Fortification and Elevated Alcohol Concentration Affect the Concentration of Rotundone and Volatiles in Vitis vinifera cv. Shiraz Wine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Vineyard and Winery Site
2.3. Small-Scale Winemaking Trial
3. Commercial-Scale Winemaking Trial
3.1. Headspace Solid-Phase Microextraction and Gas Chromatography Mass Spectrometry (HS-SPME-GC-MS) Analysis of Rotundone in Grapes and Wine
3.2. Headspace Solid-Phase Microextraction and Gas Chromatography Mass Spectrometry (HS-SPME-GC-MS) Analysis of Wine Volatiles
4. Statistical Analysis
5. Results and Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dunlevy, J.D.; Kalua, C.M.; Keyzers, R.A.; Boss, P.K. The production of flavour & aroma compounds in grape berries. In Grapevine Molecular Physiology & Biotechnology, 2nd ed.; Roubelakis-Angelakis, K., Ed.; Springer: Dordrecht, the Netherlands, 2009; pp. 293–340. [Google Scholar]
- Maicas, S.; Mateo, J.J. Hydrolysis of terpenyl glycosides in grape juice and other fruit juices: A review. Appl. Microbiol. Biotechnol. 2005, 67, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, D.; Siebert, T.; Capone, D.; Pardon, K.; Van Leeuwen, K.; Solomon, M. Grape and wine pepper aroma–analytically challenging but we sniff it out in the end. In Technical review; The Australian Wine Research Institute: Urrbrae, SA, Australia, 2009; pp. 11–16. [Google Scholar]
- Herderich, M.J.; Siebert, T.E.; Parker, M.; Capone, D.L.; Mayr, C.; Zhang, P.; Geffroy, O.; Williamson, P.; Francis, I.L. Synthesis of the ongoing works on rotundone, an aromatic compound responsible for the peppery notes in wines. Internet J. Enol. Vitic. 2013, 6, 1–6. [Google Scholar]
- Herderich, M.J.; Siebert, T.E.; Parker, M.; Capone, D.L.; Jeffery, D.W.; Osidacz, P.; Francis, I.L. Spice up your life: Analysis of key aroma compounds in Shiraz. In Flavor Chemistry of Wine and Other Alcoholic Beverages; American Chemical Society: Washington, DC, USA, 2012; pp. 3–13. [Google Scholar]
- Siebert, T.E.; Wood, C.; Elsey, G.M.; Pollnitz, A.P. Determination of rotundone, the pepper aroma impact compound, in grapes and wine. J. Agric. Food Chem. 2008, 56, 3745–3748. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.; Siebert, T.E.; Parker, M.; Capone, D.L.; Elsey, G.M.; Pollnitz, A.P.; Eggers, M.; Meier, M.; Vössing, T.; Widder, S.; et al. From wine to pepper: Rotundone, an obscure sesquiterpene, is a potent spicy aroma compound. J. Agric. Food Chem. 2008, 56, 3738–3744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Howell, K.; Krstic, M.; Herderich, M.; Barlow, E.W.R.; Fuentes, S. Environmental factors and seasonality affect the concentration of rotundone in vitis vinifera l. Cv. Shiraz wine. PLoS ONE 2015, 10, e0133137. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.; Nicholson, E.L.; Böttcher, C.; Burbidge, C.A.; Bastian, S.E.; Harvey, K.E.; Huang, A.-C.; Taylor, D.K.; Boss, P.K. Shiraz wines made from grape berries (vitis vinifera) delayed in ripening by plant growth regulator treatment have elevated rotundone concentrations and “pepper” flavor and aroma. J. Agric. Food Chem. 2015, 63, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Geffroy, O.; Dufourcq, T.; Carcenac, D.; Siebert, T.; Herderich, M.; Serrano, E. Effect of ripeness and viticultural techniques on the rotundone concentration in red wine made from vitis vinifera L. cv. Duras. Aust. J. Grape Wine Res. 2014, 20, 401–408. [Google Scholar] [CrossRef]
- Geffroy, O.; Siebert, T.; Herderich, M.; Mille, B.; Serrano, E. On-vine grape drying combined with irrigation allows to produce red wines with enhanced phenolic and rotundone concentrations. Sci. Hortic. 2016, 207, 208–217. [Google Scholar] [CrossRef]
- Geffroy, O.; Yobrégat, O.; Dufourcq, T.; Siebert, T.; Serrano, E. Certified clone and powdery mildew impact rotundone in red wine from vitis vinifera L. cv. Duras N. OENO One 2015, 49, 231–240. [Google Scholar] [CrossRef]
- Herderich, M.; Barter, S.; Black, C.A.; Bramley, R.; Capone, D.; Dry, P.; Siebert, T.; Zhang, P. Terroir effects on grape and wine aroma compounds. In Advances in wine research; American Chemical Society: Washington, DC, USA, 2015; pp. 131–146. [Google Scholar]
- May, B.; Lange, B.M.; Wüst, M. Biosynthesis of sesquiterpenes in grape berry exocarp of vitis vinifera l.: Evidence for a transport of farnesyl diphosphate precursors from plastids to the cytosol. Phytochemistry 2013, 95, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Scarlett, N.J.; Bramley, R.G.V.; Siebert, T.E. Within-vineyard variation in the ‘pepper’ compound rotundone is spatially structured and related to variation in the land underlying the vineyard. Aust. J. Grape Wine Res. 2014, 20, 214–222. [Google Scholar] [CrossRef]
- Zhang, P.; Barlow, S.; Krstic, M.; Herderich, M.; Fuentes, S.; Howell, K. Within-vineyard, within-vine, and within-bunch variability of the rotundone concentration in berries of vitis vinifera L. Cv. Shiraz. J. Agric. Food Chem. 2015, 63, 4276–4283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Fuentes, S.; Siebert, T.; Krstic, M.; Herderich, M.; Barlow, E.W.R.; Howell, K. Terpene evolution during the development of vitis vinifera L. cv. Shiraz grapes. Food Chem. 2016, 204, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Fuentes, S.; Siebert, T.; Krstic, M.; Herderich, M.; Barlow, E.W.R.; Howell, K. Comparison data of common and abundant terpenes at different grape development stages in shiraz wine grapes. Data Brief 2016, 8, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Fuentes, S.; Wang, Y.; Deng, R.; Krstic, M.; Herderich, M.; Barlow, E.W.R.; Howell, K. Distribution of rotundone and possible translocation of related compounds amongst grapevine tissues in vitis vinifera l. Cv. Shiraz. In Front. Plant Sci.; 2016. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4914589/ (accessed on 27 May 2017).
- Siebert, T.E.; Solomon, M.R. Rotundone: Development in the grape and extraction during fermentation. In Proceedings of the fourteenth Australian wine industry technical conference, Adelaide, Australia, July 2010; pp. 307–308. [Google Scholar]
- Caputi, L.; Carlin, S.; Ghiglieno, I.; Stefanini, M.; Valenti, L.; Vrhovsek, U.; Mattivi, F. Relationship of changes in rotundone content during grape ripening and winemaking to manipulation of the ‘peppery’character of wine. J. Agric. Food Chem. 2011, 59, 5565–5571. [Google Scholar] [CrossRef] [PubMed]
- Capone, D.L.; Jeffery, D.W.; Sefton, M.A. Vineyard and fermentation studies to elucidate the origin of 1,8-cineole in australian red wine. J. Agric. Food Chem. 2012, 60, 2281–2287. [Google Scholar] [CrossRef] [PubMed]
- Geffroy, O.; Siebert, T.; Silvano, A.; Herderich, M. Impact of winemaking techniques on classical enological parameters and rotundone in red wine at the laboratory scale. Am. J. Enol. Vitic. 2017, 68, 141–146. [Google Scholar] [CrossRef]
- Bramley, R.; Siebert, T.; Herderich, M.; Krstic, M. Patterns of within-vineyard spatial variation in the ‘pepper’compound rotundone are temporally stable from year to year. Aust. J. Grape Wine Res. 2017, 23, 42–47. [Google Scholar] [CrossRef]
- Kilmister, R.L.; Mazza, M.; Baker, N.K.; Faulkner, P.; Downey, M.O. A role for anthocyanin in determining wine tannin concentration in shiraz. Food Chem. 2014, 152, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, X.; Needs, S.; Fuentes, S.; Liu, D.; Howell, K. Influences of apical and basal defoliation on canopy structure and the biochemical composition of vitis vinifera cv. Shiraz grape and wine. Front. Chem. 2017, 5. [Google Scholar] [CrossRef]
- Iland, P. Chemical Analysis of Grapes and Wine: Techniques and Concepts; Patrick Iland Wine Promotions: Campbelltown, Australia, 2004. [Google Scholar]
- Siebert, T.E.; Smyth, H.E.; Capone, D.L.; Neuwohner, C.; Pardon, K.H.; Skouroumounis, G.K.; Herderich, M.J.; Sefton, M.A.; Pollnitz, A.P. Stable isotope dilution analysis of wine fermentation products by HS-SPME-GC-MS. Anal. Bioanal. Chem. 2005, 381, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Lisanti, M.T.; Gambuti, A.; Genovese, A.; Piombino, P.; Moio, L. Partial dealcoholization of red wines by membrane contactor technique: Effect on sensory characteristics and volatile composition. Food Bioprocess Technol. 2013, 6, 2289–2305. [Google Scholar] [CrossRef]
- Motta, S.; Guaita, M.; Petrozziello, M.; Ciambotti, A.; Panero, L.; Solomita, M.; Bosso, A. Comparison of the physicochemical and volatile composition of wine fractions obtained by two different dealcoholization techniques. Food Chem. 2017, 221, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gambuti, A.; Rinaldi, A.; Lisanti, M.T.; Pessina, R.; Moio, L. Partial dealcoholisation of red wines by membrane contactor technique: Influence on colour, phenolic compounds and saliva precipitation index. Eur. Food Res. Technol. 2011, 233, 647. [Google Scholar] [CrossRef]
- Gil, M.; Estévez, S.; Kontoudakis, N.; Fort, F.; Canals, J.; Zamora, F. Influence of partial dealcoholization by reverse osmosis on red wine composition and sensory characteristics. Eur. Food Res. Technol. 2013, 237, 481–488. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.F. Volatile aroma components of australian port wines. J. Sci. Food Agric. 1980, 31, 214–222. [Google Scholar] [CrossRef]
- Mayson, R. Port and the Douro; Infinite Ideas: Oxford, UK, 2012. [Google Scholar]
- Lund, S.T.; Bohlmann, J. The molecular basis for wine grape quality-a volatile subject. Science 2006, 311, 804–805. [Google Scholar] [CrossRef] [PubMed]
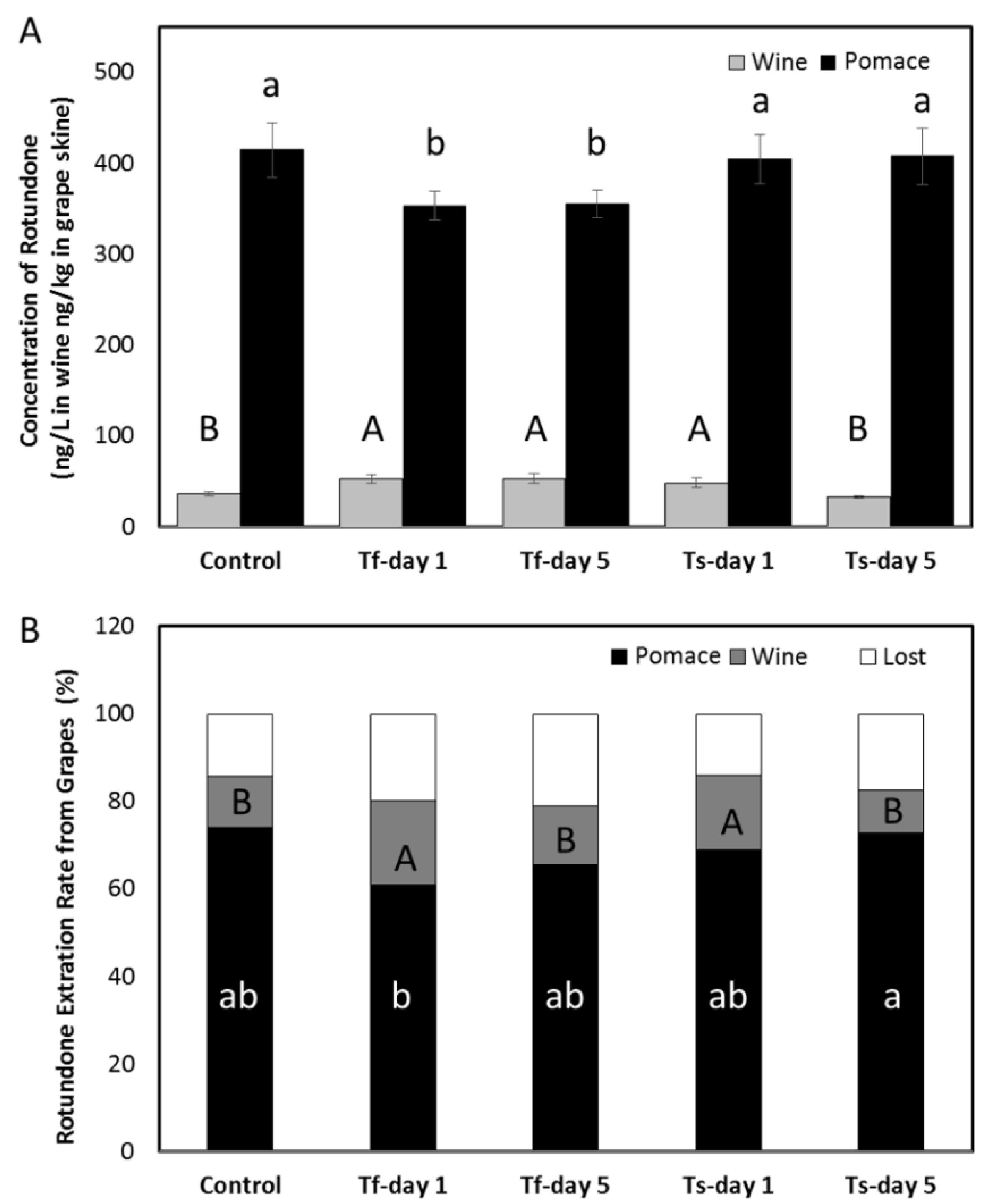
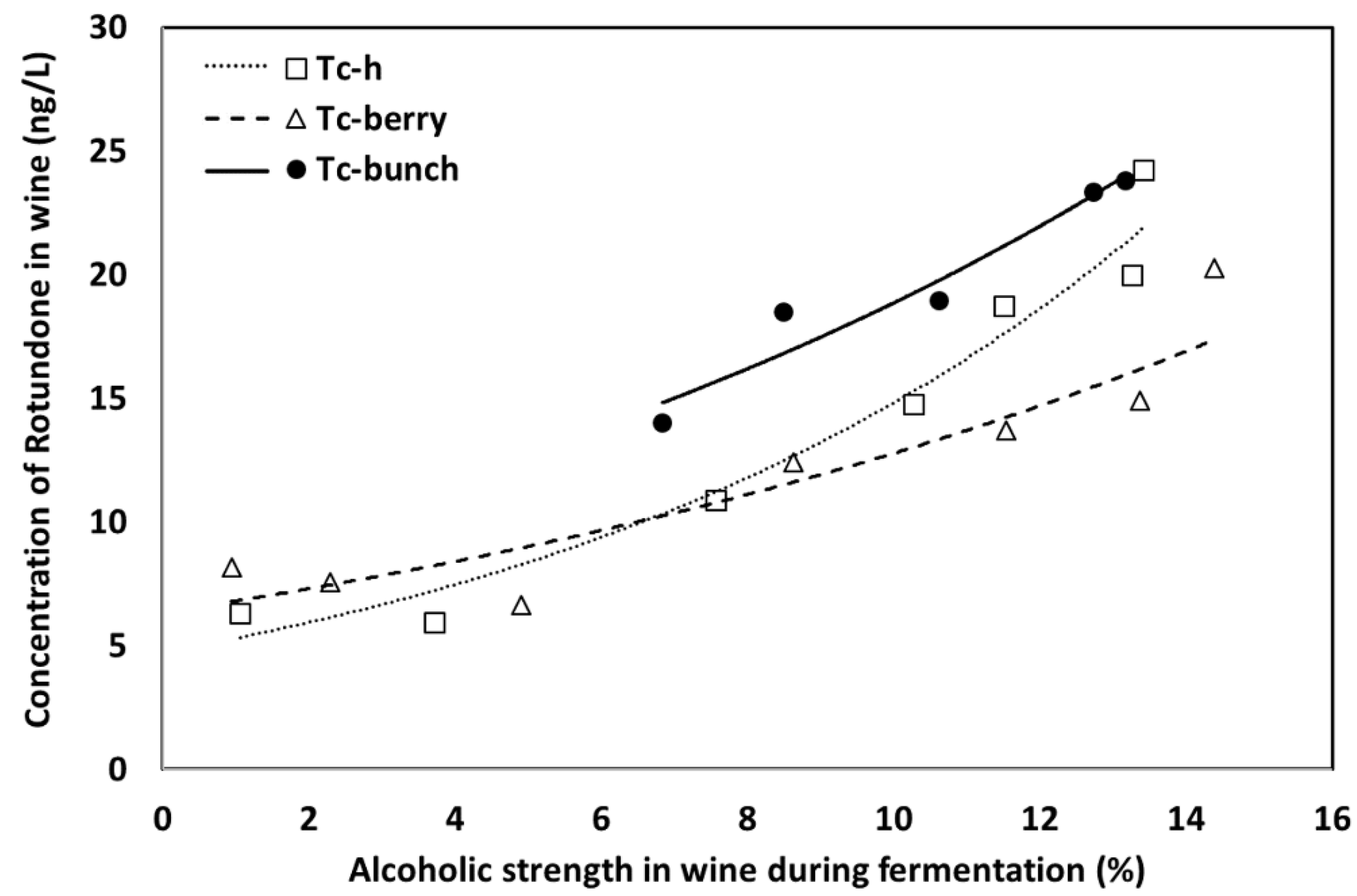
| Wine Parameters | Control | Tf-day 1 | Tf-day 5 | Ts-day 1 | Ts-day 5 |
|---|---|---|---|---|---|
| Alcohol (%v/v) | 12.7 ± 0.4 c | 16.8 ± 0.5 a | 16.9 ± 0.1 a | 15.2 ± 0.6 b | 15.3 ± 0.2 b |
| Total phenolics (a.u.) | 36.2 ± 1.8 b | 44.3 ± 1.1 a | 43.0 ± 1.2 a | 44.0 ± 0.5 a | 43.9 ± 0.7 a |
| Total red pigments (a.u.) | 14.3 ± 0.1 c | 20.1 ± 0.8 a | 18.9 ± 0.4 b | 19.0 ± 0.1 b | 18.1 ± 1.1 b |
| Wine colour density | 9.1 ± 0.8 | 10.6 ± 1.3 | 9.1 ± 1.4 | 11.0 ± 0.5 | 9.4 ± 0.5 |
| Wine colour hue | 0.63 ± 0.02 a | 0.54 ± 0.04 b | 0.60 ± 0.03 ab | 0.53 ± 0.02 b | 0.60 ± 0.00 ab |
| Degree of red pigment colouration (%) | 13.9 ± 0.9 | 14.3 ± 2.3 | 12.1 ± 1.8 | 15.0 ± 0.8 | 12.2 ± 0.5 |
| Peak Number | LRI-Lit 1 | LRI-Act 2 | Compound Name | Odour 3 | Concentration of Major Volatile in Studied Wine Samples 4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Tf-day 1 | Tf-day 5 | Ts-day 1 | Ts-day 5 | |||||
| Acetate | |||||||||
| 1 5 | 880 | 841 | Ethyl acetate (mg/L) | Fruity, pineapple | 3.8 ± 0.3 b 6 | 5.7 ± 0.2 a | 4.6 ± 0.1 b | 4 ± 0.7 b | 4 ± 0.6 b |
| 4 | 1115 | 1125 | 3-Methylbutyl acetate (mg/L) | Fruity, banana | 1.4 ± 0.3 c | 5.6 ± 1 a | 0.9 ± 0.2 c | 3.5 ± 0.7 b | 1.5 ± 0.8 c |
| 8 | 1275 | 1272 | Hexyl acetate (µg/L) | Red fruit, herb | tr 7 | 53 ± 4 | tr | tr | tr |
| Straight chain esters | |||||||||
| 2 | 1035 | 1042 | Ethyl butanoate (mg/L) | Ripe kiwi, ripe strawberry, cheese | 1.5 ± 0.2 ab | 1.9 ± 0.2 a | 1.3 ± 0.1 b | 2.0 ± 0.2 a | 1.4 ± 0.3 b |
| 7 | 1240 | 1235 | Ethyl hexanoate (mg/L) | Fruity, green apple | 2.1 ± 0.2 a | 1.2 ± 0.2 b | 0.4 ± 0.0 b | 2.3 ± 0.2 a | 1 ± 0.8 b |
| 11 | 1440 | 1435 | Ethyl octanoate (µg/L) | Waxy, apple skin, fruity | 688 ± 70 a | 407 ± 35 b | 137 ± 12 b | 795 ± 49 a | 352 ± 271 b |
| 14 | 1643 | 1638 | Ethyl decanoate (µg/L) | Waxy, fruity | 83 ± 17 | 72 ± 27 | 56 ± 4 | 113 ± 6 | 77 ± 46 |
| Other esters | |||||||||
| 12 | 1491 | 1491 | Methyl nonanoate (µg/L) | Coconut | 109 ± 19 ab | 53 ± 11 b | 74 ± 24 b | 59 ± 36 b | 135 ± 25 a |
| Alcohol | |||||||||
| 3 | 1090 | 1100 | Isobutanol (µg/L) | Fruity | 405 ± 48 | 368 ± 45 | 480 ± 27 | 448 ± 27 | 462 ± 62 |
| 5 | 1134 | 1151 | 1-Butanol (µg/L) | Fusel, spirituous, medicine | 6.0 ± 0.8 | 7.9 ± 2.5 | 5.9 ± 2.4 | 5.0 ± 2 | 4.2 ± 0.4 |
| 6 | 1185 | 1214 | Isopentanol (mg/L) | Earthy, burnt | 682 ± 12 b | 701 ± 36 ab | 708 ± 9 ab | 779 ± 14 a | 780 ± 57 a |
| 9 | 1360 | 1360 | 1-Hexanol (mg/L) | Green, floral, spice | 2.4 ± 0.3 | 1.4 ± 1.3 | 0.8 ± 1.2 | 1.7 ± 0.2 | 1.8 ± 0.4 |
| 15 | 1912 | 1917 | Phenylethyl Alcohol (mg/L) | Floral, rose | 6.9 ± 0.5 c | 6.1 ± 0.3 d | 8.4 ± 0.5 b | 8.8 ± 0.1 b | 10.1 ± 0.1 a |
| Miscellaneous compounds | |||||||||
| 13 | 1543 | 1550 | 2, 3-Butanediol (µg/L) | Odorless | tr | 44 ± 9 | 44 ± 34 | 59 ± 0 | 50 ± 4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Luo, F.; Howell, K. Fortification and Elevated Alcohol Concentration Affect the Concentration of Rotundone and Volatiles in Vitis vinifera cv. Shiraz Wine. Fermentation 2017, 3, 29. https://doi.org/10.3390/fermentation3030029
Zhang P, Luo F, Howell K. Fortification and Elevated Alcohol Concentration Affect the Concentration of Rotundone and Volatiles in Vitis vinifera cv. Shiraz Wine. Fermentation. 2017; 3(3):29. https://doi.org/10.3390/fermentation3030029
Chicago/Turabian StyleZhang, Pangzhen, Fangping Luo, and Kate Howell. 2017. "Fortification and Elevated Alcohol Concentration Affect the Concentration of Rotundone and Volatiles in Vitis vinifera cv. Shiraz Wine" Fermentation 3, no. 3: 29. https://doi.org/10.3390/fermentation3030029