Fermentation Assisted by Pulsed Electric Field and Ultrasound: A Review
Abstract
:1. Introduction
2. Ultrasound
2.1. Basic Concepts of Ultrasound
2.2. Physical and Chemical Effects of Ultrasound
2.3. Production of Biomolecules from Fermented Foods
3. Pulsed Electric Fields
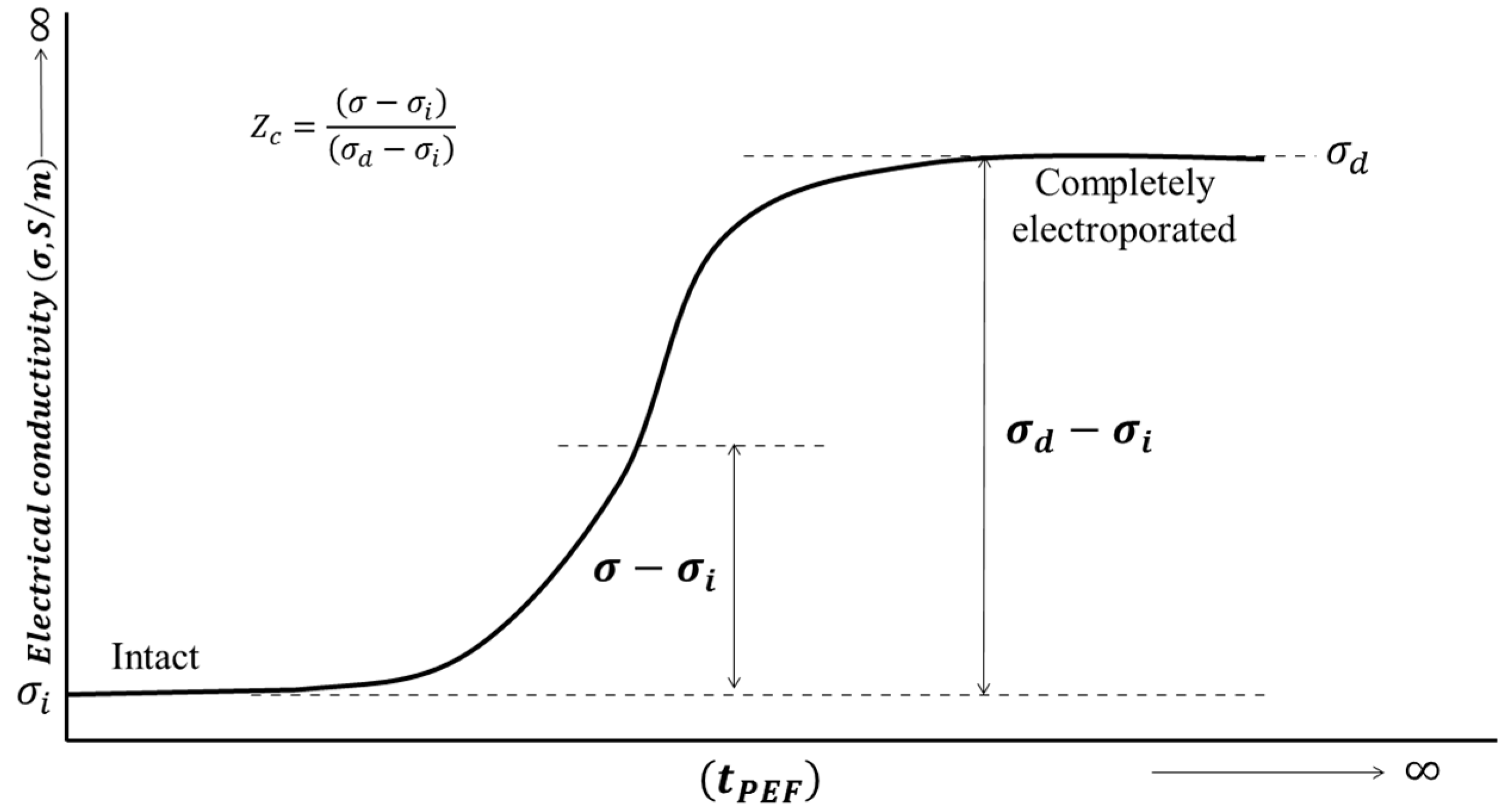
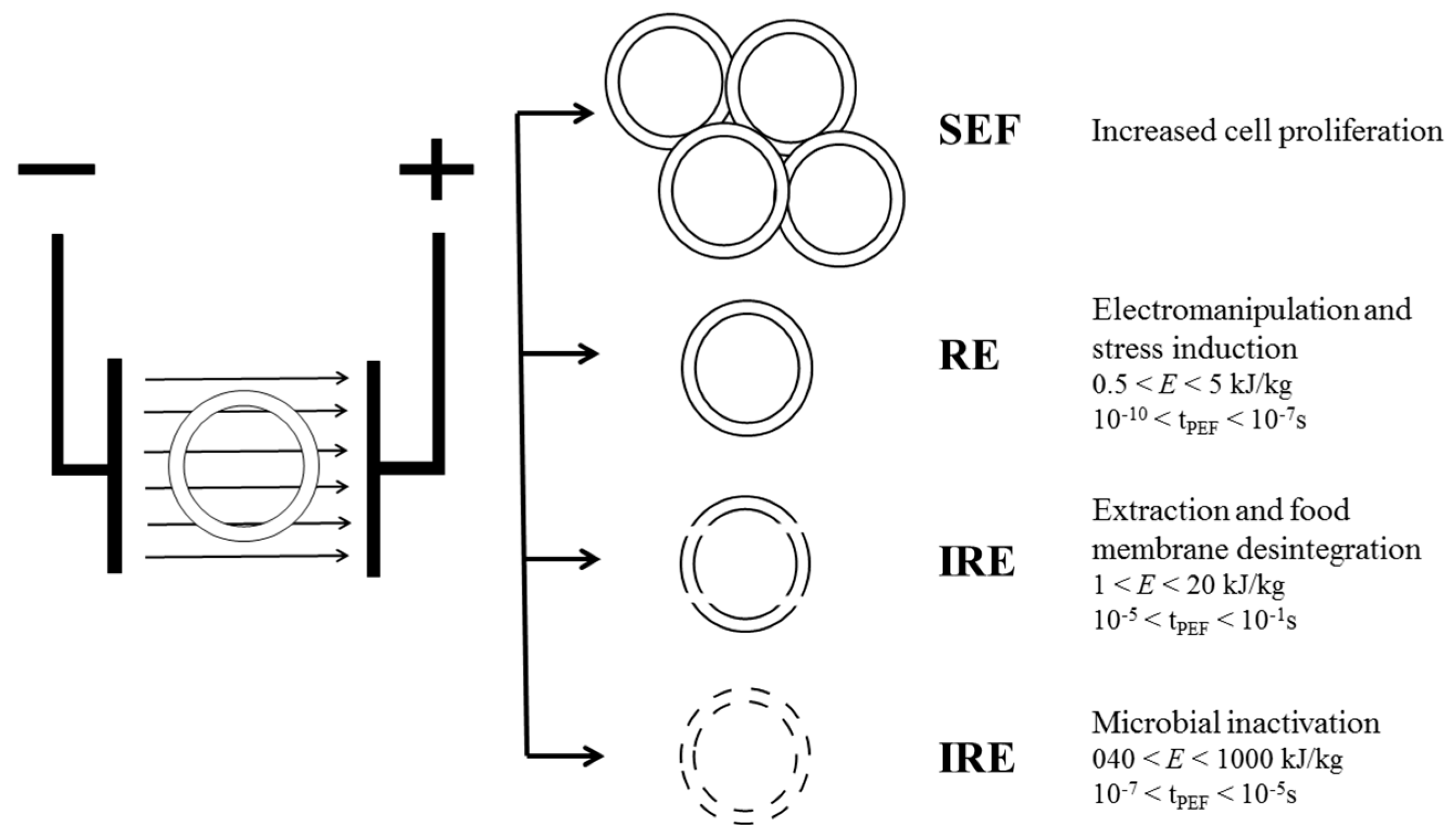
3.1. Basic Concepts of PEF on Fermentation Process
3.2. Factors Affecting Pulsed Electric Field Treatments
3.3. Production of Biomolecules from Fermented Foods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cutzu, R.; Bardi, L. Production of bioethanol from agricultural wastes using residual thermal energy of a cogeneration plant in the distillation phase. Fermentation 2017, 3, 24. [Google Scholar] [CrossRef]
- Limón, R.I.; Peñas, E.; Torino, M.I.; Martínez-Villaluenga, C.; Dueñas, M.; Frias, J. Fermentation enhances the content of bioactive compounds in kidney bean extracts. Food Chem. 2015, 172, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Mañas, P.; Pagán, R. Microbial inactivation by new technologies of food preservation. J. Appl. Microbiol. 2005, 98, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.N.; Vicente, A.A. Environmental impact of novel thermal and non-thermal technologies in food processing. Food Res. Int. 2010, 43, 1936–1943. [Google Scholar] [CrossRef] [Green Version]
- Ojha, K.S.; Mason, T.J.; O’Donnell, C.P.; Kerry, J.P.; Tiwari, B.K. Ultrasound technology for food fermentation applications. Ultrason. Sonochem. 2017, 34, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Wedzik, M. Effect of ultrasound treatment on microstructure, colour and carotenoid content in fresh and dried carrot tissue. Appl. Acoust. 2016, 103, 163–171. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M. K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.J.; Riera, E.; Vercet, A.; Lopez-Buesa, P. Application of ultrasound. In Emerging Technologies for Food Processing, 1st ed.; Sun, D.W., Ed.; Elsevier Academic Press: London, UK, 2005; pp. 323–351. ISBN 0-12-676757-2. [Google Scholar]
- Esclapez, M.D.; Garcia-Perez, J.V.; Mulet, A.; Cárcel, J.A. Ultrasound-assisted extraction of natural products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- O’Donnell, C.P.; Tiwari, B.K.; Bourke, P.; Cullen, P.J. Effect of ultrasonic processing on food enzymes of industrial importance. Trends Food Sci. Technol. 2010, 21, 358–367. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Bhaskaracharya, R.; Kentish, S.; Lee, J.; Palmer, M.; Zisu, B. The ultrasonic processing of dairy products—An overview. Dairy Sci. Technol. 2010, 90, 147–168. [Google Scholar] [CrossRef]
- Awad, T.S.; Moharram, H.A.; Shaltout, O.E.; Asker, D.; Youssef, M.M. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 2012, 48, 410–427. [Google Scholar] [CrossRef]
- Nguyen, T.M.P.; Lee, Y.K.; Zhou, W. Stimulating fermentative activities of bifidobacteria in milk by high intensity ultrasound. Int. Dairy J. 2009, 19, 410–416. [Google Scholar] [CrossRef]
- Nguyen, T.M.P.; Lee, Y.K.; Zhou, W. Effect of high intensity ultrasound on carbohydrate metabolism of bifidobacteria in milk fermentation. Food Chem. 2012, 130, 866–874. [Google Scholar] [CrossRef]
- Yeo, S.-K.; Liong, M.-T. Effect of ultrasound on the growth of probiotics and bioconversion of isoflvones in prebiotic-supplemented soymilk. J. Agric. Food Chem. 2011, 59, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Barukčić, I.; Jakopović, K.L.; Herceg, Z.; Karlović, S.; Božanić, R. Influence of high intensity ultrasound on microbial reduction, physico-chemical characteristics and fermentation of sweet whey. Innov. Food Sci. Emerg. Technol. 2015, 27, 94–101. [Google Scholar] [CrossRef]
- Lentacker, I.; De Cock, I.; Deckers, R.; De Smedt, S.C.; Moonen, C.T. Understanding ultrasound induced sonoporation: Definitions and underlying mechanisms. Adv. Drug Deliv. Rev. 2014, 72, 49–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Gu, N.; Chen, D.; Xi, X.; Zhang, D.; Li, Y.; Wu, J. Experimental study on cell self-sealing during sonoporation. J. Control. Release 2008, 131, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.-K.; Liong, M.-T. Effects and applications of sub-lethal ultrasound, electroporation and UV radiations in bioprocessing. Ann. Microbiol. 2012, 63, 813–824. [Google Scholar] [CrossRef]
- Yeo, S.-K.; Liong, M.-T. Effect of ultrasound on bioconversion of isoflavones and probiotic properties of parent organisms and subsequent passages of Lactobacillus. LWT—Food Sci. Technol. 2013, 51, 289–295. [Google Scholar] [CrossRef]
- Guzel, B.H.; Arroyo, C.; Condón, S.; Pagán, R.; Bayindirli, A.; Alpas, H. Inactivation of Escherichia coli and Listeria monocytogenes by ultrasonic waves under pressure at nonlethal (manosonication) and lethal temperatures (manothermosonication) in acidic fruit juices. Food Bioprocess Technol. 2014, 7, 1701–1712. [Google Scholar] [CrossRef]
- Terefe, N.S.; Buckow, R.; Versteeg, C. Quality-related enzymes in plant-based products: Effects of novel food-processing technologies, Part 3: Ultrasonic processing. Crit. Rev. Food Sci. Nutr. 2015, 55, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Barteri, M.; Diociaiuti, M.; Pala, A.; Rotella, S. Low frequency ultrasound induces aggregation of porcine fumarase by free radicals production. Biophys. Chem. 2004, 111, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Guiseppi-Elie, A.; Choi, S.H.; Geckeler, K.E. Ultrasonic processing of enzymes: Effect on enzymatic activity of glucose oxidase. J. Mol. Catal. B Enzym. 2009, 58, 118–123. [Google Scholar] [CrossRef]
- Ozuna, C.; Paniagua-Martínez, I.; Castaño-Tostado, E.; Ozimek, L.; Amaya-Llano, S.L. Innovative applications of high-intensity ultrasound in the development of functional food ingredients: Production of protein hydrolysates and bioactive peptides. Food Res. Int. 2015, 77, 685–696. [Google Scholar] [CrossRef]
- Rice, S.; Koziel, J.A.; Dharmadhikari, M.; Fennell, A. Evaluation of tannins and anthocyanins in marquette, frontenac, and St. croix cold-hardy grape cultivars. Fermentation 2017, 3, 47. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Z.; Sun, D.-W. Experimental and modeling studies of ultrasound-assisted release of phenolics from oak chips into model wine. Ultrason. Sonochem. 2014, 21, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Katina, K.; Liukkonen, K.H.; Kaukovirta-Norja, A.; Adlercreutz, H.; Heinonen, S.M.; Lampi, A.M.; Pihlava, J.M.; Poutanen, K. Fermentation-induced changes in the nutritional value of native or germinated rye. J. Cereal Sci. 2007, 46, 348–355. [Google Scholar] [CrossRef]
- Fernández-Orozco, R.; Frías, J.; Zielinski, H.; Muñoz, R.; Piskula, M.K.; Kozlowska, H.; Vidal-Valverde, C. Evaluation of bioprocesses to improve the antioxidant properties of chickpeas. LWT–Food Sci. Technol. 2009, 42, 885–892. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Galván-D’Alessandro, L.; Vandendriessche, P.; Chollet, S. Effect of germination and fermentation process on the antioxidant compounds of quinoa seeds. Plant Foods Hum. Nutr. 2016, 71, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, D.J.; Karabarbounis, A.; Margaritis, L.H. Mechanism for action of electromagnetic fields on cells. Biochem. Biophys. Res. Commun. 2002, 298, 95–102. [Google Scholar] [CrossRef]
- Lebovka, N.; Vorobiev, E. Techniques and procedures to detect electroporation in food cellular tissues. In Proceedings of the School on Applications of Pulsed Electric Fields for Food Processing, Zaragoza, Spain, 20–23 January 2014; pp. 46–47. [Google Scholar]
- Koubaa, M.; Roselló-Soto, E.; Šic Žlabur, J.; Režek Jambrak, A.; Brnčić, M.; Grimi, N.; Boussetta, N.; Barba, F.J. Current and new insights in the sustainable and green recovery of nutritionally valuable compounds from Stevia rebaudiana bertoni. J. Agric. Food Chem. 2015, 63, 6835–6846. [Google Scholar] [CrossRef] [PubMed]
- Vorobiev, E.; Lebovka, N. Enhanced extraction from solid foods and biosuspensions by pulsed electrical energy. Food Eng. Rev. 2010, 2, 95–108. [Google Scholar] [CrossRef]
- Jaeger, H. PEF Process Performance Analysis. In Proceedings of the School on Applications of Pulsed Electric Fields for Food Processing, Zaragoza, Spain, 20–23 January 2014; pp. 87–93. [Google Scholar]
- Puértolas, E.; Luengo, E.; Alvarez, I.; Raso, J. Improving mass transfer to soften tissues by pulsed electric fields: Fundamentals and applications. Annu. Rev. Food Sci. Technol. 2012, 3, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, S.; Castle, G.S.P.; Margaritis, A. Effects of high electric field pulses on Lactobacillus brevis at elevated temperatures. In Proceedings of the Conference on Electrical Insulation and Dielectric Phenomena, Dearborn, MI, USA, 18–21 October 1992; pp. 674–681. [Google Scholar] [CrossRef]
- Pliquett, U. Bioimpedance: A Review for Food Processing. Food Eng. Rev. 2010, 2, 74–94. [Google Scholar] [CrossRef]
- Lebovka, N.I.; Bazhal, M.I.; Vorobiev, E. Estimation of characteristic damage time of food materials in pulsed electric fields. J. Food Eng. 2002, 4, 337–346. [Google Scholar] [CrossRef]
- Ben Ammar, J.; Lanoisellée, J.L.; Lebovka, N.I.; Van Hecke, E.; Vorobiev, E. Impact of a pulsed electric field on damage of plant tissues: Effects of cell size and tissue electrical conductivity. J. Food Sci. 2011, 76, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Shamtsyan, M. The influence of electric field on microbial growth. J. Eur. Hyg. Eng. Des. Group 2012, 1, 88–92. [Google Scholar]
- Zhang, Q.H.; Qin, B.L.; Barbosa-Cánovas, G.V.; Swanson, B.G. Inactivation of Escherichia coli for food pasteurization by highstrength pulsed electric fields. J. Food Process. Preserv. 1995, 19, 103–118. [Google Scholar] [CrossRef]
- Qin, B.L.; Pothakamury, U.R.; Barbosa-Cánovas, G.V.; Swanson, B.G. Nonthermal pasteurization of liquid foods using high-intensity pulsed electric field. Crit. Rev. Food Sci. Nutr. 1996, 36, 603–627. [Google Scholar] [CrossRef] [PubMed]
- Pothakamury, U.R.; Vega, H.; Zhang, Q.; Barbosa-Canovas, G.V.; Swanson, B.G. Effect of growth stage and processing temperature on the inactivation of E. coli by pulsed electric fields. J. Food Prot. 1996, 59, 1167–1171. [Google Scholar] [CrossRef]
- Alvarez, I.; Palop, J.R.A.; Sala, F.J. Influence of different factors on the inactivation of Salmonella senftenberg by pulsed electric fields. Int. J. Food Microbiol. 2000, 55, 143–146. [Google Scholar] [CrossRef]
- Harrison, S.L.; Barbosa-Cánovas, G.V.; Swanson, B.G. Saccharomyces cerevisiae structural changes induced by pulsed electric field treatment. LWT—Food Sci. Technol. 1997, 30, 236–240. [Google Scholar] [CrossRef]
- Heinz, V.; Toepfl, S.; Knorr, D. Impact of temperature on lethality and energy efficiency of apple juice pasteurization by pulsed electric fields treatment. Innov. Food Sci. Emerg. 2003, 4, 167–175. [Google Scholar] [CrossRef]
- Toepfl, S.; Heinz, V.; Knorr, D. High intensity pulsed electric fields applied for food preservation. Chem. Eng. Process. 2007, 46, 537–546. [Google Scholar] [CrossRef]
- Barbosa-Cánovas, G.V.; Gongora-Nieto, M.M.; Pothakamury, U.R.; Swanson, B.G. Preservation of Foods with Pulsed Electric Fields, 1st ed.; Academic Press: London, UK, 1999; ISBN 0-12-078149-2. [Google Scholar]
- Frey, W.; Flickinger, B.; Berghoefer, T.; Eing, C.; Liu, Q.; Nick, P. Electropermeabilization versus nsPEF-stimulation—Pulsed electric fields can stimulate the growth of plants and fungi. In Proceedings of the 10th International Conference of the European Bioelectromagnetic Association, Roma, Italy, 21–24 February 2011. [Google Scholar]
- Fiedler, U.; Gröbner, U.; Berg, H. Electrostimulation of yeast proliferation. Bioelectrochem. Bioenerg. 1995, 38, 423–425. [Google Scholar] [CrossRef]
- Hunt, R.W.; Zavalin, A.; Bhatnagar, A.; Chinnasamy, S.; Das, K.C. Electromagnetic biostimulation of living cultures for biotechnology, biofuel and bioenergy applications. Int. J. Mol. Sci. 2009, 10, 4515–4558. [Google Scholar] [CrossRef] [PubMed]
- Araujo, O.Q.F.; Coelho, M.A.Z.; Margarit, I.C.P.; Vaz-Junior, C.A.; Rocha-Leão, M.H.M. Electrical stimulation of Saccharomyces cerevisiae cultures. Braz. J. Microbiol. 2004, 35, 97–103. [Google Scholar] [CrossRef]
- Rocha-Leão, M.H.; Coelho, M.A.Z.; Margarit, I.P.; Catarino, A.A.; Gandelman, R.A.; Vaz, C.A.; Araújo, O.Q.F. Biochemical cell responses to electrical stress stimulation. In Proceedings of the 2nd Mercosur Congress on Chemical Engineering 4th Mercosur Congress on Process Systems Engineering, Rio de Janeiro, Brazil, 14–18 August 2005. [Google Scholar]
- Tanino, T.; Sato, S.; Oshige, M.; Ohshima, T. Analysis of the stress response of yeast Saccharomyces cerevisiae toward pulsed electric field. J. Electrostat. 2012, 70, 212–216. [Google Scholar] [CrossRef]
- Fologea, D.; Vassu-Dimovb, T.; Stoicab, I.; Csutakb, O.; Radua, M. Increase of Saccharomyces cerevisiae plating efficiency after treatment with bipolar electric pulses. Bioelectrochem. Bioenerg. 1998, 46, 285–287. [Google Scholar] [CrossRef]
- Shynkaryk, M.V.; Lebovka, N.I.; Lanoisellé, J.-L.; Nonus, M.; Bedel-Clotour, C.; Vorobiev, E. Electrically-assisted extraction of bio-products using high pressure disruption of yeast cells (Saccharomyces cerevisiae). J. Food Eng. 2009, 92, 189–195. [Google Scholar] [CrossRef]
- Loghavi, L.; Sastry, S.K.; Yousef, A.E. Effect of moderate electric field frequency on growth kinetics and metabolic activity of Lactobacillus acidophilus. Biotechnol. Progr. 2008, 24, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lebovka, N.I.; Vorobiev, E. Impact of electric pulse treatment on selective extraction of intracellular compounds from Saccharomyces cerevisiae yeasts. Food Bioprocess Technol. 2013, 6, 576–584. [Google Scholar] [CrossRef]
- Mahnic-Kalamiza, S.; Vorobiev, E.; Miklavcic, D. Electroporation in food processing and biorefinery. J. Membr. Biol. 2014, 247, 1279–1304. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galván-D’Alessandro, L.; Carciochi, R.A. Fermentation Assisted by Pulsed Electric Field and Ultrasound: A Review. Fermentation 2018, 4, 1. https://doi.org/10.3390/fermentation4010001
Galván-D’Alessandro L, Carciochi RA. Fermentation Assisted by Pulsed Electric Field and Ultrasound: A Review. Fermentation. 2018; 4(1):1. https://doi.org/10.3390/fermentation4010001
Chicago/Turabian StyleGalván-D’Alessandro, Leandro, and Ramiro Ariel Carciochi. 2018. "Fermentation Assisted by Pulsed Electric Field and Ultrasound: A Review" Fermentation 4, no. 1: 1. https://doi.org/10.3390/fermentation4010001





