A Pichia anomala Strain (P. anomala M1) Isolated from Traditional Greek Sausage is an Effective Producer of Extracellular Lipolytic Enzyme in Submerged Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Isolation
2.2. Molecular Characterization of an Isolated Pichia Anomala Strain
2.3. Yeast Cultivation
2.4. Analytical Methods
3. Results and Discussion
3.1. Strain Isolation and Identification
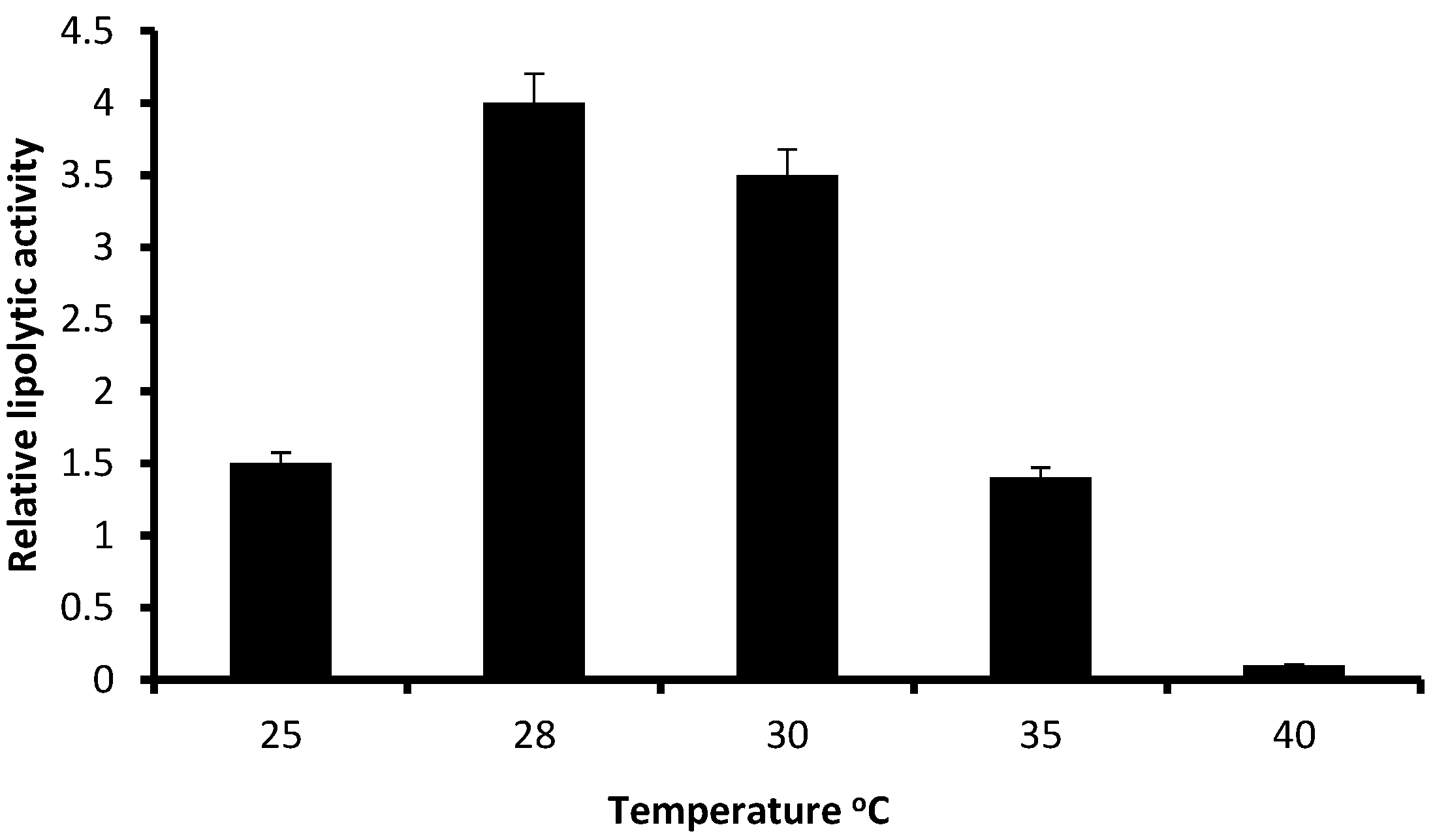
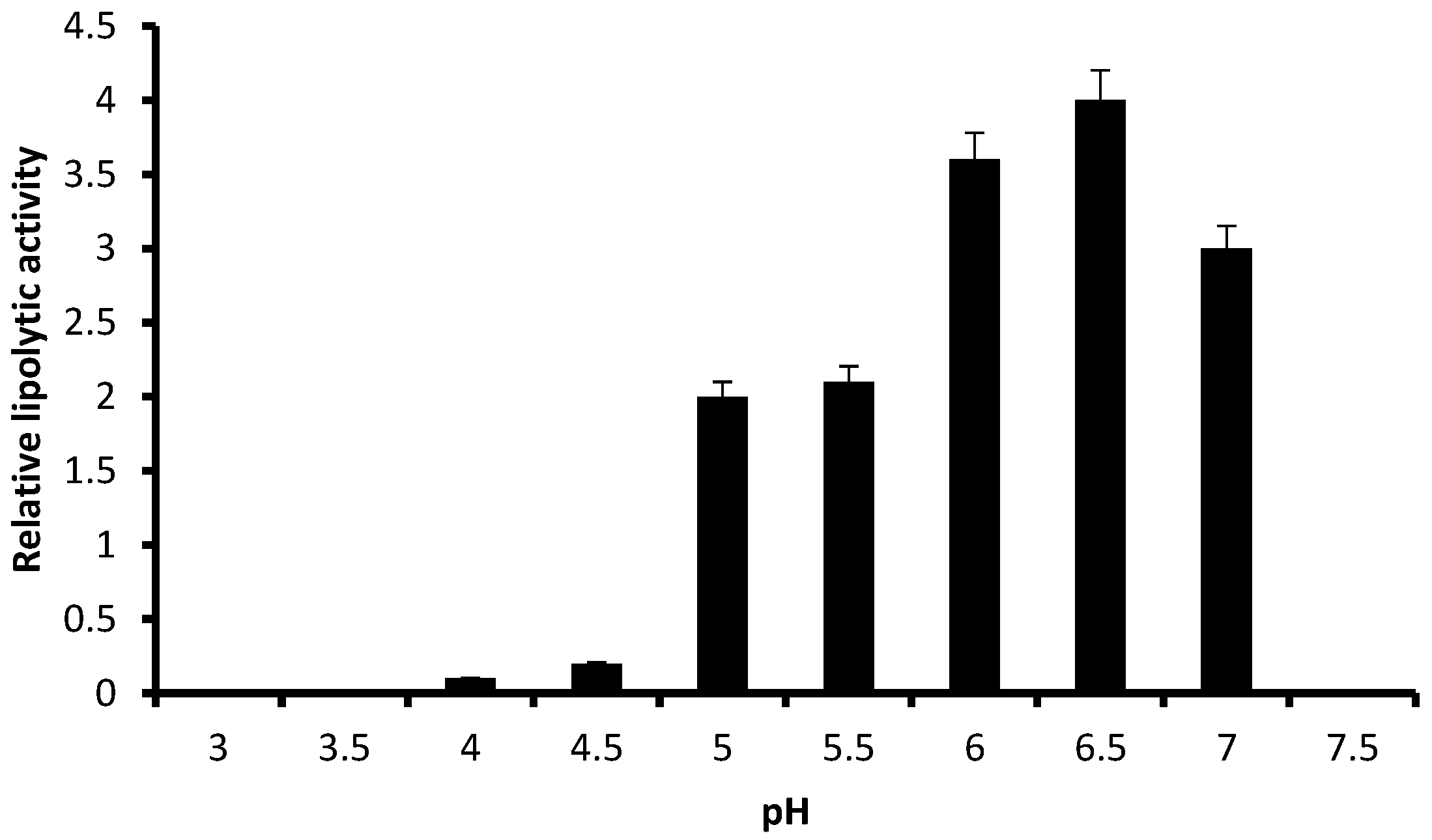
3.2. Relative Lipolytic Activity in Solid State Cultivation
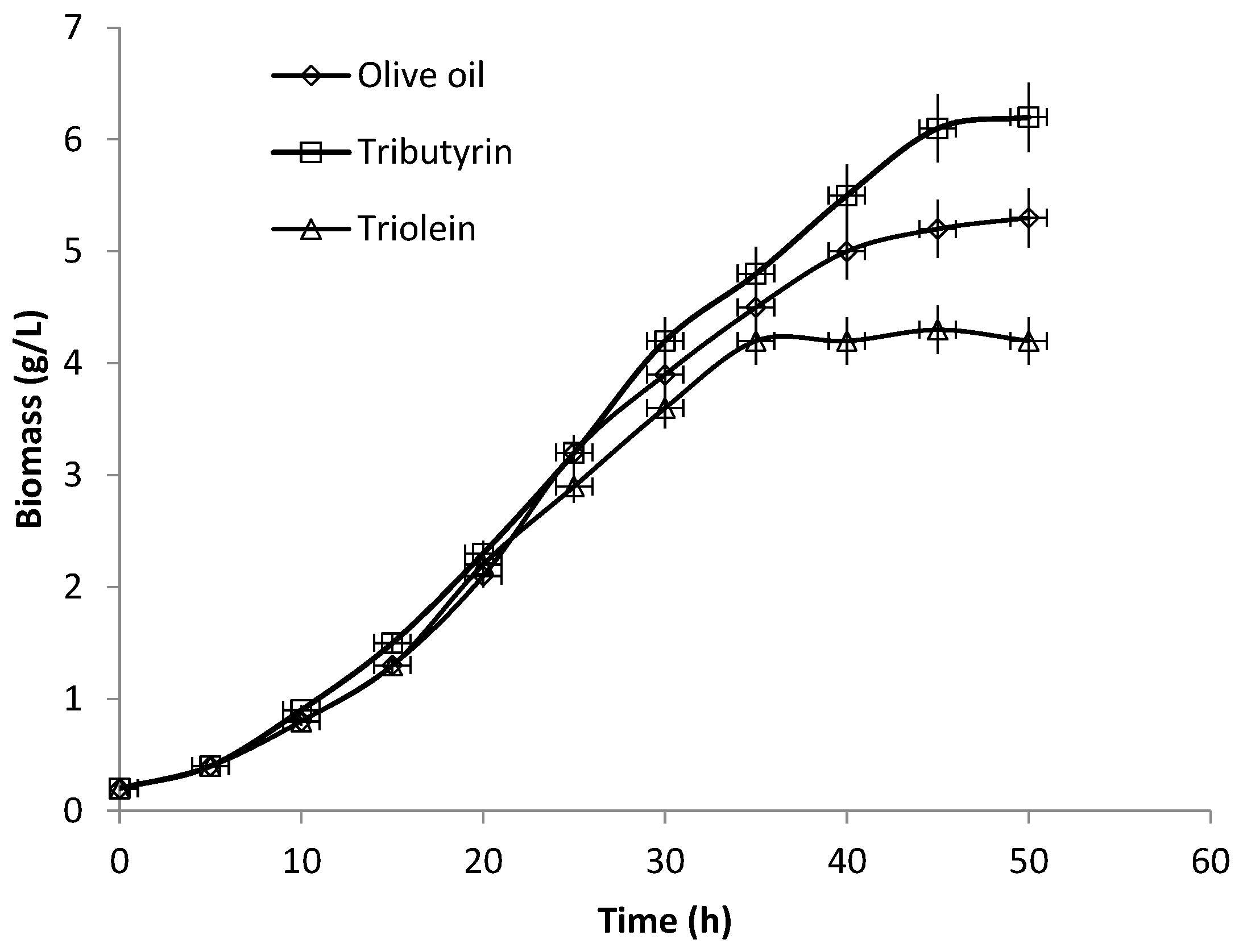
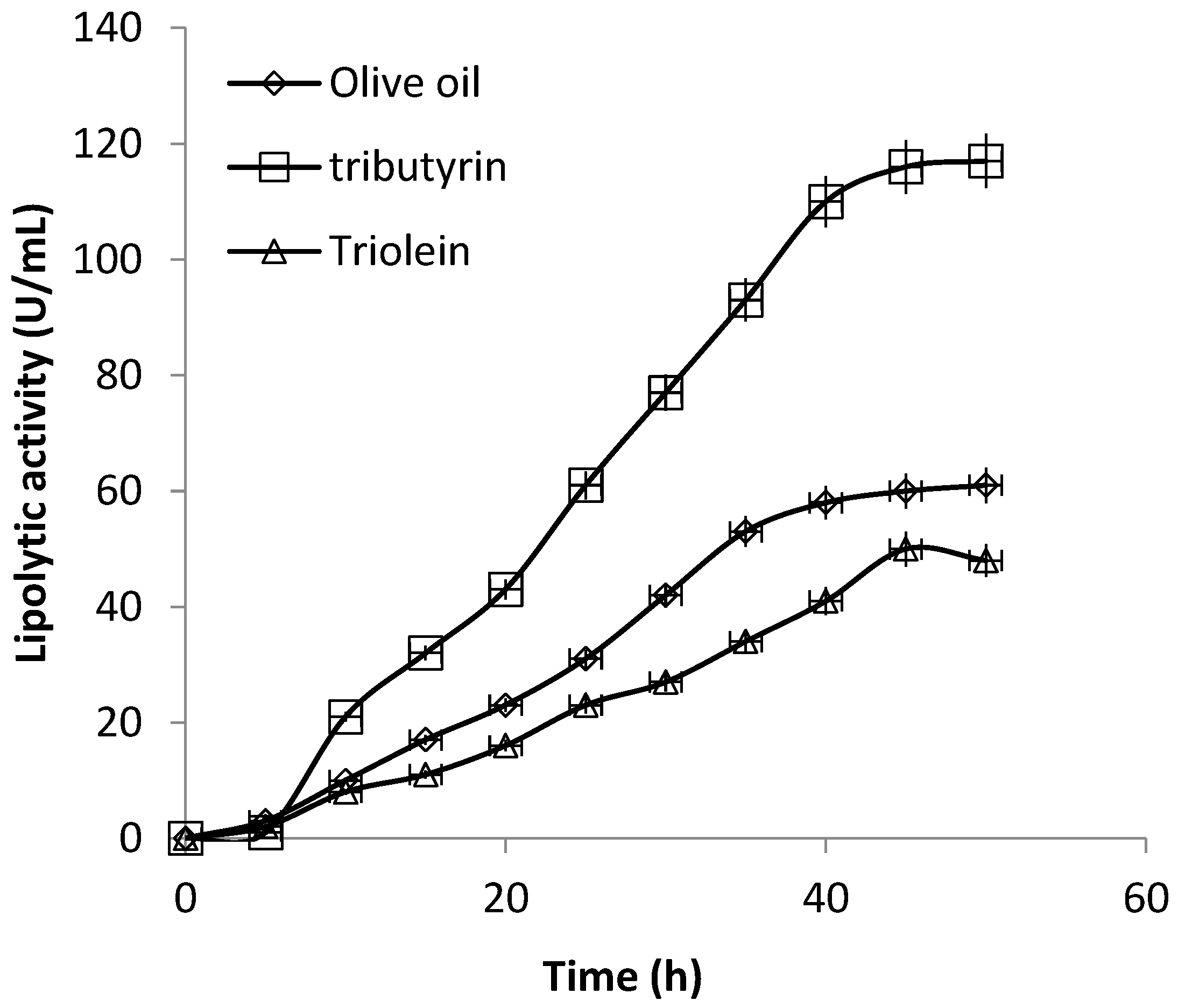
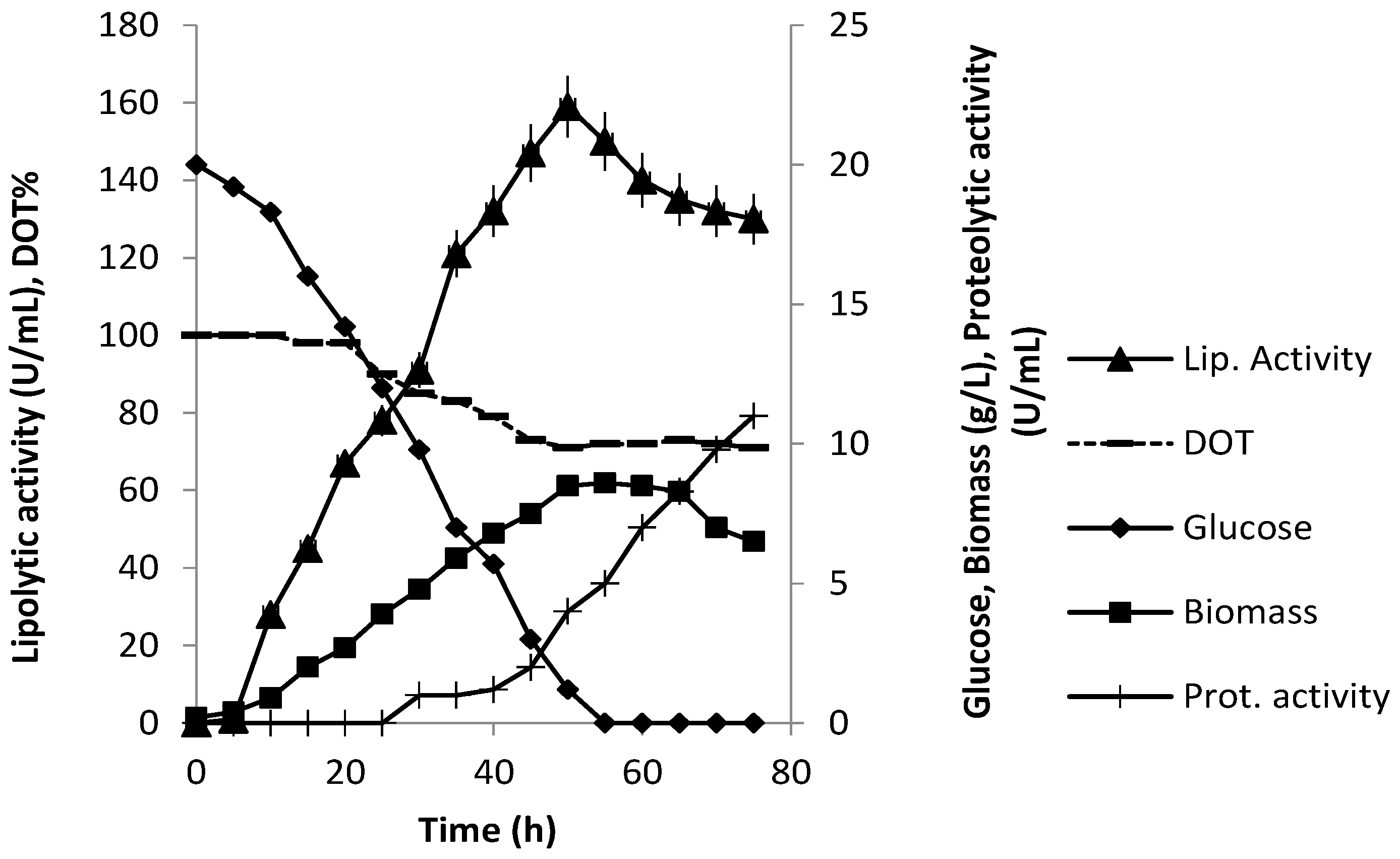
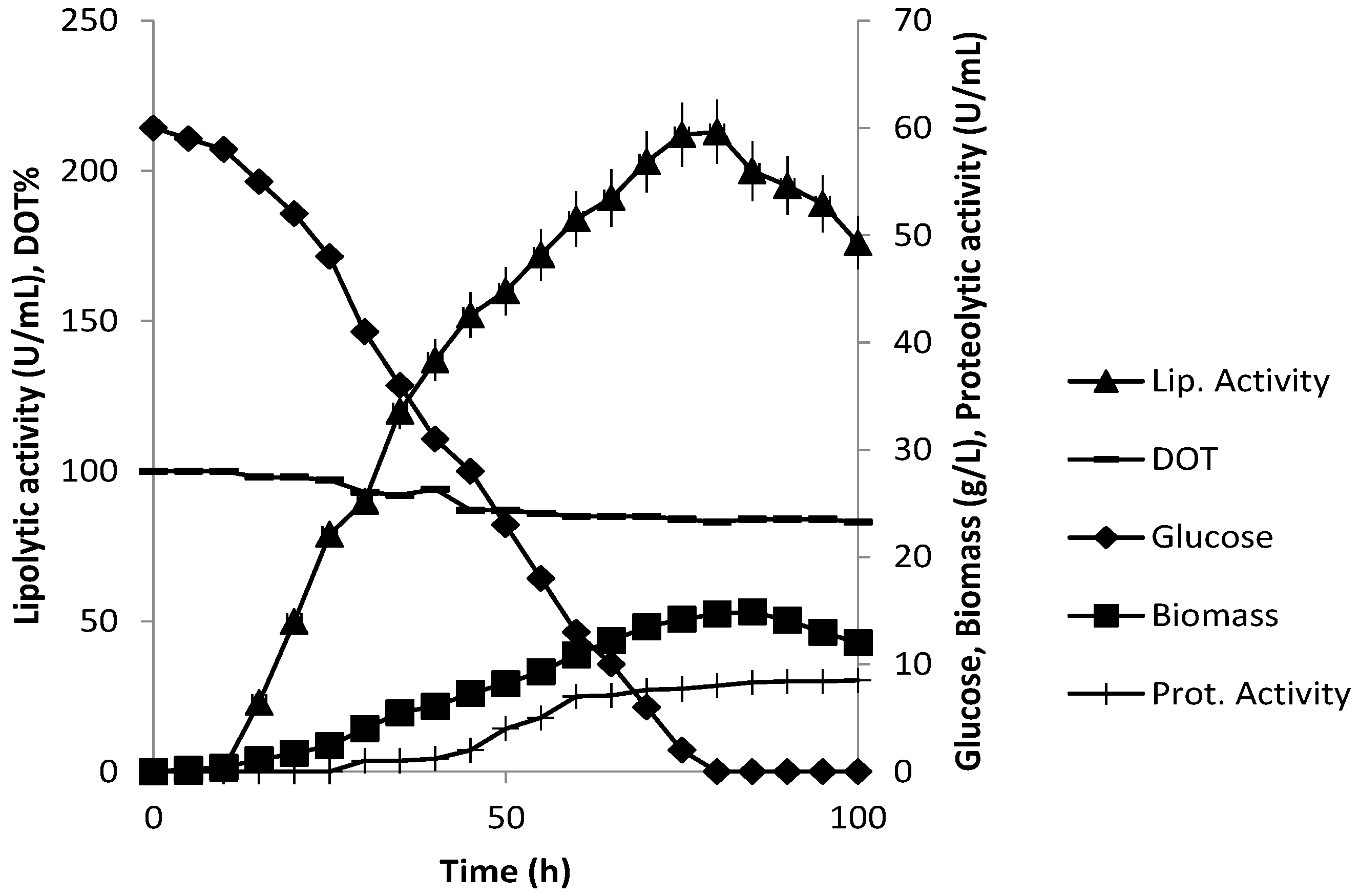
3.3. Production of Extracellular Lipase in Submerged Fermentation
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Kurtzman, C.P.; Fell, J.W. (Eds.) The Yeasts, a Taxonomic Study; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Passoth, V.; Fredlund, E.; Druvefors, U.Ä.; Schnürer, J. Biotechnology, physiology and genetics of the yeast Pichia anomala. FEMS Yeast Res. 2006, 6, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.M. Pichia anomala: Cell physiology and biotechnology relative to other yeasts. Antonie van Leeuwenhoek 2011, 99, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.; Feizi, A.; Kim, S.; Siewers, V.; Nielsen, J. RNA-Seq analysis of Pichia anomala reveals important mechanisms required for survival at low pH. Microb. Cell Fact. 2015, 14, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passoth, V.; Olstorpe, M.; Schnürer, J. Past, present and future research directions with Pichia anomala. Antonie Van Leeuwenhoek 2011, 99, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Rupp, O.; Trost, E.; Jaenicke, S.; Passoth, V.; Goesmann, A.; Tauch, A.; Brinkrolf, K. Genome sequence of Wickerhamomyces anomalus DSM 6766 reveals genetic basis of biotechnologically important antimicrobial activities. FEMS Yeast Res. 2012, 12, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, F.A.G.; Colen, G.; Takahasi, J.A. Yarrowia lipolytica and its multiple applications in the biotechnological industry. Sci. World J. 2014. [Google Scholar] [CrossRef]
- Satyanarayana, T.; Kunze, G. Yeast Biotechnology: Diversity and Applications; Springer: Berlin, Germany, 2009. [Google Scholar]
- Stergiou, P.Y.; Foukis, A.; Sklivaniti, H.; Zacharaki, P.; Papagianni, M.; Papamichael, E.M. Experimental investigation and optimization of process variables affecting the production of extracellular lipase by Kluyveromyces marxianus IFO 0288. Appl. Biochem. Biotechnol. 2012, 168, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Sengupta, I.; Majumdar, S.K. Lipase Production by Hansenula anomala var.Schnegii. J. Food Sci. Technol. 1985, 22, 137–139. [Google Scholar]
- Ionita, A.; Moscovici, M.; Popa, C.; Vamanu, A.; Popa, O.; Dinu, L. Screening of yeast and fungal strains for lipolytic potential and determination of some biochemical properties of microbial lipases. J. Mol. Catal. B. Enzym. 1997, 3, 147–151. [Google Scholar] [CrossRef]
- De Miranda, A.S.; Miranda, L.S.M.; de Suza, R.O.M.A. Lipases: Valuable catalysts for dynamic kinetic resolutions. Biotechnol. Adv. 2015, 33, 372–393. [Google Scholar] [CrossRef] [PubMed]
- Faber, K. Biotransformations in Organic Chemistry, 6th ed.; Springer: Berlin, Germany, 2011. [Google Scholar]
- Fredlund, E.; Blank, L.M.; Schnürer, J.; Sauer, U.; Passoth, V. Oxygen- and glucose-dependent regulation of central carbon metabolism in Pichia anomala. Appl. Environ. Microbiol. 2004, 70, 5905–5911. [Google Scholar] [CrossRef] [PubMed]
- Fredlund, E.; Druvefors, U.; Ligsten, K.J.; Boysen, M.E.; Schnürer, J. Physiological characteristics of the biocontrol yeast Pichia anomala J121. FEMS Yeast Res. 2002, 2, 395–402. [Google Scholar] [PubMed]
- Kreger-van Rij, N.J.W. The Yeasts: A Taxonomic Study, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Caggia, C.; Restuccia, C.; Pulvirenti, A.; Giudici, P. Identification of Pichia anomala isolated from yoghurt by RFLP of the ITS region. Int. J. Food Microbiol. 2001, 71, 71–73. [Google Scholar] [CrossRef]
- Tao, N.; Gao, Y.; Liu, X. Isolation and characterization of a Pichia anomala strain: A promising candidate for bioethanol production. Braz. J. Microbiol. 2011, 42, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, M. An evaluation of the proteolytic and lipolytic potential of Penicillium spp. isolated from traditional Greek sausages in submerged fermentation. Appl. Biochem. Biotechnol. 2014, 172, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Winkler, U.K.; Stuckmann, M. Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J. Bacteriol. 1979, 138, 663–670. [Google Scholar] [PubMed]
- Sigma-Aldrich. Universal Protease Activity Assay: Casein as a Substrate. 2013. Available online: http://www.sigmaaldrich.com/life-science/learning-center/life-science-video/universal-protease.html. (accessed on 1 July 2017).
- Reichard, U.; Buttner, S.; Eifferst, H.; Staib, F.; Ruchel, R. Purification and characterisation of an extracellular serine proteinase from Aspergillus fumigatus and its detection in tissue. J. Med. Microbiol. 1990, 33, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Kunst, A.; Draeger, B.; Ziegenhom, J. Colorimetric methods with glucose oxidase. Methods Enzym. Anal. 1986, 6, 178–185. [Google Scholar]
- Gardini, F.; Suzzi, G.; Lombardi, A.; Galgano, F.; Crudele, M.A.; Andrighetto, C.; Schirone, M.; Totalo, R. A survey of yeast in traditional sausages of southern Italy. FEMS Yeast Res. 2001, 1, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Mendoça, R.C.S.; Gouvêa, D.M.; Hungaro, H.M.; Sodré, A.F.; Querol-Simon, A. Dynamics of the yeast flora in artisanal country style and industrial dry cured sausage (yeast in fermented sausage). Food Control 2013, 29, 143–148. [Google Scholar] [CrossRef]
- Durá, M.A.; Flores, M.; Toldrá, F. Effect of growth phase and dry-cured sausage processing conditions on Debaryomyces spp. generation of volatile compounds from branched-chain amino acids. Food Chem. 2004, 86, 391–399. [Google Scholar] [CrossRef]
- Bussamara, R.; Fuentefria, A.M.; de Oliveira, E.S.; Broetto, L.; Simcikowa, M.; Valente, P.; Schrank, A.; Vainstein Henning, M. Isolation of a lipase-secreting yeast for enzyme production in a pilot-plant scale batch fermentation. Biores. Technol. 2010, 101, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Kar, T.; Delvigne, F.; Masson, M.; Destain, J.; Thonart, P. Investigation of the effect of different extracellular factors on the lipase production by Yarrowia lipolytica on the basis of a scale-down approach. J. Ind. Microbiol. Biotechnol. 2008, 35, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Hiol, A.; Jonzo, M.D.; Bruet, D.; Comeu, L. Production, purification and characterization of an extracellular lipase from Mucor hiemalis f. hiemalis. Enzyme Microb. Technol. 1999, 25, 80–87. [Google Scholar] [CrossRef]
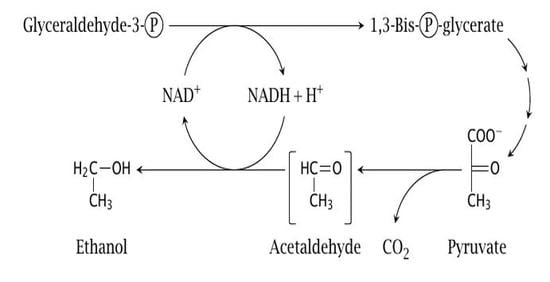
- Kiers, J.; Zeeman, A.M.; Luttic, C.; Thiele, C.; Castrillo, J.I.; Steensma, H.Y.; van Dijken, J.P.; Pronk, J.T. Regulation of alcoholic fermentation in batch and chemostat cultures of Kluyveromyces lactis CBS 2359. Yeast 1998, 14, 459–469. [Google Scholar] [CrossRef]

| Parameter | 2 L Bioreactor 20 g/L Glucose | 10 L Bioreactor 60 g/L Glucose |
|---|---|---|
| Maximum Specific Growth Rate (h−1) | 0.17 ± 0.02 | 0.12 ± 0.03 |
| Maximum Specific Consumption Rate of Glucose (mmol/g/h) | 1.9 ± 0.2 | 1.3 ± 0.3 |
| Specific Production Rate of | ||
| Lipolytic activity (U/g/h) | 7 ± 0.8 | 51 ± 1.3 |
| Ethanol (mmol/g/h) | 0.12 ± 0.02 | 0.09 ± 0.03 |
| Acetate (mmol/g/h) | 0.14 ± 0.04 | 0.11 ± 0.01 |
| Glycerol (mmol/g/h) | 0.11 ± 0.03 | 0.10 ± 0.02 |
| Yield on Glucose (g/g) | ||
| Biomass | 0.43 ± 0.14 | 0.24 ± 0.02 |
| Ethanol | 0.03 ± 0.002 | 0.05 ± 0.01 |
| Acetate | 0.05 ± 0.01 | 0.04 ± 0.02 |
| Glycerol | 0.02 ± 0.009 | 0.02 ± 0.001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papagianni, M.; Papamichael, E.M. A Pichia anomala Strain (P. anomala M1) Isolated from Traditional Greek Sausage is an Effective Producer of Extracellular Lipolytic Enzyme in Submerged Fermentation. Fermentation 2017, 3, 43. https://doi.org/10.3390/fermentation3030043
Papagianni M, Papamichael EM. A Pichia anomala Strain (P. anomala M1) Isolated from Traditional Greek Sausage is an Effective Producer of Extracellular Lipolytic Enzyme in Submerged Fermentation. Fermentation. 2017; 3(3):43. https://doi.org/10.3390/fermentation3030043
Chicago/Turabian StylePapagianni, Maria, and Emmanuel M. Papamichael. 2017. "A Pichia anomala Strain (P. anomala M1) Isolated from Traditional Greek Sausage is an Effective Producer of Extracellular Lipolytic Enzyme in Submerged Fermentation" Fermentation 3, no. 3: 43. https://doi.org/10.3390/fermentation3030043