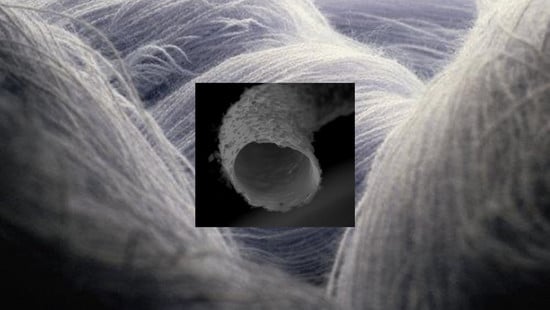
Supercapacitor Electrode Based on Activated Carbon Wool Felt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Activated Carbon Felt Preparation
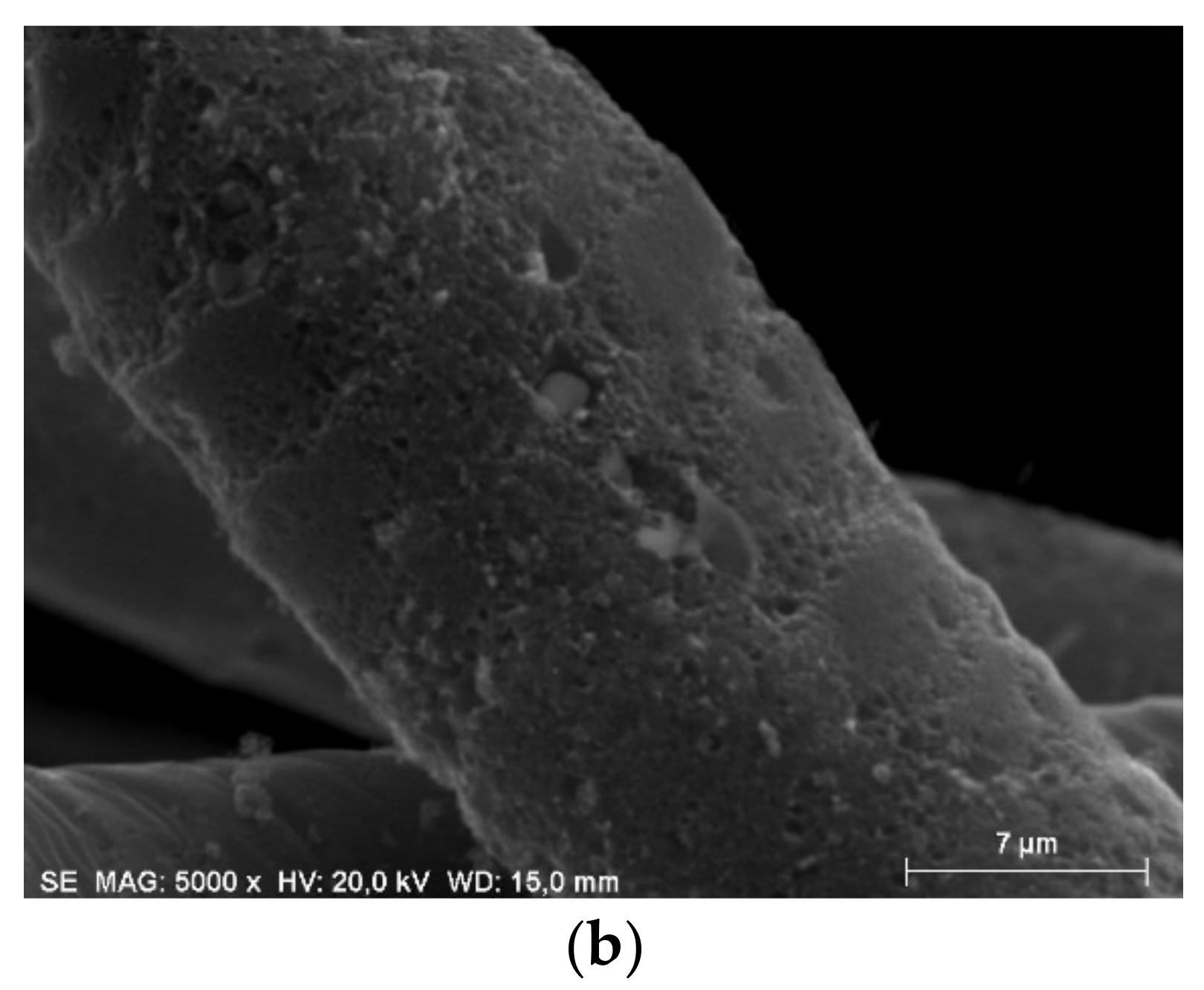
2.2. Scanning Electron Microscopy (SEM)
2.3. Chemical and Electrochemical Characterizations
3. Results and Discussion
3.1. Sample Obtention
3.2. SEM Microscopy
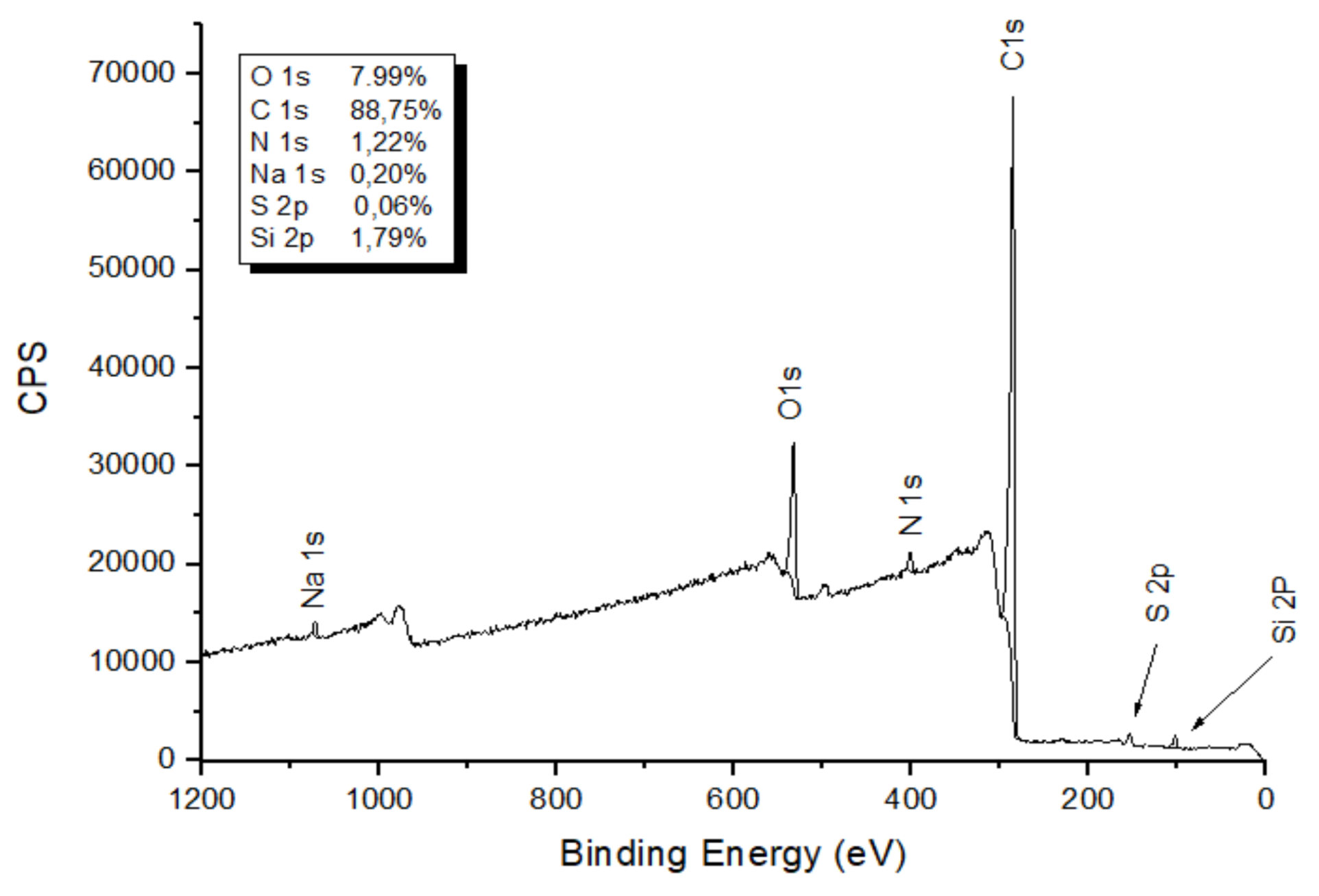
3.3. Elemental Analysis
3.4. Textural Analysis
3.5. X-ray Photoelectron Spectroscopy
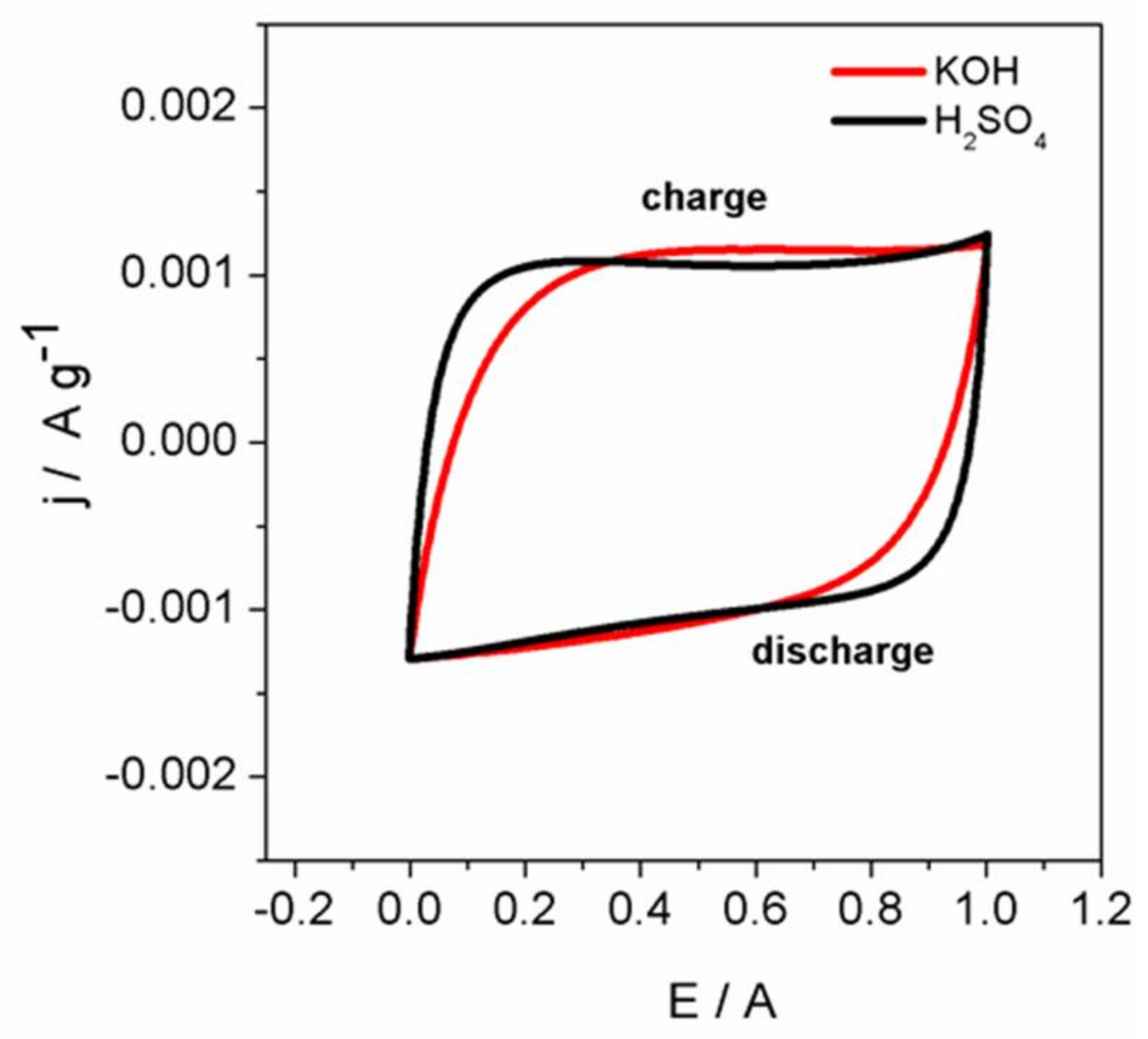
3.6. Electrochemical Analysis
3.6.1. Charge-Discharge Curves
3.6.2. Voltammograms
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Béguin, F.; Frąckowiak, E. Supercapacitors: Materials, Systems, and Applications; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2013. [Google Scholar]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Centeno, T.A.; Hahn, M.; Fernández, J.; Kotz, R.; Stoeckli, F. Correlation between capacitances of porous carbons in acidic and aprotic EDLC electrolytes. Electrochem. Commun. 2007, 9, 1242–1246. [Google Scholar] [CrossRef] [Green Version]
- Ujjain, S.K.; Ahuja, P.; Bhatia, R.; Attri, P. Printable multi-walled carbon nanotubes thin film for high performance all solid state flexible supercapacitors. Mater. Res. Bull. 2016, 83, 167–171. [Google Scholar] [CrossRef]
- Savilov, S.V.; Strokova, N.E.; Ivanov, A.S.; Arkhipova, E.A.; Desyatov, A.V.; Hui, X.; Aldoshin, S.M.; Lunin, V.V. Nanoscale carbon materials from hydrocarbons pyrolysis: Structure, chemical behavior, utilization for non-aqueous supercapacitors. Mater. Res. Bull. 2015, 69, 13–19. [Google Scholar] [CrossRef]
- Dolah, B.N.M.; Deraman, M.; Othman, M.A.R.; Farma, R.; Taer, E.M.; Awitdrus, A.; Basri, N.H.; Talib, I.A.; Omar, R.; Nor, N.S.M. A method to produce binder less supercapacitor electrode monoliths from biomass carbon and carbon nanotubes. Mater. Res. Bull. 2014, 60, 10–19. [Google Scholar] [CrossRef]
- Xi, S.; Kang, Y.; Qu, S.; Han, S. Flexible supercapacitors on chips with interdigital carbon nanotube fiber electrodes. Mater. Lett. 2016, 175, 126–130. [Google Scholar] [CrossRef]
- Shen, H.; Liu, E.; Xiang, X.; Huang, Z.; Tian, Y.; Wu, Y.; Wu, Z.; Xie, H. A novel activated carbon for supercapacitors. Mater. Res. Bull. 2012, 47, 662–666. [Google Scholar] [CrossRef]
- Cuña, A.; Tancredi, N.A.; Bussi, J.; Deiana, A.C.; Sardella, M.-F.; Barranco, V.; Rojo, J.M. Eucaliptus grandis as a biocarbon precursor for supercapacitor electrode application. Waste Biomass Valoriz. 2014, 5, 305–313. [Google Scholar] [CrossRef]
- Cuña, A.; Ortega, M.R.; da Silva, E.L.; Tancredi, N.; Radtke, C.; Malfatti, C.F. Nitric acid functionalization of carbon monoliths for supercapacitors: Effect on the electrochemical properties. Int. J. Hydrogen Energy 2016, 41, 12127–12135. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Solano, A.L.; Carzola-Amorós, D. Adsorption on Activated Carbon Fiber: Adsorption by Carbons; Elsevier: New York, NY, USA, 2008; pp. 431–454. [Google Scholar]
- Newcomb, B.A. Processing, structure, and properties of carbon fibers. Compos. Part A Appl. Sci. Manuf. 2016, 91, 262–282. [Google Scholar] [CrossRef]
- Edie, D.D. The effect of processing on the structure and properties of carbon fibers. Carbon 1998, 4, 345–362. [Google Scholar] [CrossRef]
- Pickering, A.K.; Efendi, M.; Le, T. A review of recent developments in natural fiber composites and their mechanical performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Lang, J.; Xu, S.; Wang, X. Nitrogen-containing activated carbon fibers derived from silk fibers for CO2 capture. Mater. Lett. 2015, 152, 145–147. [Google Scholar] [CrossRef]
- Lee, H.-M.; Kwac, L.-K.; An, K.-H.; Park, S.-J.; Kim, B.-J. Electrochemical behavior of pitch-based activated carbon fibers for electrochemical capacitors. Energy Conver. Manag. 2016, 125, 347–352. [Google Scholar] [CrossRef]
- Li, T.; Zhang, W.; Zhi, L.; Yu, H.; Dang, L.; Shi, F.; Xu, H.; Hu, F.; Liu, Z.; Lei, Z.; et al. High energy asymmetric electrochemical capacitors based on oxides functionalized hollow carbon fibers electrodes. Nano Energy 2016, 30, 9–17. [Google Scholar] [CrossRef]
- Marcuzzo, J.S.; Cuña, A.; Tancredi, N.; Mendez, E.; Bernardi, H.H.; Baldan, M. Microporous activated carbon felt from Brazilian textile PAN fiber: Preparation, characterization and application as super capacitor electrode. Rev. Bras. Apliçacões Vácuo 2016, 35, 58–63. [Google Scholar] [CrossRef]
- Hassan, M.M.; Schiermeister, L.; Staiger, M.P. Sustainable Production of Carbon Fiber: Effect of Crosslinking in Wool Fiber on Carbon Yields and Morphologies of Derived Carbon Fiber. ACS Sustain. Chem. Eng. 2015, 311, 2660–2668. [Google Scholar] [CrossRef]
- Hassan, M.M.; Schiermeister, L.; Staiger, M.P. Thermal, chemical and morphological properties of carbon fibers derived from chemically pre-treated wool fibers. RSC Adv. 2015, 5, 55353–55362. [Google Scholar] [CrossRef]
- Macias-Garcia, A.; Cuerda-Correa, E.; Olivares-Marinb, M.; Diaz-Paralejo, A.Y.; Diaz-Dieza, M.A. Development and characterization of carbon-honeycomb monoliths from kenaf natural fibers: A preliminary study. Ind. Crop. Prod. 2012, 35, 105–110. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W. Preparation and characterization of activated carbon fibers from liquefied poplar bark. Mater. Lett. 2013, 112, 26–28. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, E.; Zhao, G. Thermal and structure analysis on reaction mechanisms during the preparation of activated carbon fibers by KOH activation from liquefied wood-base fibers. Ind. Crop. Prod. 2015, 69, 447–455. [Google Scholar] [CrossRef]
- Chai, X.; Mi, H.; Zhu, C.; He, C.; Xu, J.; Zhou, X.; Liu, J. Low-temperature thermal stabilization of polyacrylonitrile-based precursor fibers towards efficient preparation of carbon fibers with improved mechanical properties. Polymer 2015, 76, 131–139. [Google Scholar] [CrossRef]
- Chen, W.; Liu, X.; He, R.L.; Lin, T.; Zeng, Q.F.; Wang, X.G. Activated carbon powders from wool fibers. Powder Technol. 2013, 234, 76–83. [Google Scholar] [CrossRef]
- Marcuzzo, J.; Pina, A.C.; García, L.; Tancredi, N.; Amaya, A. Production and characterization of carbon felt from wool. In Proceedings of the Carbon 2015—Innovation with Carbon Materials, Dresden, Alemania, 12–17 July 2015. [Google Scholar]
- Brunauer, S.; Emmett, P.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K.S.W. Adsorption by Powders and Porous Solids: Principle, Methodology and Applications, 2nd ed.; Academic Press: New York, NY, USA, 2013. [Google Scholar]
- Moreno, C.; López, M.V.; Carrasco, F. Changes in Surface chemistry of activated carbons by wet oxidation. Carbon 2000, 38, 1995–2001. [Google Scholar] [CrossRef]
- Biniak, S.; Szymánski, G.; Siedlewski, J.; Swia, A. The characterization of activated carbons with oxygen and nitrogen surface groups. Carbon 1997, 35, 1799–1810. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Ania, C.O. Chapter 4 Surface chemistry of activated carbons and its characterization. Int. Sci. Technol. 2006, 7, 159–229. [Google Scholar]
- Breitkopf, C.; Swider-Lyons, K. Handbook of Electrochemical Energy; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Conway, B.E. Electrochemical supercapacitors. In Scientific Fundamentals and Technological Applications; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999. [Google Scholar]
- Diez, N.; Díaz, P.; Álvarez, P.; González, Z.; Granda, M.; Blanco, C.; Santamaría, R.; Menéndez, R. Activated carbon fibers prepared directly from stabilized fibers for use as electrodes in supercapacitors. Mater. Lett. 2014, 136, 214–217. [Google Scholar] [CrossRef]
- Lei, D.; Devarayan, K.; Seo, M.K.; Kim, Y.G.; Kim, B.-S. Flexible polyaniline-decorated carbon fiber nanocomposite mats as supercapacitors. Mater. Lett. 2015, 154, 173–176. [Google Scholar] [CrossRef]
- Pico, F.; Morales, E.; Fernandez, J.A.; Centeno, T.A.; Ibañez, J.; Rojas, R.M.; Amarilla, J.M.; Rojo, J.M. Ruthenium oxide/carbon composites with microporous or mesoporous carbon as support and prepared by two procedures. A comparative study as supercapacitor electrodes. Electrochim. Acta 2009, 54, 2239–2245. [Google Scholar] [CrossRef]
- Zhang, G.; Xiao, X.; Li, B.; Gu, P.; Xue, H.; Pang, H. Transition metal oxides with one-dimensional/one-dimensional-analogue nanostructures for advanced supercapacitors. J. Mater. Chem. A 2017, 5, 8155–8186. [Google Scholar] [CrossRef]
- Frackowiak, E.; Khomenko, V.; Jurewicz, K.; Lota, K.; Beguin, F. Supercapacitors based on conducting polymers/nanotubes composites. J. Power Sources 2006, 153, 413–418. [Google Scholar] [CrossRef]
- Bavio, M.A.; Acosta, G.G.; Kessler, T.; Visintin, A. Flexible symmetric and asymmetric supercapacitors based in nanocomposites of carbon cloth/polyaniline-carbon nanotubes. Energy 2017, 130, 22–28. [Google Scholar] [CrossRef]
- Kang, J.; Wen, J.; Jayaram, S.H.; Yu, A.; Wang, X. Development of an equivalent circuit model for electrochemical double layer capacitors (EDLCs) with distinct electrolytes. Electrochim. Acta 2014, 115, 587–598. [Google Scholar] [CrossRef]









| Sample | T (°C) | N (%) | C (%) | H (%) | S (%) | O (%) |
|---|---|---|---|---|---|---|
| Felt | - | 15.2 | 47.0 | 6.5 | 3.4 | 27.9 |
| Oxidized | 300 | 16.1 | 59.3 | 3.4 | 0.8 | 20.4 |
| Ox/Carbonized | 800 | 17.6 | 62.4 | 1.9 | 0.8 | 17.3 |
| ACF’f | 1000 | 17.1 | 61.4 | 1.9 | 0.8 | 18.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pina, A.C.; Amaya, A.; Marcuzzo, J.S.; Rodrigues, A.C.; Baldan, M.R.; Tancredi, N.; Cuña, A. Supercapacitor Electrode Based on Activated Carbon Wool Felt. C 2018, 4, 24. https://doi.org/10.3390/c4020024
Pina AC, Amaya A, Marcuzzo JS, Rodrigues AC, Baldan MR, Tancredi N, Cuña A. Supercapacitor Electrode Based on Activated Carbon Wool Felt. C. 2018; 4(2):24. https://doi.org/10.3390/c4020024
Chicago/Turabian StylePina, Ana Claudia, Alejandro Amaya, Jossano Saldanha Marcuzzo, Aline C. Rodrigues, Mauricio R. Baldan, Nestor Tancredi, and Andrés Cuña. 2018. "Supercapacitor Electrode Based on Activated Carbon Wool Felt" C 4, no. 2: 24. https://doi.org/10.3390/c4020024