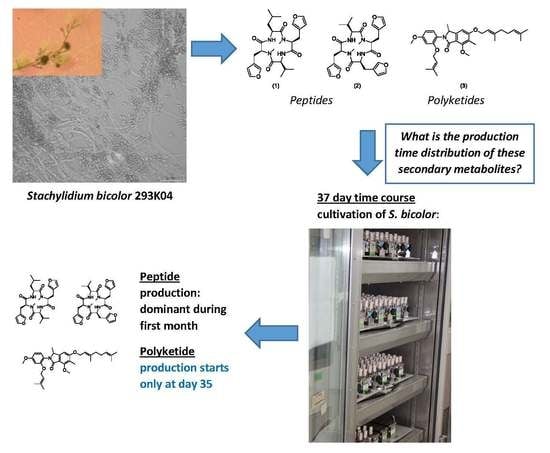
Time-Dependent Production of the Bioactive Peptides Endolides A and B and the Polyketide Mariline A from the Sponge-Derived Fungus Stachylidium bicolor 293K04
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Material
2.2. General Experimental Procedures
2.2.1. Cultivation Procedures
2.2.2. Extraction of Cultures
2.3. Ultraviolet-Liquid Chromatography-Mass Spectrometry (UV-LC-MS) Analysis
2.3.1. Low Resolution UV-LC-MS
2.3.2. High Resolution UV-LC-MS
2.4. Methods for Time Course Measurements
2.5. Radial Growth and Optimum Temperature Growth Measurements in Solid Medium
2.6. Calibration Curves
3. Results
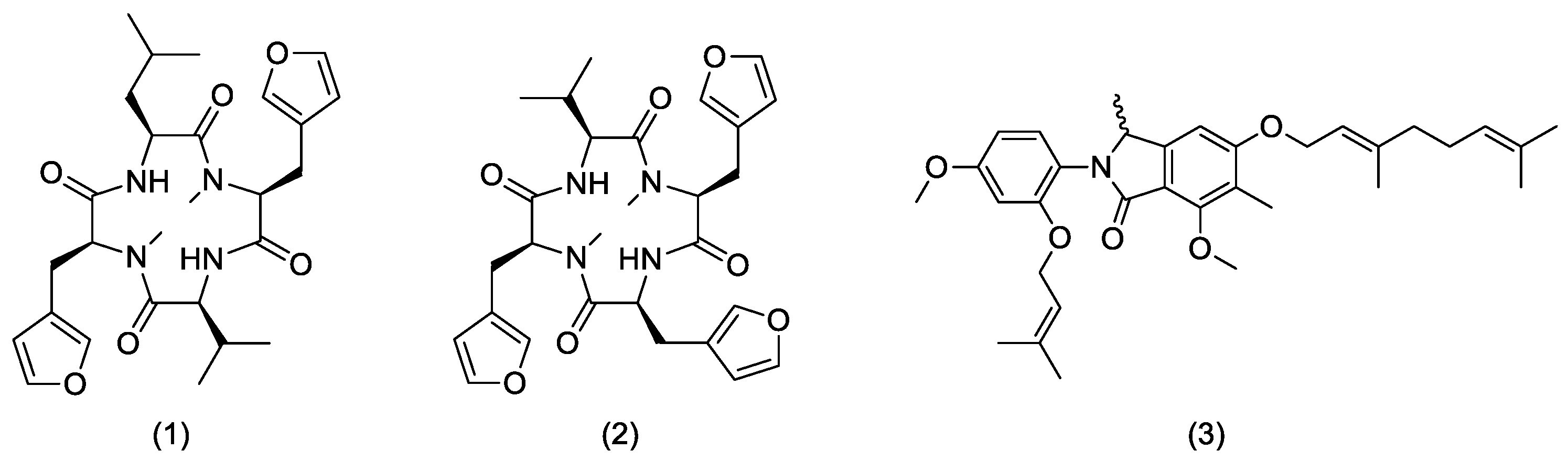
3.1. Production of Endolides by S. bicolor 293K04 during Short Incubation Times
3.2. Time-Course Study for S. bicolor 293K04
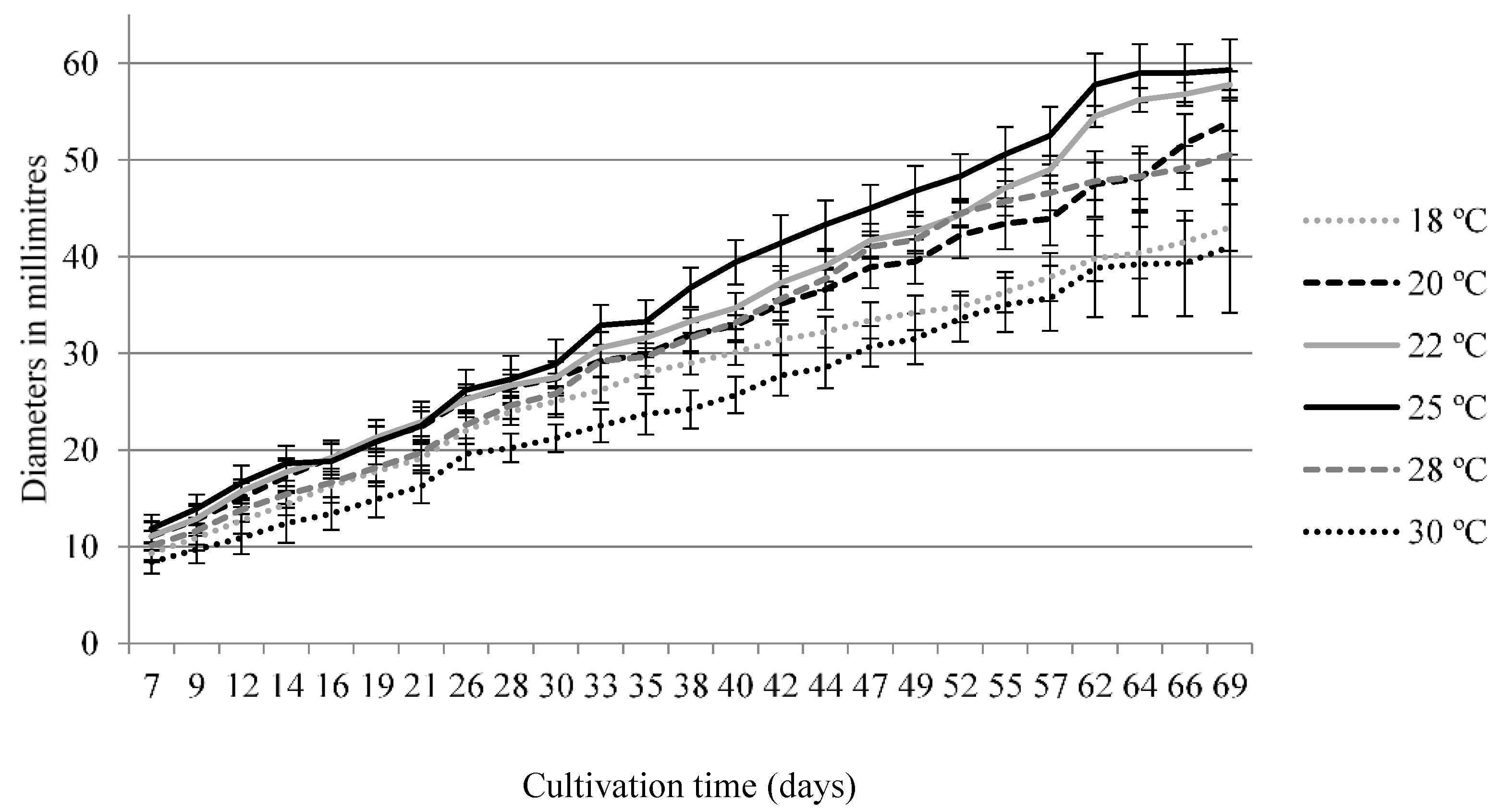
3.2.1. Optimal Temperature for Growth of S. bicolor
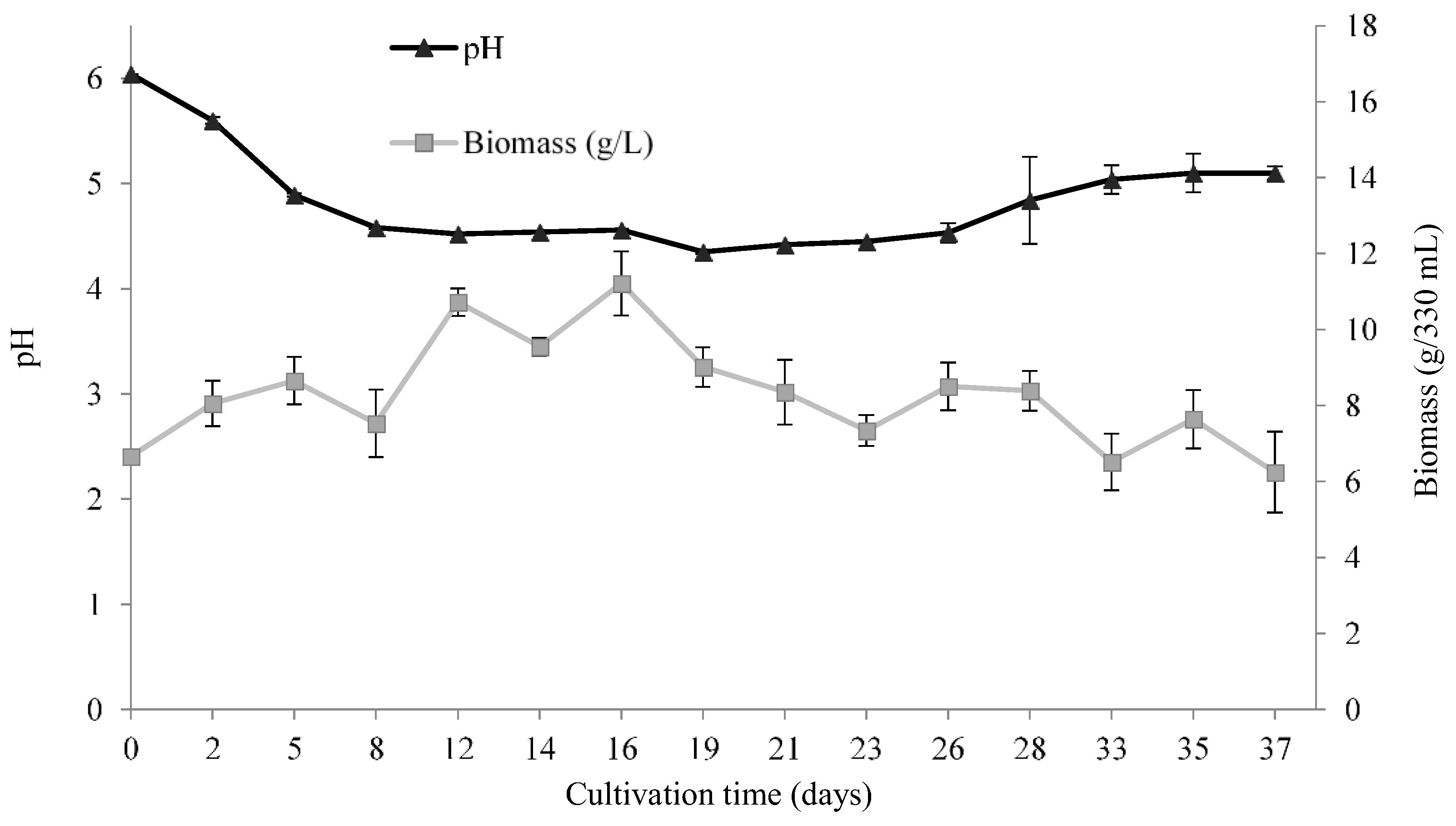
3.2.2. pH and Biomass Variation during the Time Course
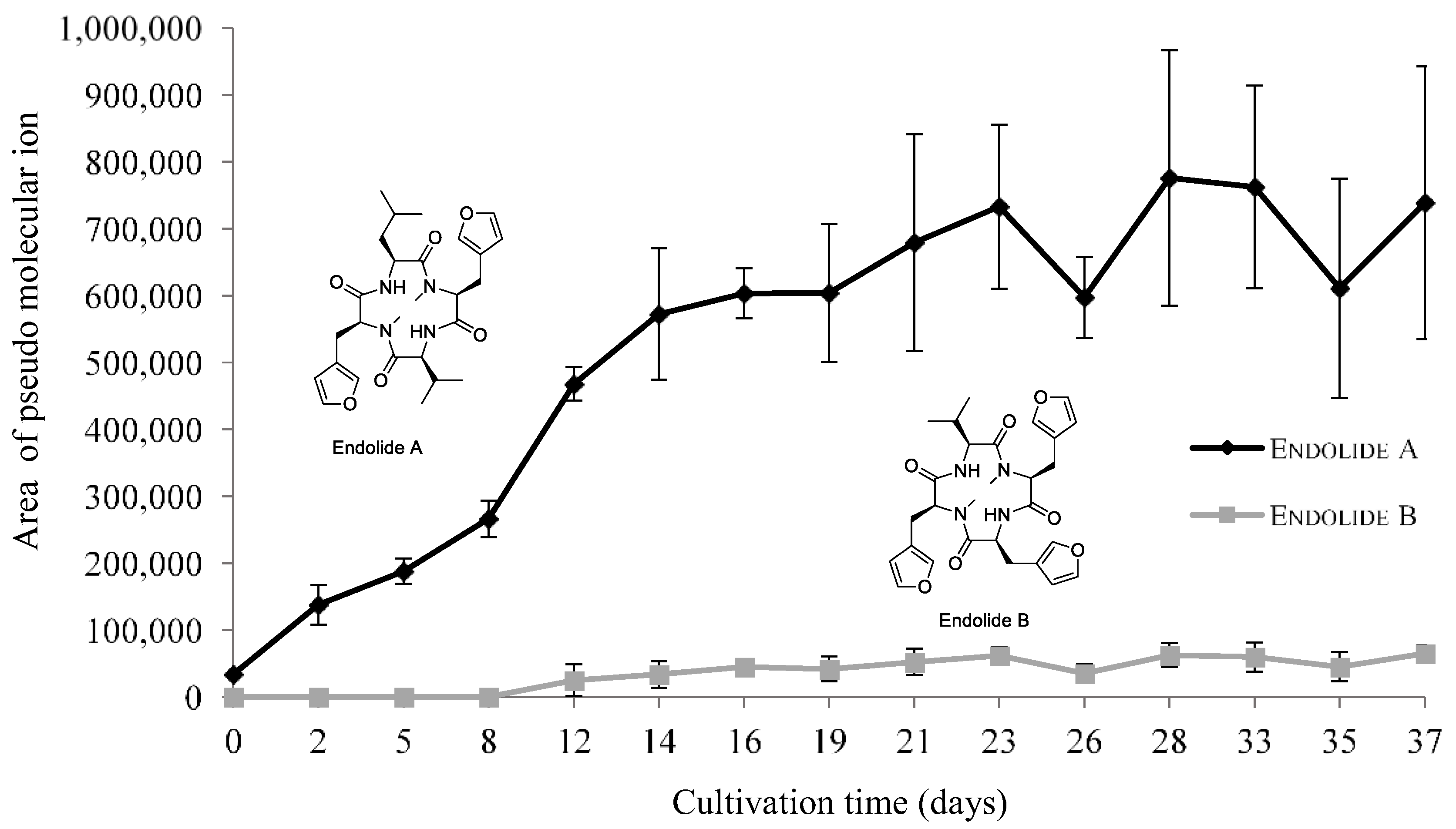
3.2.3. Endolides Production
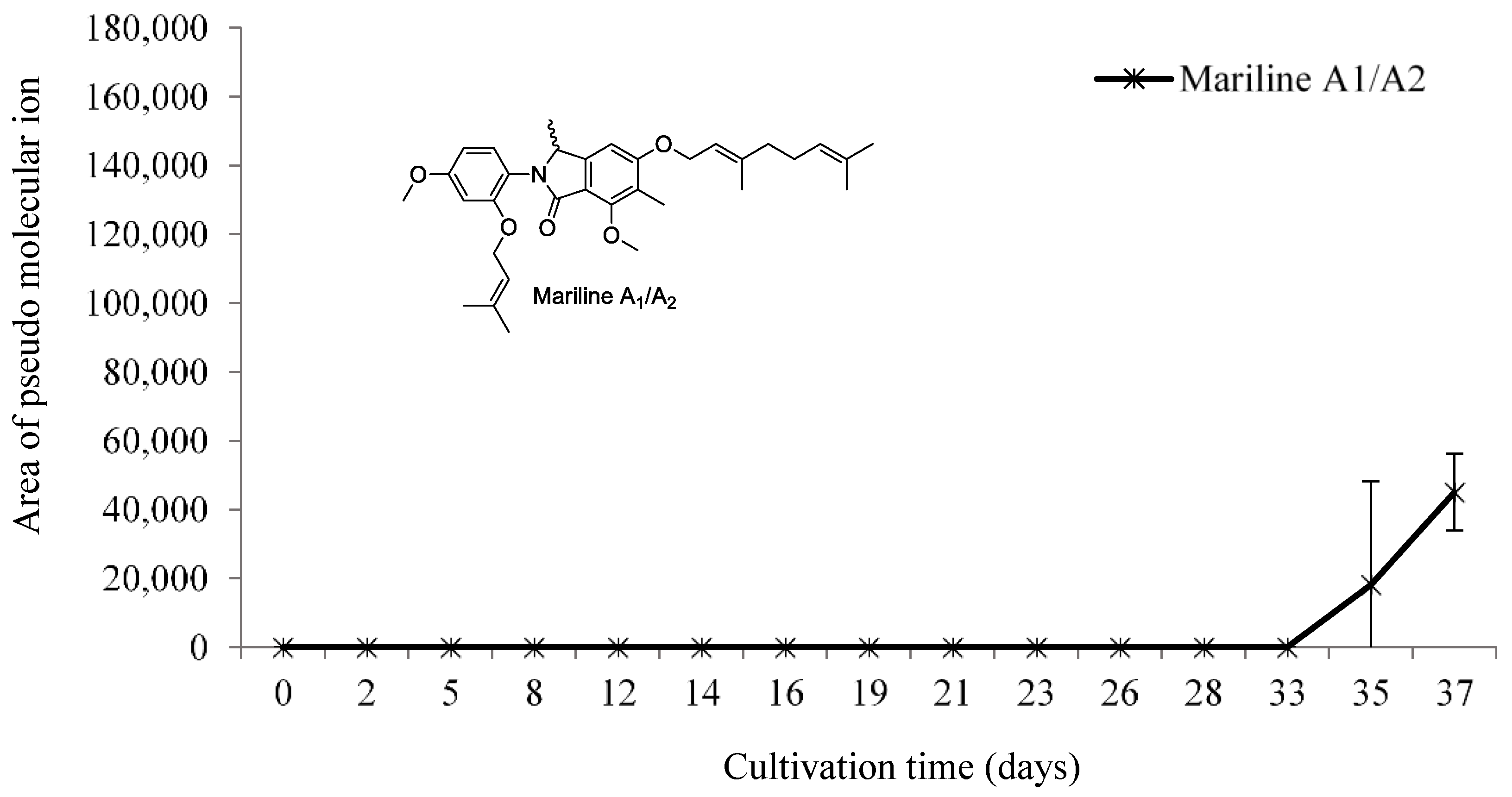
3.2.4. Mariline A1/A2 Production
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brakhage, A.A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.R.; Nielsen, J.B.; Klitgaard, A.; Petersen, L.M.; Zachariasen, M.; Hansen, T.J.; Blicher, L.H.; Gotfredsen, C.H.; Larsen, T.O.; Nielsen, K.F.; et al. Accurate prediction of secondary metabolite gene clusters in filamentous fungi. Proc. Natl. Acad. Sci. USA 2013, 110, E99–E107. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; El Maddah, F.; Kehraus, S.; Schnakenburg, G.; Konig, G.M. Endolides A and B, vasopressin and serotonin-receptor interacting n-methylated peptides from the sponge-derived fungus Stachylidium sp. Org. Lett. 2016, 18, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Potgieter, M.; Steyn, P.S.; van Heerden, F.R.; van Rooyen, P.H.; Wessels, P.L. Conformational analysis of the cyclic peptide rhizonin a in solution and crystalline state. Tetrahedron 1989, 45, 2337–2350. [Google Scholar] [CrossRef]
- Steyn, P.; Tuinman, A.A.; van Heerden, F.R.; van Rooyen, P.H.; Wessels, P.L.; Rabie, C.J. The isolation, structure, and absolute configuration of the mycotoxin, rhizonin a, a novel cyclic heptapeptide containing N-methyl-3-(3-furyl)alanine, produced by rhizopus microsporus. J. Chem. Soc. Chem. Commun. 1983, 47–49. [Google Scholar] [CrossRef]
- Partida-Martinez, L.P.; de Looss, C.F.; Ishida, K.; Ishida, M.; Roth, M.; Buder, K.; Hertweck, C. Rhizonin, the first mycotoxin isolated from the zygomycota, is not a fungal metabolite but is produced by bacterial endosymbionts. Appl. Environ. Microbiol. 2007, 73, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.S.; Wang, J.D.; Wang, X.J.; Zhang, J. Bingchamides A and B, two novel cyclic pentapeptides from the streptomyces bingchenggensis: Fermentation, isolation, structure elucidation and biological properties. J. Antibiot. 2009, 62, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Eguereva, E.; Kehraus, S.; Konig, G.M. Unprecedented polyketides from a marine sponge-associated stachylidium sp. J. Nat. Prod. 2013, 76, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Hemberger, Y.; Schmitt, S.M.; Bouhired, S.; Natesan, L.; Kehraus, S.; Dimas, K.; Gutschow, M.; Bringmann, G.; Konig, G.M. Marilines A-C: Novel phthalimidines from the sponge-derived fungus Stachylidium sp. Chemistry 2012, 18, 8827–8834. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Kehraus, S.; Prudencio, M.; Konig, G.M. Marilones a-c, phthalides from the sponge-derived fungus Stachylidium sp. Beilstein J. Org. Chem. 2011, 7, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Part, N.; Bouhired, S.; Kehraus, S.; Konig, G.M. Stachylines A-D from the sponge-derived fungus Stachylidium sp. J. Nat. Prod. 2011, 74, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Hahn-Hägerdal, B.; Karhumaa, K.; Larsson, C.U.; Gorwa-Grauslund, M.; Görgens, J.; van Zyl, W.H. Role of cultivation media in the development of yeast strains for large scale industrial use. Microb. Cell Fact. 2005, 4, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Menéndez, V.; Asensio, F.; Moreno, C.; de Pedro, N.; Monteiro, M.C.; de la Cruz, M.; Vicente, F.; Bills, G.F.; Reyes, F.; Genilloud, O.; et al. Assessing the effects of adsorptive polymeric resin additions on fungal secondary metabolite chemical diversity. Mycology 2014, 5, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Harms, H.; Orlikova, B.; Ji, S.; Nesaei-Mosaferan, D.; Konig, G.M.; Diederich, M. Epipolythiodiketopiperazines from the marine derived fungus dichotomomyces cejpii with nf-kappab inhibitory potential. Mar. Drugs 2015, 13, 4949–4966. [Google Scholar] [CrossRef] [PubMed]
- Serrano, R.; González-Menéndez, V.; Rodríguez, L.; Martín, J.; Tormo, J.R.; Genilloud, O. Co-culturing of fungal strains against botrytis cinerea as a model for the induction of chemical diversity and therapeutic agents. Front. Microbiol. 2017, 8, 649. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz, M.; Martin, J.; Gonzalez-Menendez, V.; Perez-Victoria, I.; Moreno, C.; Tormo, J.R.; El Aouad, N.; Guarro, J.; Vicente, F.; Reyes, F.; et al. Chemical and physical modulation of antibiotic activity in emericella species. Chem. Biodivers. 2012, 9, 1095–1113. [Google Scholar] [CrossRef] [PubMed]
- El Aouad, N.; Perez-Moreno, G.; Sanchez, P.; Cantizani, J.; Ortiz-Lopez, F.J.; Martin, J.; Gonzalez-Menendez, V.; Ruiz-Perez, L.M.; Gonzalez-Pacanowska, D.; Vicente, F.; et al. Lasionectrin, a naphthopyrone from a lasionectria sp. J. Nat. Prod. 2012, 75, 1228–1230. [Google Scholar] [CrossRef] [PubMed]
- Basilio, A.; Justice, M.; Harris, G.; Bills, G.; Collado, J.; de la Cruz, M.; Diez, M.T.; Hernandez, P.; Liberator, P.; Nielsen kahn, J.; et al. The discovery of moriniafungin, a novel sordarin derivative produced by morinia pestalozzioides. Bioorg. Med. Chem. 2006, 14, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Moreira Neto, S.L.; Matheus, D.R.; Machado, K.M.G. Influence of ph on the growth, laccase activity and rbbr decolorization by tropical basidiomycetes. Braz. Arch. Biol. Technol. 2009, 52, 1075–1082. [Google Scholar] [CrossRef]
- Yamanaka, R.; Soares, C.F.; Matheus, D.R.; Machado, K.M.G. Lignolytic enzymes produced by trametes villosa ccb176 under different culture conditions. Braz. J. Microbiol. 2008, 39, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Khalil, Z.G.; Kalansuriya, P.; Capon, R.J. Lipopolysaccharide (lps) stimulation of fungal secondary metabolism. Mycology 2014, 5, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Khalil, Z.G.; Huang, X.C.; Raju, R.; Piggott, A.M.; Capon, R.J. Shornephine a: Structure, chemical stability, and p-glycoprotein inhibitory properties of a rare diketomorpholine from an australian marine-derived aspergillus sp. J. Org. Chem. 2014, 79, 8700–8705. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.H. Fungal Physiology, 2nd ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 1995. [Google Scholar]
- Kettering, M.; Sterner, O.; Anke, T. Antibiotics in the chemical communication of fungi. Z. Naturforsch. C J. Biosci. 2004, 59, 816–823. [Google Scholar] [CrossRef]
- Weber, D.; Sterner, O.; Anke, T. Mollisianitrile, a new antibiotic from mollisia sp. A59–96. Z. Naturforsch. C J. Biosci. 2007, 62, 567–570. [Google Scholar] [CrossRef]
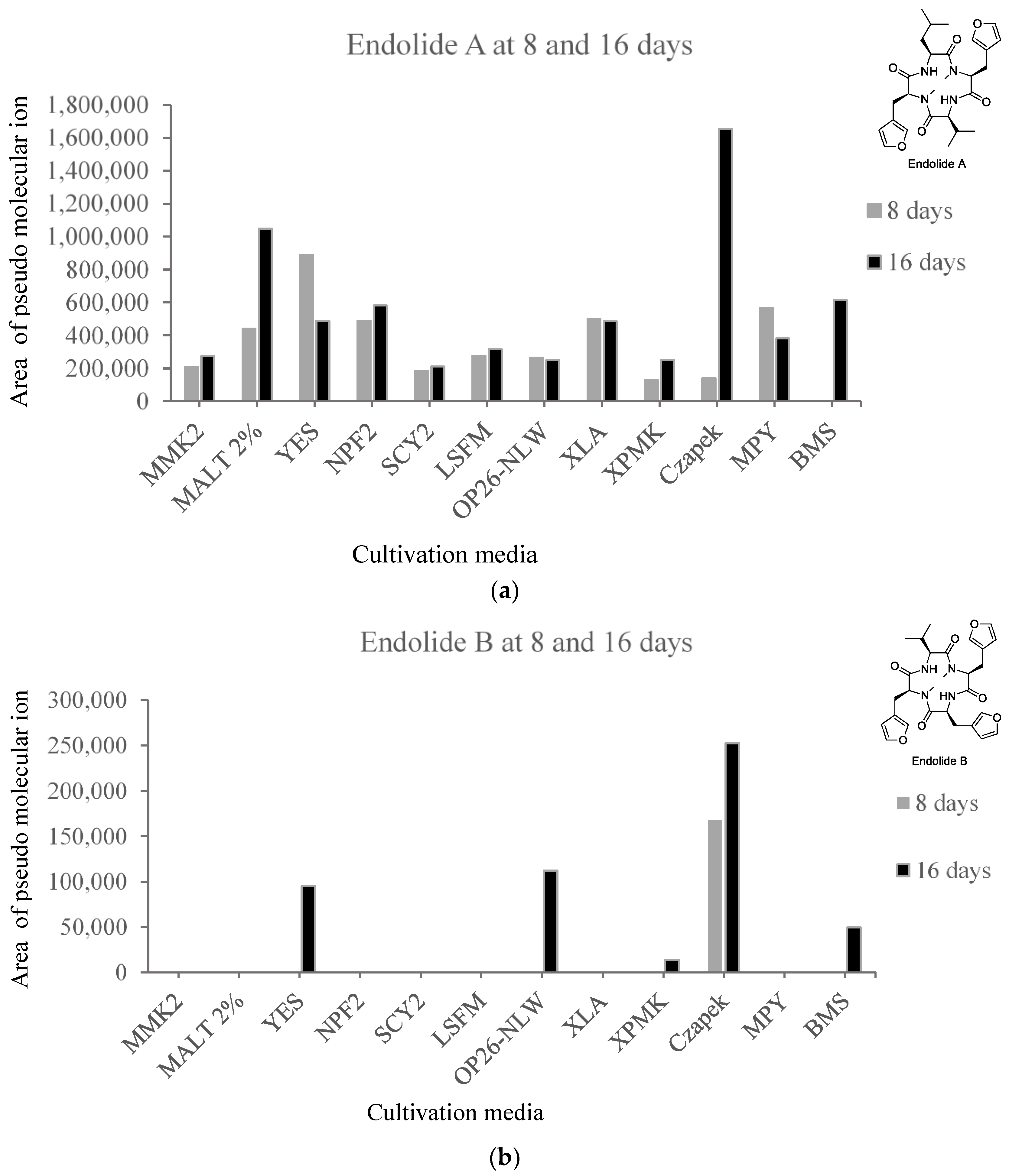
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, C.; Bills, G.; González-Menéndez, V.; Martin, J.; Tormo, J.R.; Genilloud, O. Time-Dependent Production of the Bioactive Peptides Endolides A and B and the Polyketide Mariline A from the Sponge-Derived Fungus Stachylidium bicolor 293K04. Fermentation 2017, 3, 45. https://doi.org/10.3390/fermentation3030045
Almeida C, Bills G, González-Menéndez V, Martin J, Tormo JR, Genilloud O. Time-Dependent Production of the Bioactive Peptides Endolides A and B and the Polyketide Mariline A from the Sponge-Derived Fungus Stachylidium bicolor 293K04. Fermentation. 2017; 3(3):45. https://doi.org/10.3390/fermentation3030045
Chicago/Turabian StyleAlmeida, Celso, Gerald Bills, Víctor González-Menéndez, Jesús Martin, José R. Tormo, and Olga Genilloud. 2017. "Time-Dependent Production of the Bioactive Peptides Endolides A and B and the Polyketide Mariline A from the Sponge-Derived Fungus Stachylidium bicolor 293K04" Fermentation 3, no. 3: 45. https://doi.org/10.3390/fermentation3030045