Assessment of Acidified Fibrous Immobilization Materials for Improving Acetone-Butanol-Ethanol (ABE) Fermentation
Abstract
:1. Introduction
2. Experimental Section
2.1. Microorganism and Culture Environment
2.2. Immobilization Materials and Pretreating Process
2.3. Analytical Methods
2.4. In Vitro Performance of the Materials
3. Results
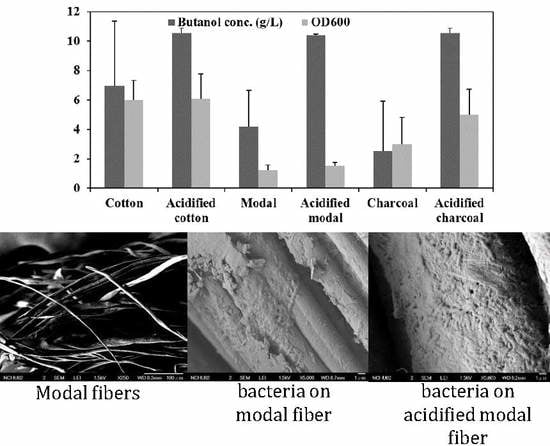
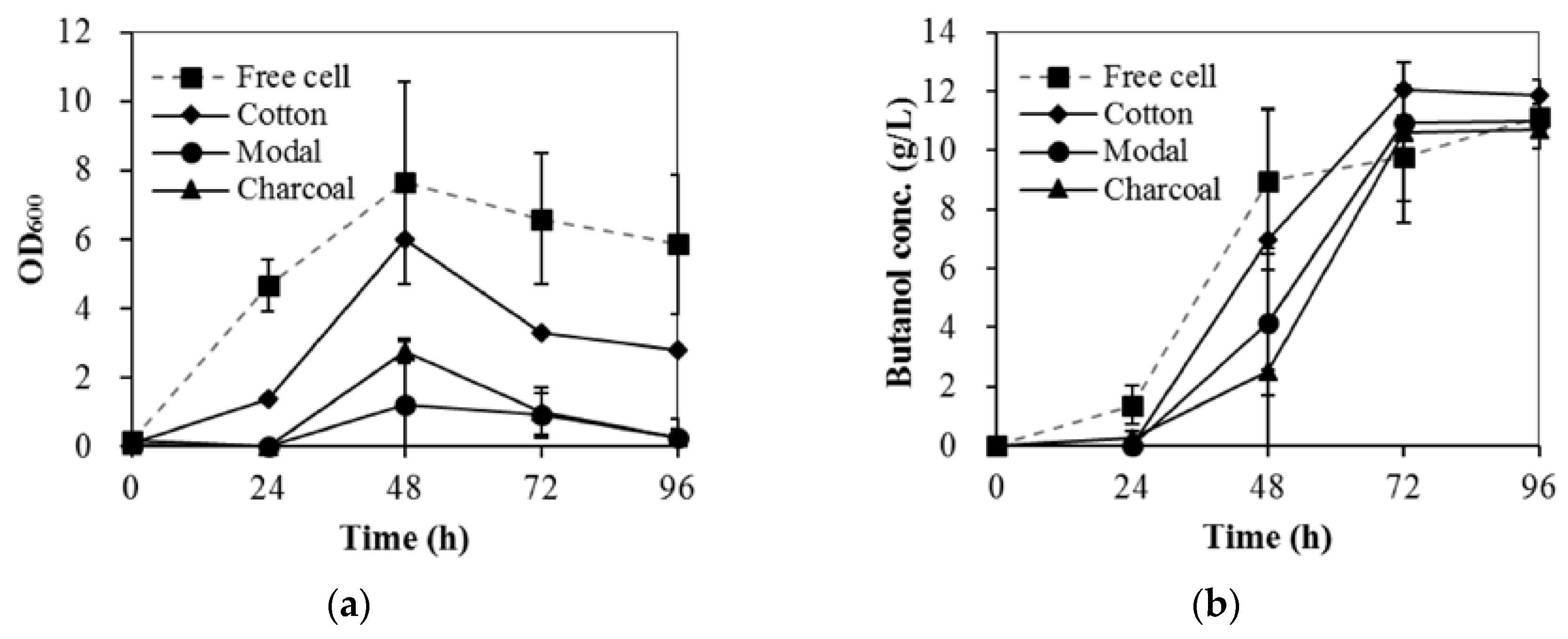
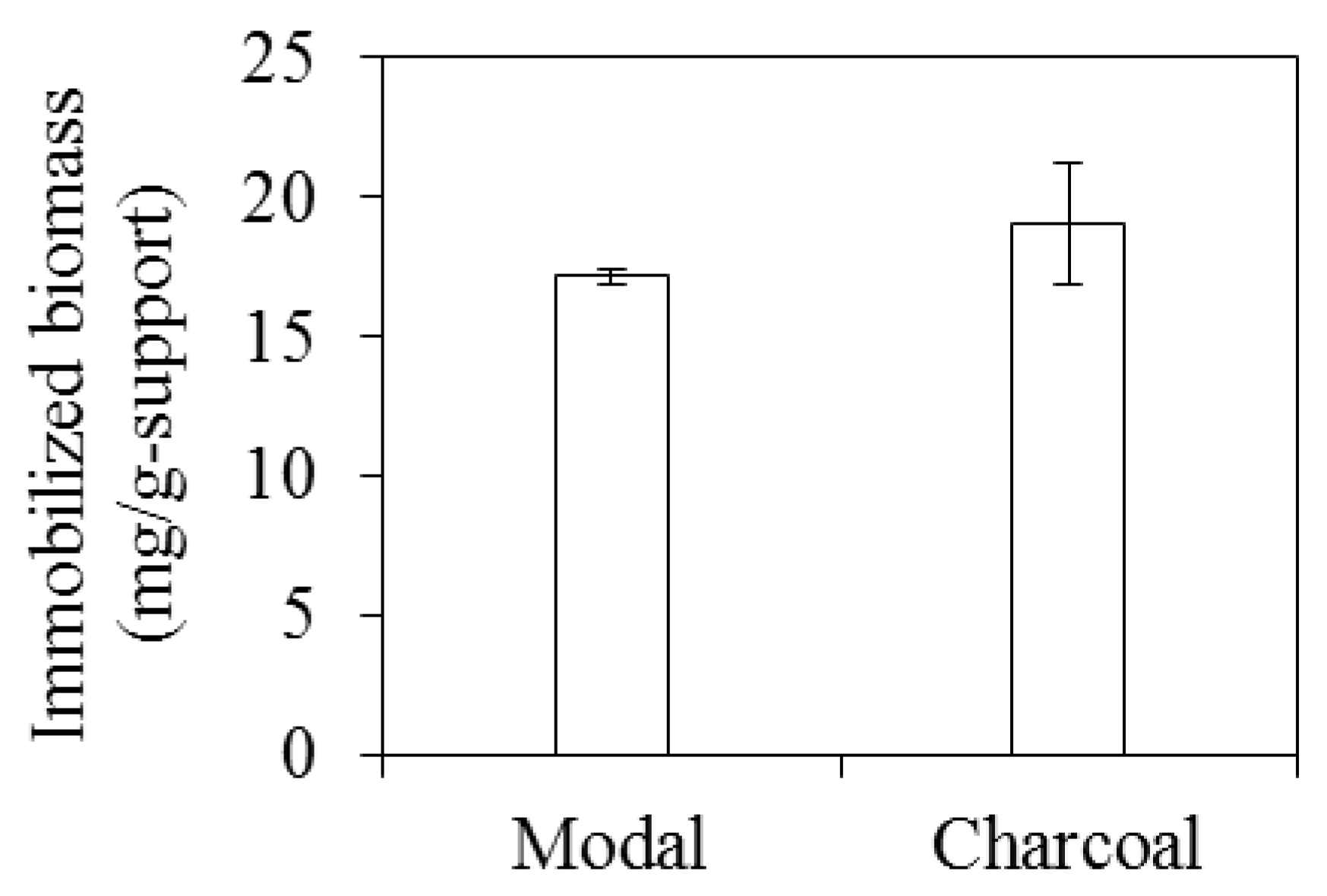
3.1. The Performance of Immobilized Materials for ABE Fermentation
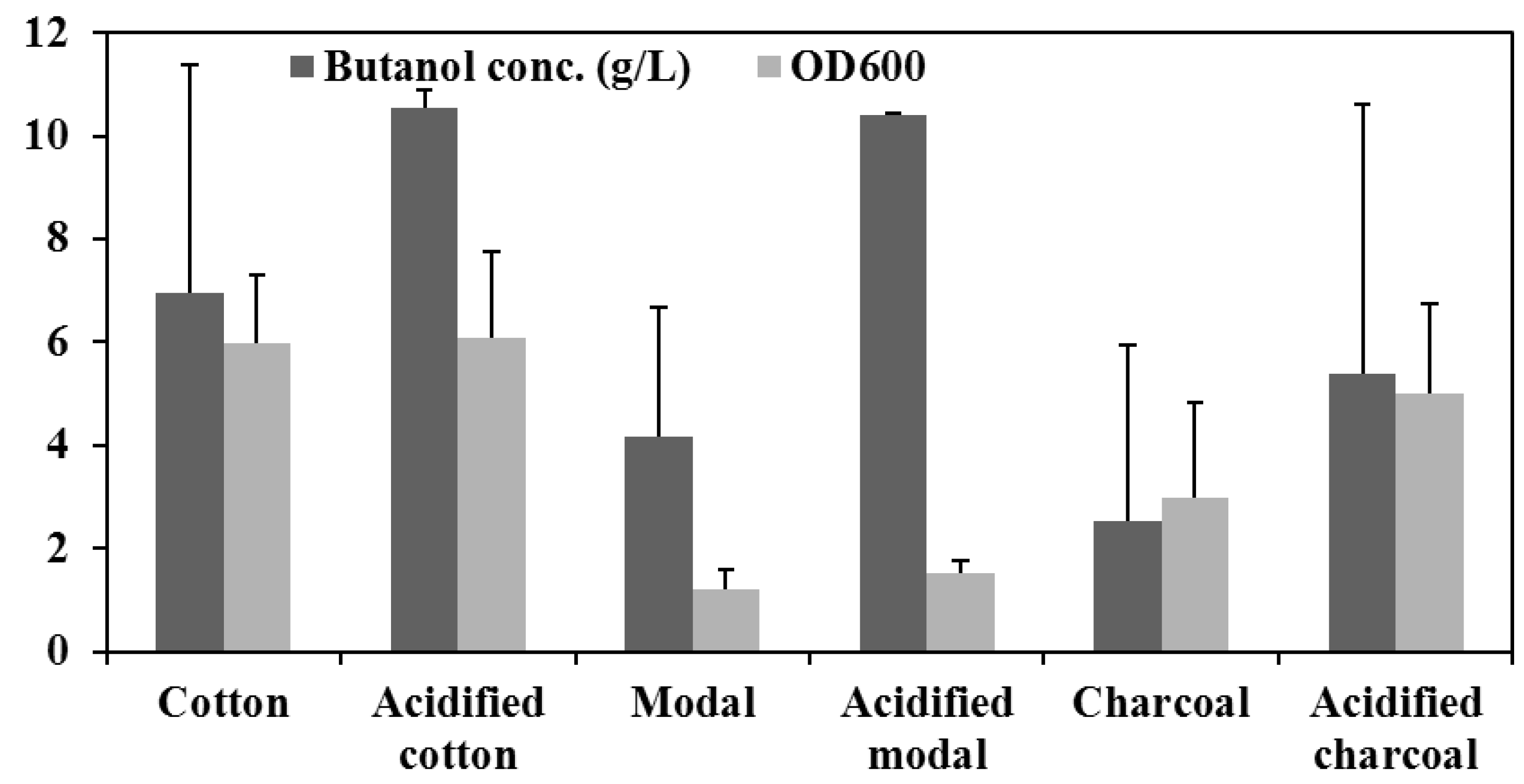
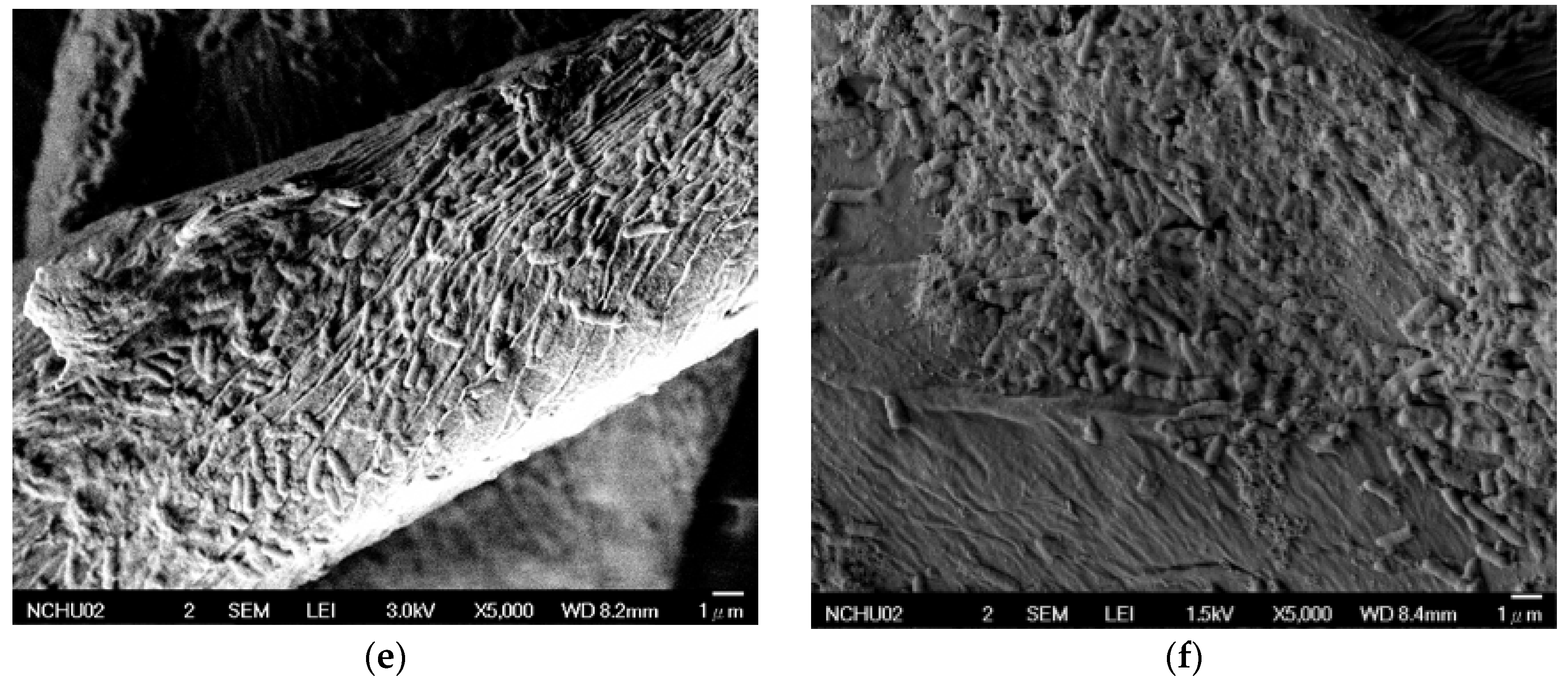
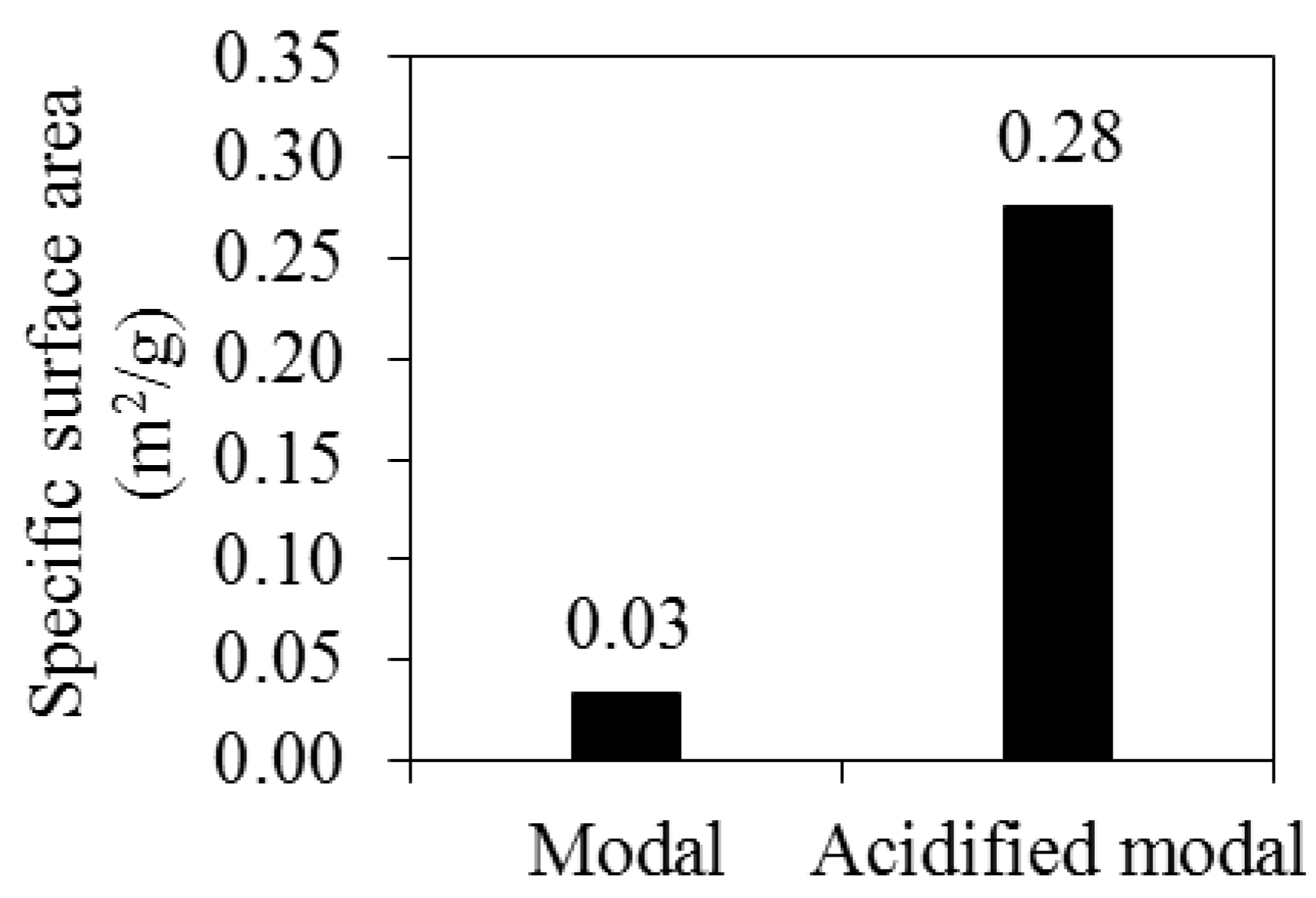
3.2. The Effects of Acid Pretreatment
3.3. In Vitro Performance of the Materials
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chang, Z.; Cai, D.; Wang, Y.; Chen, C.; Fu, C.; Wang, G.; Qin, P.; Wang, Z.; Tan, T. Effective multiple stages continuous acetone-butanol-ethanol fermentation by immobilized bioreactors: Making full use of fresh corn stalk. Bioresour. Technol. 2016, 205, 82–89. [Google Scholar] [CrossRef]
- Wang, Y.-R.; Chiang, Y.-S.; Chuang, P.-J.; Chao, Y.-P.; Li, S.-Y. Direct in situ butanol recovery inside the packed bed during continuous acetone-butanol-ethanol (ABE) fermentation. Appl. Microbiol. Biotechnol. 2016, 100, 7449–7456. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Chiang, C.-J.; Tseng, I.T.; He, C.-R.; Chao, Y.-P. Bioreactors and in situ product recovery techniques for acetone-butanol-ethanol fermentation. FEMS Microbiol. Lett. 2016, 363, fnw107. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.W.; Li, R.J.; Ma, T.W. The development process for a continuous acetone-butanol-ethanol (ABE) fermentation by immobilized Clostridium acetobutylicum. J. Taiwan Inst. Chem. Eng. 2011, 42, 902–907. [Google Scholar] [CrossRef]
- Kheyrandish, M.; Asadollahi, M.A.; Jeihanipour, A.; Doostmohammadi, M.; Rismani-Yazdi, H.; Karimi, K. Direct production of acetone-butanol-ethanol from waste starch by free and immobilized Clostridium acetobutylicum. Fuel 2015, 142, 129–133. [Google Scholar] [CrossRef]
- Shamsudin, S.; Kalil, M.; Yusoff, W. Production of acetone, butanol and ethanol (ABE) by Clostridium saccharoperbutylacetonicum N1–4 with different immobilization systems. Pak. J. Biol. Sci. 2006, 9, 1923–1928. [Google Scholar]
- Qureshi, N.; Li, X.-L.; Hughes, S.; Saha, B.C.; Cotta, M.A. Butanol production from corn fiber xylan using Clostridium acetobutylicum. Biotechnol. Prog. 2006, 22, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Kittithanesuan, N.; Phisalaphong, M. Enhanced acetone-butanol production from sugarcane juice by immobilized Clostridium acetobutylicum (ATCC 824) on thin-shell silk cocoons. Biotechnol. Bioprocess Eng. 2015, 20, 599–607. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Lu, K.-M.; Chiang, Y.-S.; Wang, Y.-R.; Chein, R.-Y.; Li, S.-Y. Performance of fed-batch acetone-butanol-ethanol (ABE) fermentation coupled with the integrated in situ extraction-gas stripping process and the fractional condensation. J. Taiwan Inst. Chem. Eng. 2016, 60, 119–123. [Google Scholar] [CrossRef]
- Lu, K.-M.; Li, S.-Y. An integrated in situ extraction-gas stripping process for acetone-butanol-ethanol (ABE) fermentation. J. Taiwan Inst. Chem. Eng. 2014, 45, 2106–2110. [Google Scholar] [CrossRef]
- Chen, S.-K.; Chin, W.-C.; Tsuge, K.; Huang, C.-C.; Li, S.-Y. Fermentation approach for enhancing 1-butanol production using engineered butanologenic Escherichia coli. Bioresour. Technol. 2013, 145, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, H.-S.; He, C.-R.; Yen, A.T.-C.; Wu, T.-M.; Li, S.-Y. Assessment of Acidified Fibrous Immobilization Materials for Improving Acetone-Butanol-Ethanol (ABE) Fermentation. Fermentation 2017, 3, 3. https://doi.org/10.3390/fermentation3010003
Zeng H-S, He C-R, Yen AT-C, Wu T-M, Li S-Y. Assessment of Acidified Fibrous Immobilization Materials for Improving Acetone-Butanol-Ethanol (ABE) Fermentation. Fermentation. 2017; 3(1):3. https://doi.org/10.3390/fermentation3010003
Chicago/Turabian StyleZeng, Hong-Sheng, Chi-Ruei He, Andy Tien-Chu Yen, Tzong-Ming Wu, and Si-Yu Li. 2017. "Assessment of Acidified Fibrous Immobilization Materials for Improving Acetone-Butanol-Ethanol (ABE) Fermentation" Fermentation 3, no. 1: 3. https://doi.org/10.3390/fermentation3010003