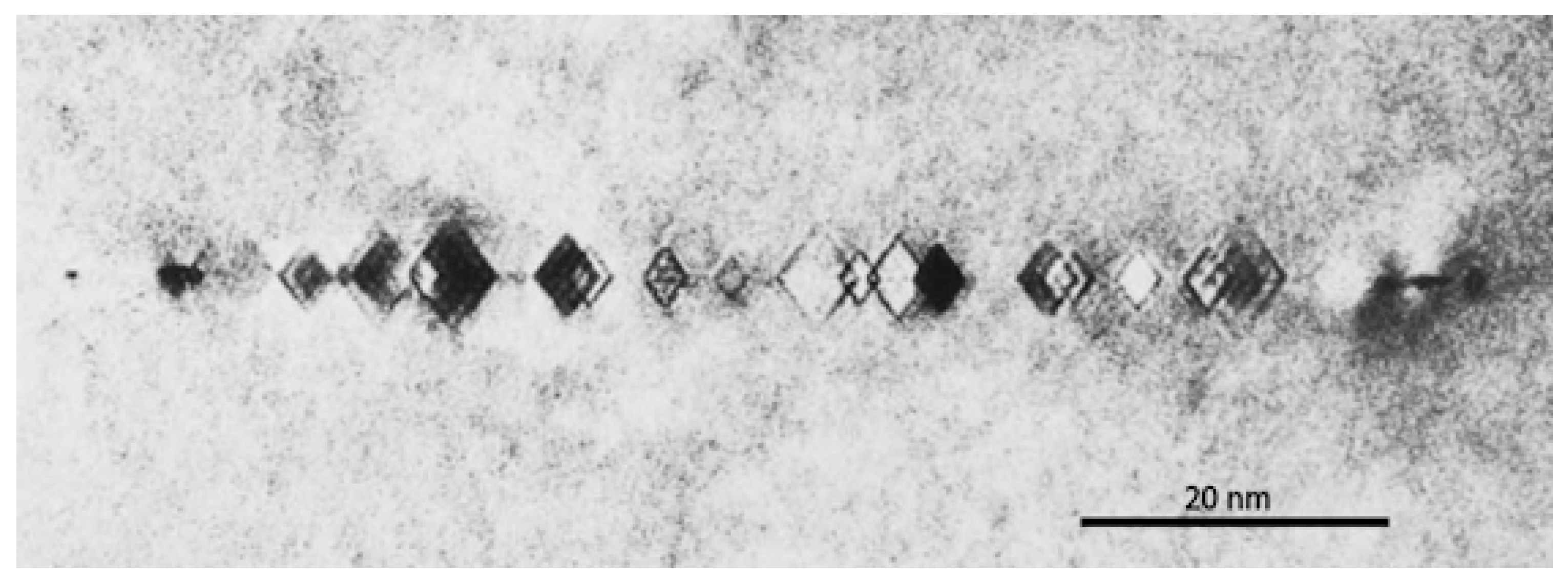
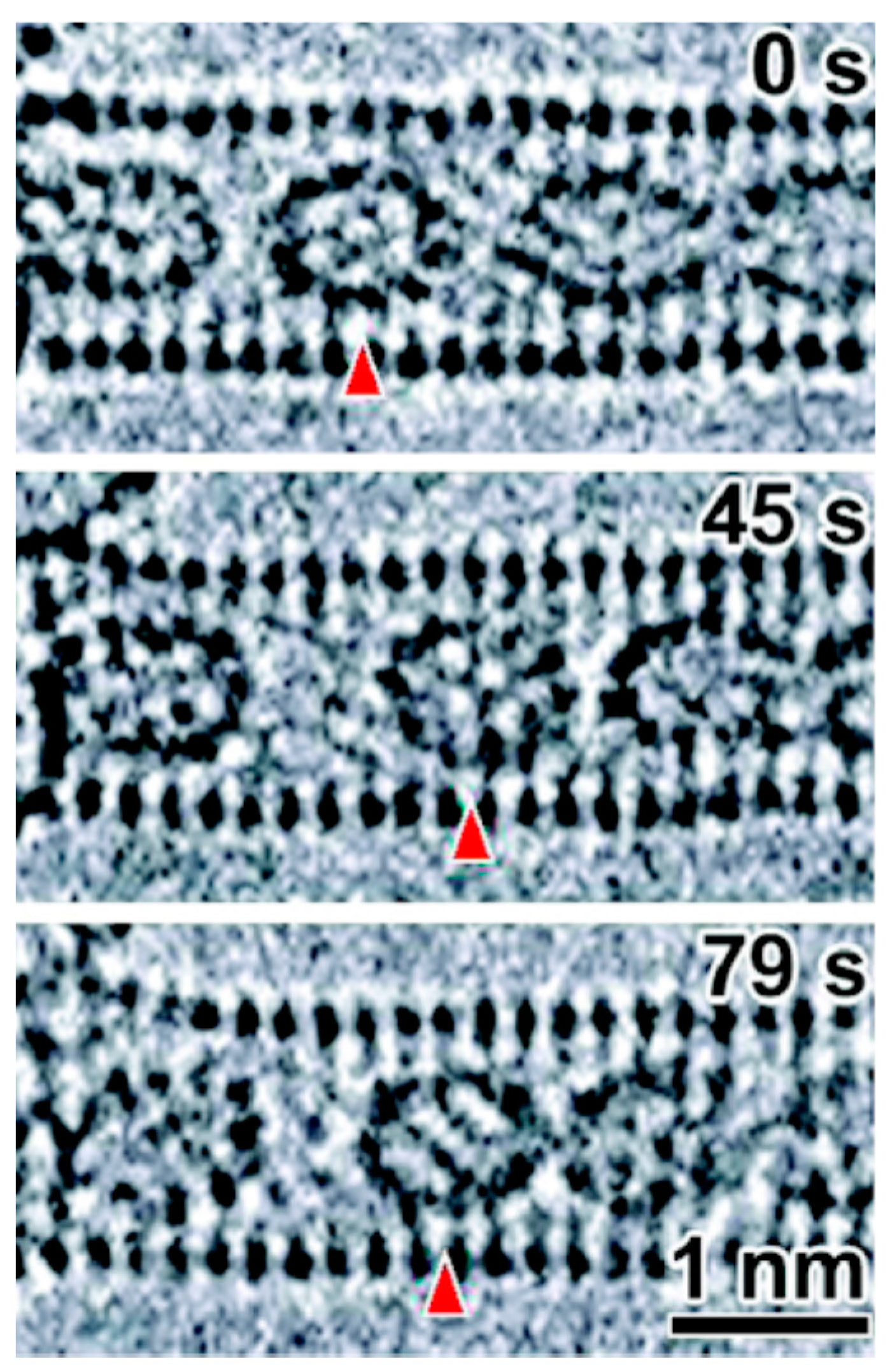
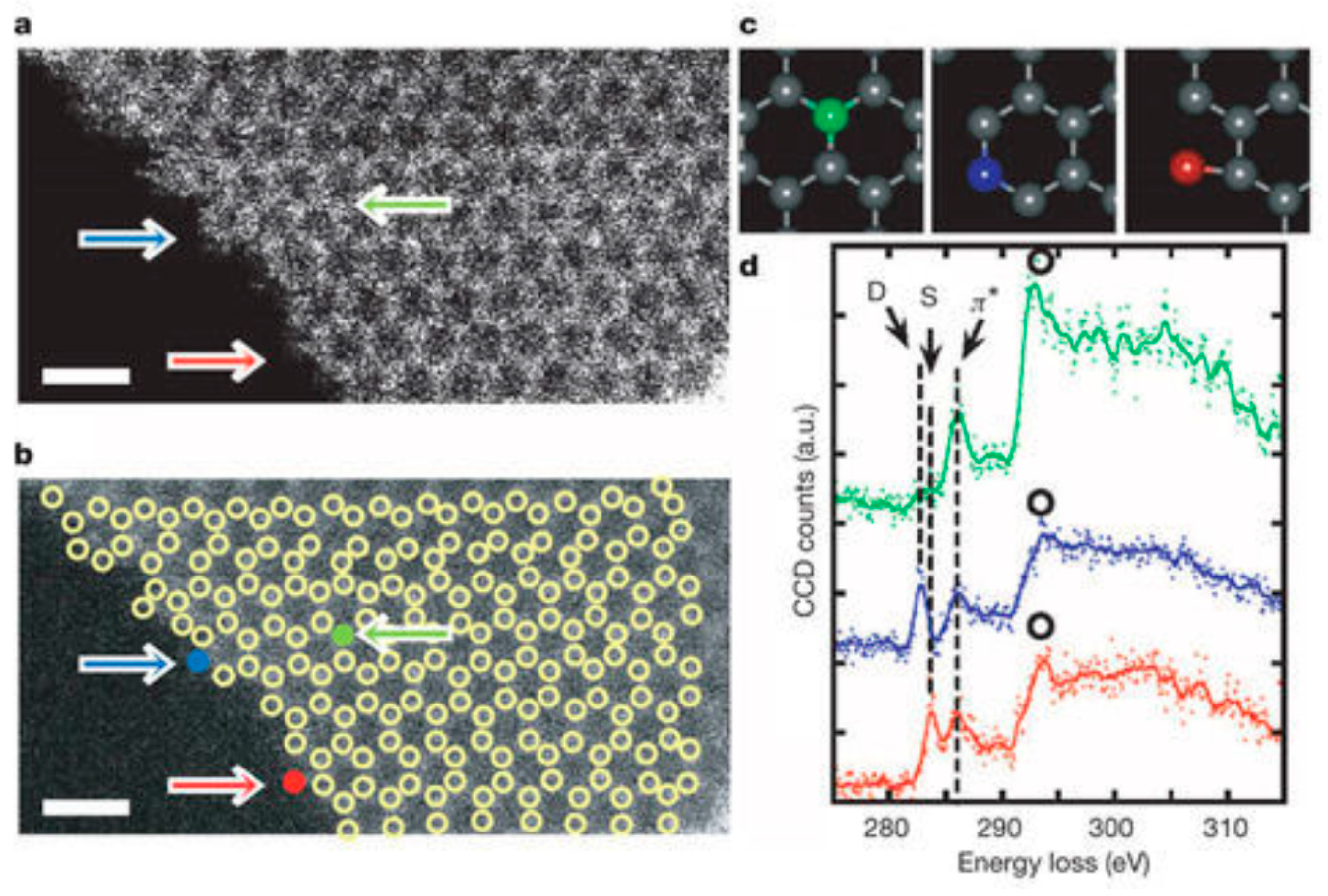
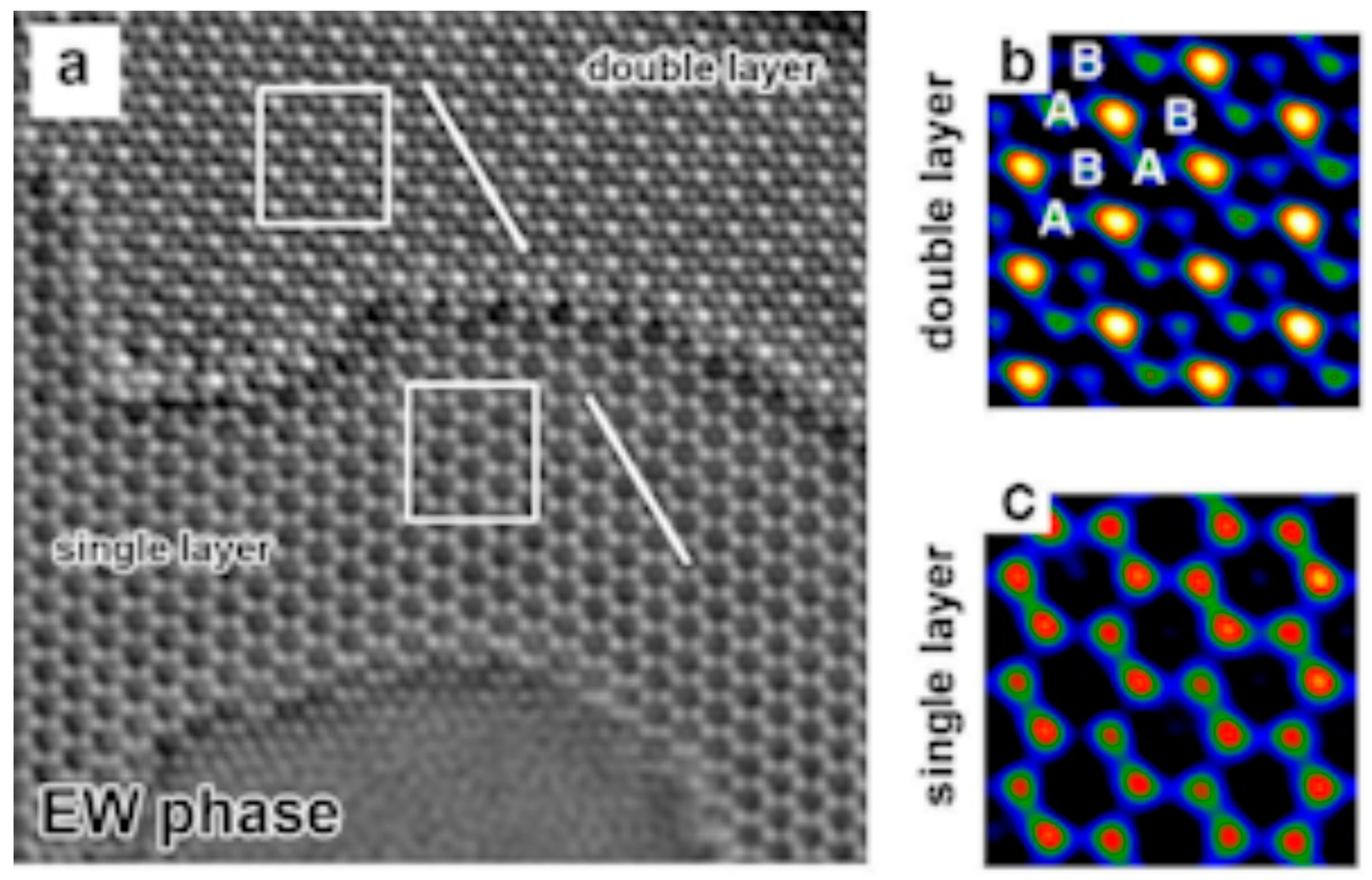
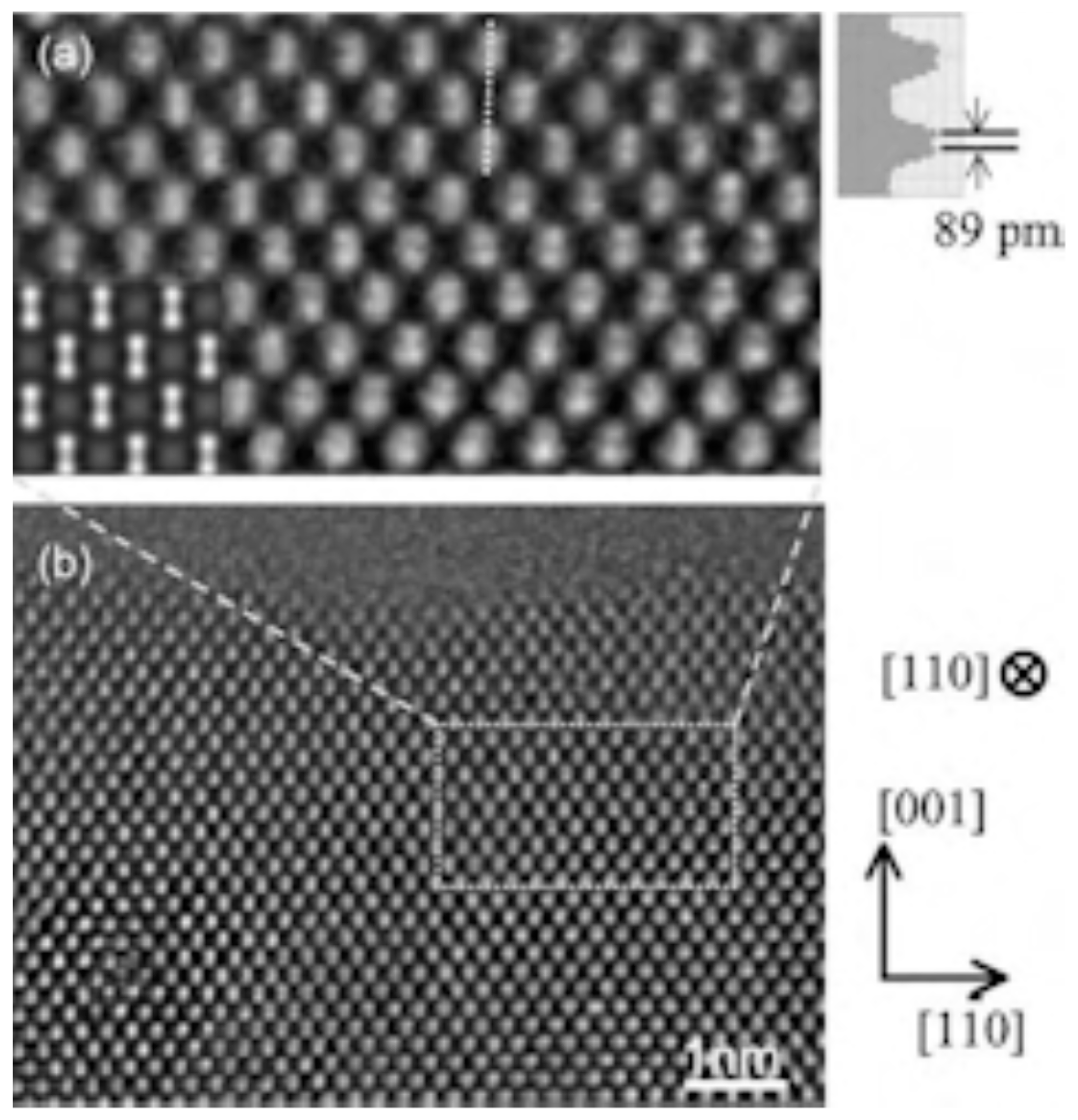
3.4. 2000 to Present
4. Discussion
Acknowledgments
Conflicts of Interest
References
- Ruska, E.; Knoll, M. Das Elektronenmikroskop. Z. Phys. 1932, 78, 318–339. [Google Scholar]
- Scherzer, O. Über einige Fehler von Elektronenlinsen. Z. Phys. 1936, 101, 593–603. [Google Scholar] [CrossRef]
- Heidenreich, R.D. Electron microscope and diffraction study of metal crystal textures by means of thin sections. J. Appl. Phys. 1949, 20, 993–1010. [Google Scholar] [CrossRef]
- Hirsch, P.B.; Horne, R.W.; Whelan, M.J. Direct observations of the arrangement and motion of dislocations in aluminium. Philos. Mag. 1956, 1, 677–684. [Google Scholar] [CrossRef]
- Bollmann, W. Interference effects in the electron microscopy of thin crystal foils. Phys. Rev. 1956, 103, 1588–1589. [Google Scholar] [CrossRef]
- Menter, J.W. The direct study by electron microscopy of crystal lattices and their imperfections. Proc. R. Soc. A 1956, 236, 119–135. [Google Scholar] [CrossRef]
- Suito, E.; Uyeda, N.; Watanabe, H.; Komoda, T. Lattice image of twin structure observed directly by electron microscope in a crystal of copper phthalocyanine. Nature 1958, 181, 332–333. [Google Scholar] [CrossRef]
- Komoda, T. Observation of lattice planes of 2.35 Å spacing with an electron microscope. Jpn. J. Appl. Phys. 1966, 3, 122–123. [Google Scholar] [CrossRef]
- Heidenreich, R.D.; Hess, W.M.; Ban, L.L. A test object and criteria for high resolution electron microscopy. J. Appl. Crystallogr. 1968, 1, 1–19. [Google Scholar] [CrossRef]
- Von Ardenne, M. Das elektronen-rastermikroskop. Theoretische grundlagen. Z. Phys. 1938, 109, 553–572. [Google Scholar] [CrossRef]
- Crewe, A.V.; Wall, J.; Langmore, J. Visibility of single atoms. Science 1970, 168, 1338–1340. [Google Scholar] [CrossRef] [PubMed]
- Brydson, R. Electron Energy Loss Spectroscopy; Taylor & Francis: London, UK, 2001. [Google Scholar]
- Haider, M.; Rose, H.; Uhlemann, S.; Schwan, E.; Kabius, B.; Urban, K. Towards 0.1 nm resolution with the first spherically corrected transmission electron microscope. J. Electron Microsc. 1998, 47, 395–405. [Google Scholar] [CrossRef]
- Hawkes, P.W. The Beginnings of Electron Microscopy; Academic Press: Orlando, FL, USA, 1985. [Google Scholar]
- Braun, E. Mechanical properties of solids. In Out of the Crystal Maze: Chapters from the History of Solid-State Physics; Hoddeson, L., Braun, E., Teichmann, J., Weart, S., Eds.; Oxford University Press: Oxford, UK, 1991; pp. 317–358. [Google Scholar]
- Hirsch, P.B. Electron microscopy in materials science a historical perspective. In The Electron: Proceedings of the International Centennial Symposium on the Electron; Kirkland, A.I., Brown, P.D., Eds.; IOM Communications: London, UK, 1998; pp. 64–81. [Google Scholar]
- Rose, H.H. Historical aspects of aberration correction. J. Electron Microsc. 2009, 58, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Su, D.S. Transmission electron microscopy and the science of carbon nanomaterials. Small 2014, 10, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, U.; Höper, W. On the “active sites” in catalysis (in German). Naturwissenschaften 1944, 32, 225–226. [Google Scholar] [CrossRef]
- Watson, J.H.L. Filmless sample mounting for the electron microscope. J. Appl. Phys. 1946, 17, 121–127. [Google Scholar] [CrossRef]
- Hall, C.E. Dark-field electron microscopy: II. Studies of colloidal carbon. J. Appl. Phys. 1948, 19, 271–277. [Google Scholar] [CrossRef]
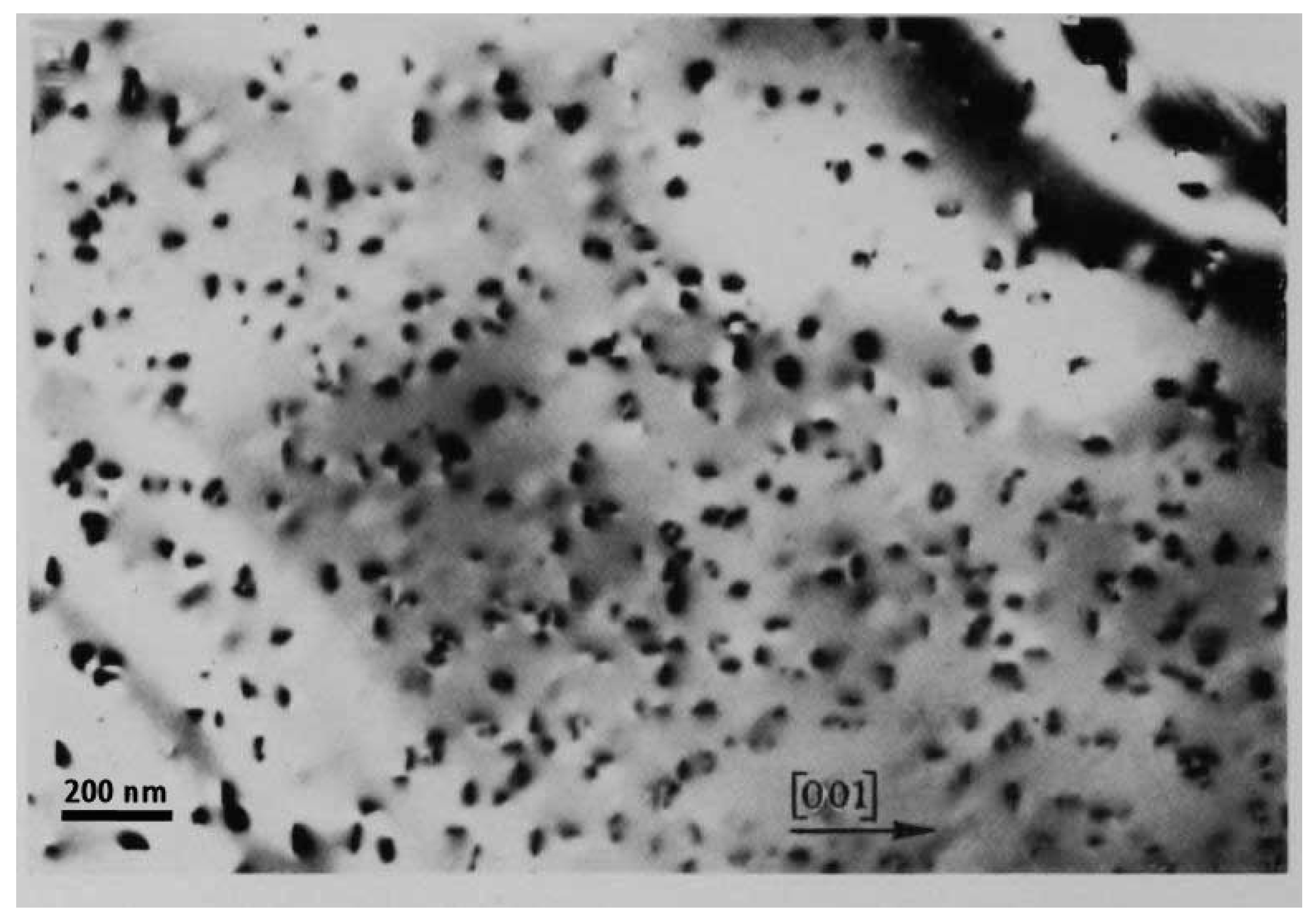
- Radushkevich, L.V.; Lukyanovich, V.M. On the carbon structure formed during thermal decomposition of carbon monoxide in the presence of iron (in Russian). Zhurnal Fizicheskoi Khimii 1952, 26, 88–95. [Google Scholar]
- Monthioux, M.; Kuznetsov, V.L. Who should be given the credit for the discovery of carbon nanotubes? Carbon 2006, 44, 1621–1623. [Google Scholar] [CrossRef]
- Davis, W.; Slawson, R.; Rigby, G.R. An unusual form of carbon. Nature 1953, 171, 756. [Google Scholar] [CrossRef]
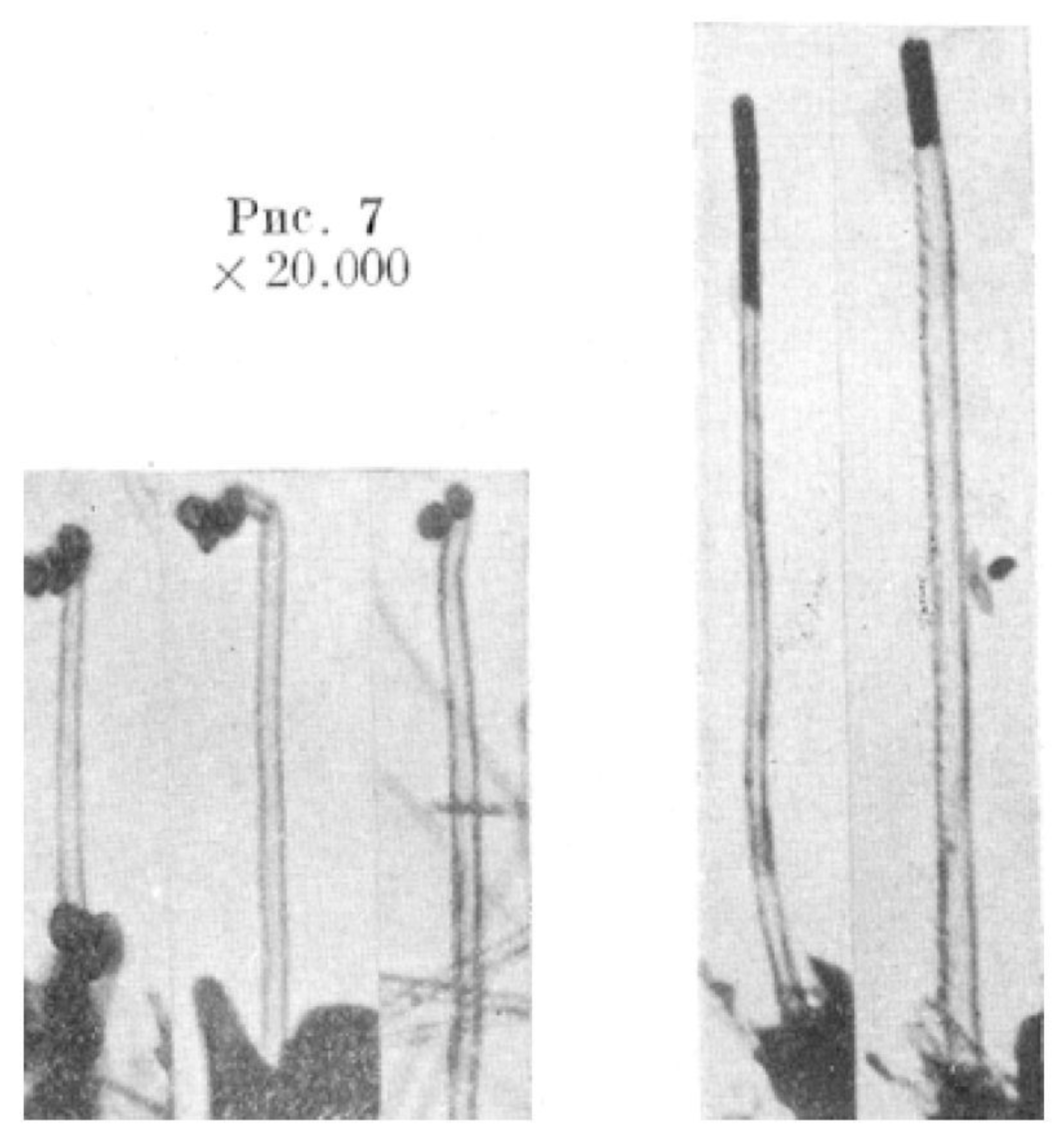
- Bacon, R. Growth, structure and properties of graphite whiskers. J. Appl. Phys. 1960, 31, 283–290. [Google Scholar] [CrossRef]
- Bollmann, W. Electron-microscopic observations on radiation damage in graphite. Philos. Mag. 1960, 5, 621–624. [Google Scholar] [CrossRef]
- Jenkins, G.M.; Turnbull, J.A.; Williamson, G.K. Electron microscope studies of graphitization and deformation in carbon film. J. Nucl. Mater. 1962, 7, 215–217. [Google Scholar] [CrossRef]
- Jenkins, G.M.; Williamson, G.K.; Barnett, J.T. The role of crystal structure in determining the mechanical properties of graphite. Carbon 1965, 3, 1–6. [Google Scholar] [CrossRef]
- Amelinckx, S.; Delavignette, P.; Heerschap, M. Dislocations and stacking faults in graphite. Chem. Phys. Carbon 1965, 1, 1–71. [Google Scholar]
- Thrower, P.A. The study of defects in graphite by transmission electron microscopy. Chem. Phys. Carbon 1969, 5, 217–319. [Google Scholar]
- Thomas, J.M. Microscopic studies of graphite oxidation. Chem. Phys. Carbon 1966, 1, 122–202. [Google Scholar]
- Evans, E.L.; Griffiths, R.J.M.; Thomas, J.M. Kinetics of single-layer graphite oxidation: Evaluation by electron microscopy. Science 1971, 171, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.T.K.; Harris, P.S. The formation of filamentous carbon. Chem. Phys. Carbon 1978, 14, 83–165. [Google Scholar]
- Marton, L.L. La microscopie électronique des objets biologiques. Bull. Acad. R. Med. Belg. 1935, 21, 553–564. [Google Scholar]
- Endo, M. Grow carbon-fibers in the vapor-phase. Chemtech 1988, 18, 568–576. [Google Scholar]
- Ban, L.L. Direct study of structural imperfections by high-resolution electron microscopy. In Surface and Defect Properties of Solids; Roberts, M.W., Thomas, J.M., Eds.; Chemical Society: London, UK, 1972; Volume 1, pp. 54–94. [Google Scholar]
- Crawford, D.; Johnson, D.J. High-resolution electron microscopy of high-modulus carbon fibres. J. Microsc. 1971, 94, 51–62. [Google Scholar] [CrossRef]
- Jenkins, G.M.; Kawamura, K. Structure of glassy carbon. Nature 1971, 231, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Ban, L.L.; Crawford, D.; Marsh, H. Lattice-resolution electron-microscopy in structural studies of non-graphitizing carbons from polyvinylidene chloride (PVDC). J. Appl. Crystallogr. 1975, 8, 415–420. [Google Scholar] [CrossRef]
- Oberlin, A.; Terriere, G. Graphitization studies of anthracites by high-resolution electron-microscopy. Carbon 1975, 13, 367–376. [Google Scholar] [CrossRef]
- Crawford, D.; Marsh, H. High resolution electron microscopy of carbon structure. J. Microsc. 1977, 109, 145–152. [Google Scholar] [CrossRef]
- Millward, G.R.; Jefferson, D.A. Lattice resolution of carbons by electron microscopy. Chem. Phys. Carbon 1978, 14, 1–82. [Google Scholar]
- Evans, T.; Phaal, C. Imperfections in type I and type II diamonds. Proc. R. Soc. A 1962, 270, 538–552. [Google Scholar] [CrossRef]
- Frank, F.C. On the X-ray diffraction spikes of diamond. Proc. R. Soc. A 1956, 237, 168–174. [Google Scholar] [CrossRef]
- Lang, A.R. A proposed structure for nitrogen impurity platelets in diamond. Proc. Phys. Soc. Lond. 1964, 84, 871–876. [Google Scholar] [CrossRef]
- Woods, G.S. Electron microscopy of ‘giant’ platelets on cube planes in diamond. Philos. Mag. A 1976, 34, 993–1012. [Google Scholar] [CrossRef]
- Stephenson, R.F. The Partial Dissociation of Nitrogen Aggregates in Diamond by High Temperature-High Pressure Treatments. Ph.D. Thesis, University of Reading, Reading, UK, 1978. [Google Scholar]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- lijima, S.; Ichihashi, T.; Ando, Y. Pentagons, heptagons and negative curvature in graphite microtubule growth. Nature 1992, 356, 776–778. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Bethune, D.S.; Kiang, C.H.; de Vries, M.S.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature 1993, 363, 605–607. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Iijima, S. Capillarity-induced filling of carbon nanotubes. Nature 1993, 361, 333–334. [Google Scholar] [CrossRef]
- Tsang, S.C.; Harris, P.J.F.; Green, M.L.H. Thinning and opening of carbon nanotubes by oxidation using carbon dioxide. Nature 1993, 362, 520–522. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Ebbesen, T.W.; Ichihashi, T.; Iijima, S.; Tanigaki, K.; Hiura, H. Opening carbon nanotubes with oxygen and implications for filling. Nature 1993, 362, 522–525. [Google Scholar] [CrossRef]
- Tsang, S.C.; Chen, Y.K.; Harris, P.J.F.; Green, M.L.H. A simple chemical method of opening and filling carbon nanotubes. Nature 1994, 372, 159–162. [Google Scholar] [CrossRef]
- Sloan, J.; Hammer, J.; Zwiefka-Sibley, M.; Green, M.L.H. The opening and filling of single walled carbon nanotubes (SWTs). Chem. Commun. 1998, 3, 347–348. [Google Scholar] [CrossRef]
- Smith, B.W.; Monthioux, M.; Luzzi, D.E. Encapsulated C60 in carbon nanotubes. Nature 1998, 396, 323–324. [Google Scholar] [CrossRef]
- Ugarte, D. Curling and closure of graphitic networks under electron-beam irradiation. Nature 1992, 359, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.J.F.; Tsang, S.C. High-resolution electron microscopy studies of non-graphitizing carbons. Philos. Mag. A 1997, 76, 667–677. [Google Scholar] [CrossRef]
- Franklin, R.E. Crystallite growth in graphitizing and non-graphitizing carbons. Proc. R. Soc. A 1951, 209, 196–218. [Google Scholar] [CrossRef]
- Harris, P.J.F. Fullerene-like models for microporous carbon. J. Mater. Sci. 2013, 48, 565–577. [Google Scholar] [CrossRef]
- Barry, J.C.; Bursill, L.A.; Hutchison, J.L.; Lang, A.R.; Rackham, G.M.; Sumida, N. On voidites: A high-resolution transmission electron microscopic study of faceted void-like defects in natural diamonds. Philos. Trans. R. Soc. Lond. Ser. A 1987, 321, 361–401. [Google Scholar] [CrossRef]
- Berger, S.D.; Pennycook, S.J. Detection of nitrogen at {100} platelets in diamond. Nature 1982, 298, 635–637. [Google Scholar] [CrossRef]
- Bruley, J.; Brown, L.M. Quantitative electron energy-loss spectroscopy microanalysis of platelet and voidite defects in natural diamond. Philos. Mag. A 1989, 59, 247–261. [Google Scholar] [CrossRef]
- Fallon, P.J.; Brown, L.M.; Barry, J.C.; Bruley, J. Nitrogen determination and characterization in natural diamond platelets. Philos. Mag. A 1995, 72, 21–37. [Google Scholar] [CrossRef]
- Humble, P. The structure and mechanism of formation of platelets in natural type Ia diamond. Proc. R. Soc. A 1982, 381, 65–81. [Google Scholar] [CrossRef]
- Hutchison, J.L.; Bursill, L.A. Fresnel fringe contrast of faceted voids within gem-quality diamond. J. Microsc. 1983, 131, 63–66. [Google Scholar] [CrossRef]
- Barry, J.C. Voidites in diamond—Do they contain nitrogen? Ultramicroscopy 1986, 20, 169–176. [Google Scholar] [CrossRef]
- Hirsch, P.B.; Hutchison, J.L.; Titchmarsh, J. Voidites in diamond: Evidence for a crystalline phase containing nitrogen. Philos. Mag. A 1986, 54, L49–L54. [Google Scholar] [CrossRef]
- Barry, J.C.; Queensland University of Technology, Brisbane, Australia. Personal communication, 2017.
- Evans, T.; Qi, Z. The kinetics of the aggregation of nitrogen atoms in diamond. Proc. R. Soc. A 1982, 381, 159–178. [Google Scholar] [CrossRef]
- Hirahara, K.; Saitoh, K.; Yamasaki, J.; Tanaka, N. Direct observation of six-membered rings in the upper and lower walls of a single-wall carbon nanotube by spherical aberration-corrected HRTEM. Nano Lett. 2006, 6, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, K.; Wakabayashi, H.; Koshino, M.; Sato, Y.; Urita, K.; Iijima, S. Imaging active topological defects in carbon nanotubes. Nat. Nanotechnol. 2007, 2, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Suenaga, K.; Iijima, S. Smallest carbon nanotube assigned with atomic resolution accuracy. Nano Lett. 2008, 8, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yanagi, K.; Miyata, Y.; Suenaga, K.; Kataura, H.; Iijima, S. Chiral-angle distribution for separated single-walled carbon nanotubes. Nano Lett. 2008, 8, 3151–3154. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Suenaga, K.; Okubo, S.; Okazaki, T.; Iijima, S. Structures of D5d-C80 and Ih-Er3N@C80 Fullerenes and Their Rotation Inside Carbon Nanotubes Demonstrated by Aberration-Corrected Electron Microscopy. Nano Lett. 2007, 7, 3704–3708. [Google Scholar] [CrossRef]
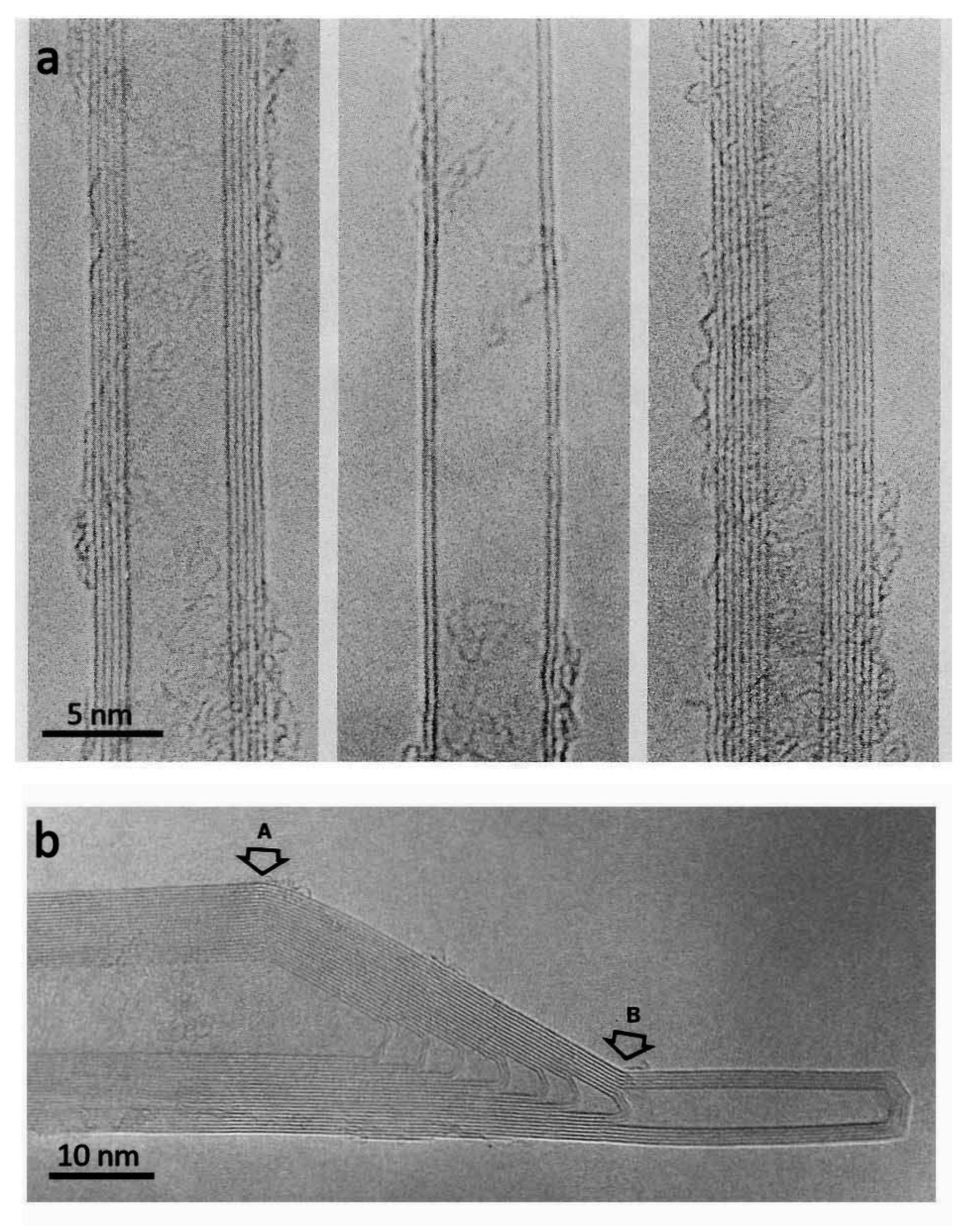
- Liu, Z.; Suenaga, K.; Harris, P.J.F.; Iijima, S. Open and closed edges of graphene layers. Phys. Rev. Lett. 2009, 102, 015501. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, K.; Koshino, M. Atom-by-atom spectroscopy at graphene edge. Nature 2010, 468, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.J.F.; Liu, Z.; Suenaga, K. Imaging the atomic structure of activated carbon. J. Phys. Condens. Matter 2008, 20, 362201. [Google Scholar] [CrossRef]
- Guo, J.; Morris, J.R.; Ihm, Y.; Contescu, C.I.; Gallego, N.C.; Duscher, G.; Pennycook, S.J.; Chisholm, M.F. Topological defects: Origin of nanopores and enhanced adsorption performance in nanoporous carbon. Small 2012, 8, 3283–3288. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.C.; Kisielowski, C.; Erni, R.; Rossell, M.D.; Crommie, M.F.; Zettl, A. Direct imaging of lattice atoms and topological defects in graphene membranes. Nano Lett. 2008, 8, 3582–3586. [Google Scholar] [CrossRef] [PubMed]
- Girit, Ç.Ö.; Meyer, J.C.; Erni, R.; Rossell, M.D.; Kisielowski, C.; Yang, L.; Park, C.-H.; Crommie, M.F.; Cohen, M.L.; Louie, S.G.; et al. Graphene at the edge: Stability and dynamics. Science 2009, 323, 1705–1708. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.C.; Kotakoski, J.; Mangler, C. Atomic structure from large-area, low-dose exposures of materials: A new route to circumvent radiation damage. Ultramicroscopy 2014, 145, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Kotakoski, J.; Mangler, C.; Meyer, J.C. Imaging atomic-level random walk of a point defect in graphene. Nat. Commun. 2014, 5, 3991. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.H.; Ruemmeli, M.H.; Gemming, T.; Buechner, B.; Briggs, G.A.D. Direct imaging of rotational stacking faults in few layer graphene. Nano Lett. 2009, 9, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.H.; Margine, E.R.; Mukai, M.; Robertson, A.W.; Giustino, F.; Kirkland, A.I. Dislocation-driven deformations in graphene. Science 2012, 337, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.W.; Allen, C.S.; Wu, Y.A.; He, K.; Olivier, J.; Neethling, J.; Kirkland, A.I.; Warner, J.H. Spatial control of defect creation in graphene at the nanoscale. Nat. Commun. 2012, 3, 1144. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Lee, S.; Hong, S.; Yoon, E.; Lee, G.D.; Warner, J.H. Point defects in turbostratic stacked bilayer grapheme. Nanoscale 2017, 9, 13725–13730. [Google Scholar] [CrossRef] [PubMed]
- Haigh, S.J.; Gholinia, A.; Jalil, R.; Romani, S.; Britnell, L.; Elias, D.C.; Novoselov, K.S.; Ponomarenko, L.A.; Geim, A.K.; Gorbachev, R. Cross-sectional imaging of individual layers and buried interfaces of graphene-based heterostructures and superlattices. Nat. Mater. 2012, 11, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Zan, R.; Ramasse, Q.M.; Jalil, R.; Tu, J.-S.; Bangert, U.; Novoselov, K.S. Imaging two dimensional materials and their heterostructures. J. Phys. Conf. Ser. 2017, 902, 012028. [Google Scholar] [CrossRef]
- Harris, P.J.F.; Slater, T.J.A.; Haigh, S.J.; Hage, F.S.; Kepaptsoglou, D.M.; Ramasse, Q.M.; Brydson, R. Bilayer graphene formed by passage of current through graphite: Evidence for a three-dimensional structure. Nanotechnology 2014, 25, 465601. [Google Scholar] [CrossRef] [PubMed]
- Jinschek, J.R.; Yucelen, E.; Calderon, H.A.; Freitag, B. Quantitative atomic 3-D imaging of single/double sheet graphene structure. Carbon 2011, 49, 556–562. [Google Scholar] [CrossRef]
- Urban, K.W. Electron microscopy: The challenges of graphene. Nat. Mater. 2011, 10, 165–166. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, I.S.; Bangert, U. An analysis of vacancy clusters and sp2 bonding in natural type IIa diamond using aberration corrected STEM and EELS. J. Phys. Conf. Ser. 2011, 281, 012024. [Google Scholar] [CrossRef]
- Erni, R.; Freitag, B.; Hartel, P.; Müller, H.; Tiemeijer, P.; van der Stam, M.; Stekelenburg, M.; Hubert, D.; Specht, P.; Garibay-Febles, V. Atomic scale analysis of planar defects in polycrystalline diamond. Microsc. Microanal. 2006, 12, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, F.; Sawada, H.; Kondo, Y.; Takayanagi, K.; Suenaga, K. Development of Cs and Cc correctors for transmission electron microscopy. Microscopy 2013, 62, 23–41. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, P.J.F. Transmission Electron Microscopy of Carbon: A Brief History. C 2018, 4, 4. https://doi.org/10.3390/c4010004
Harris PJF. Transmission Electron Microscopy of Carbon: A Brief History. C. 2018; 4(1):4. https://doi.org/10.3390/c4010004
Chicago/Turabian StyleHarris, Peter J. F. 2018. "Transmission Electron Microscopy of Carbon: A Brief History" C 4, no. 1: 4. https://doi.org/10.3390/c4010004