Adding Flavor to Beverages with Non-Conventional Yeasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Media
2.2. Fermentation Conditions
2.3. Sample Preparation for Solid-Phase MicroExtraction–Gas Chromatography/Mass Spectrometry (SPME-GC/MS) Analysis
2.4. Analytical Methods for GC/MS
2.5. Multivariate Data Analysis
3. Results
3.1. Strain Selection and Identification of Representative Isolates
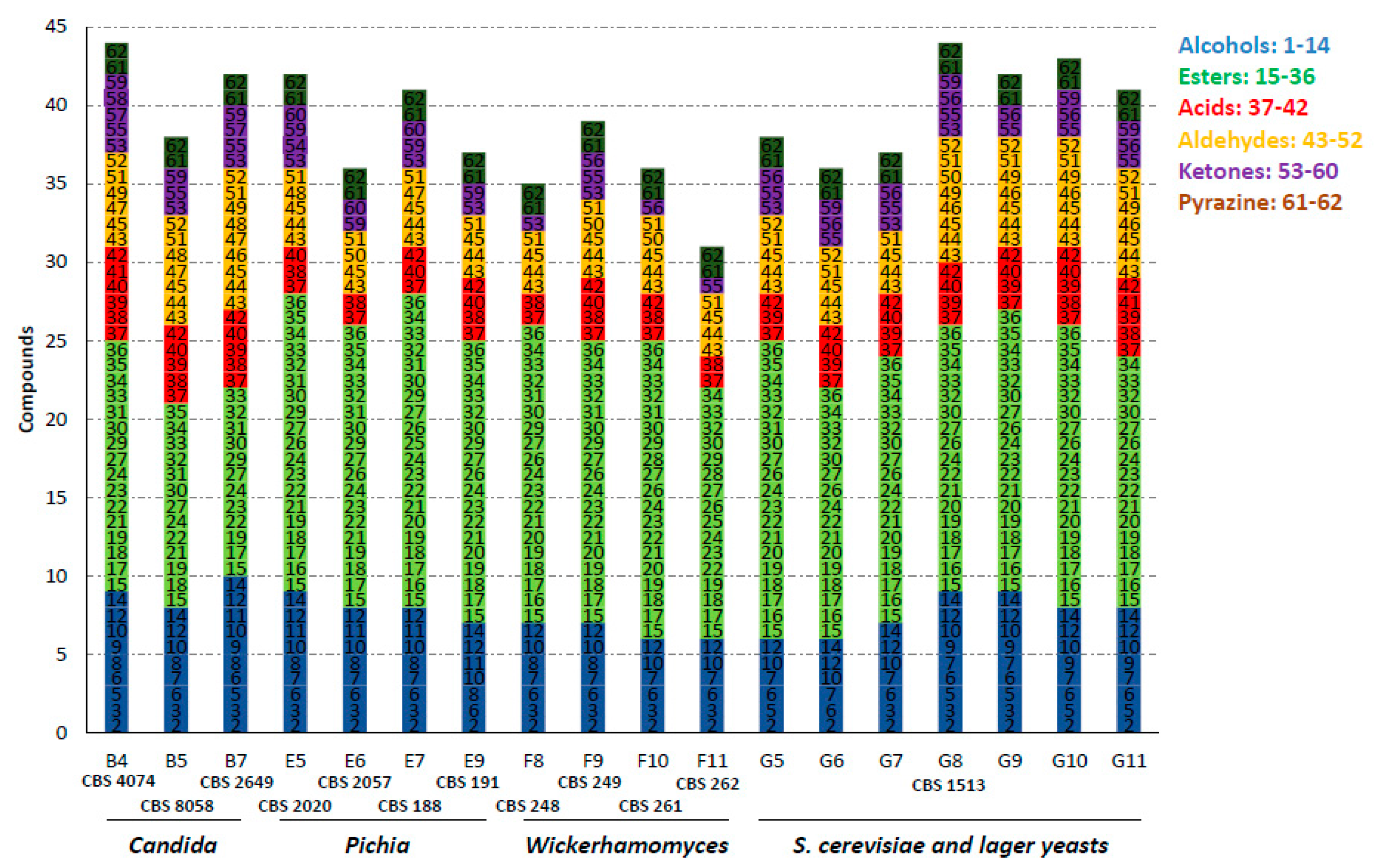
3.2. Aroma Profiles of Fermentations
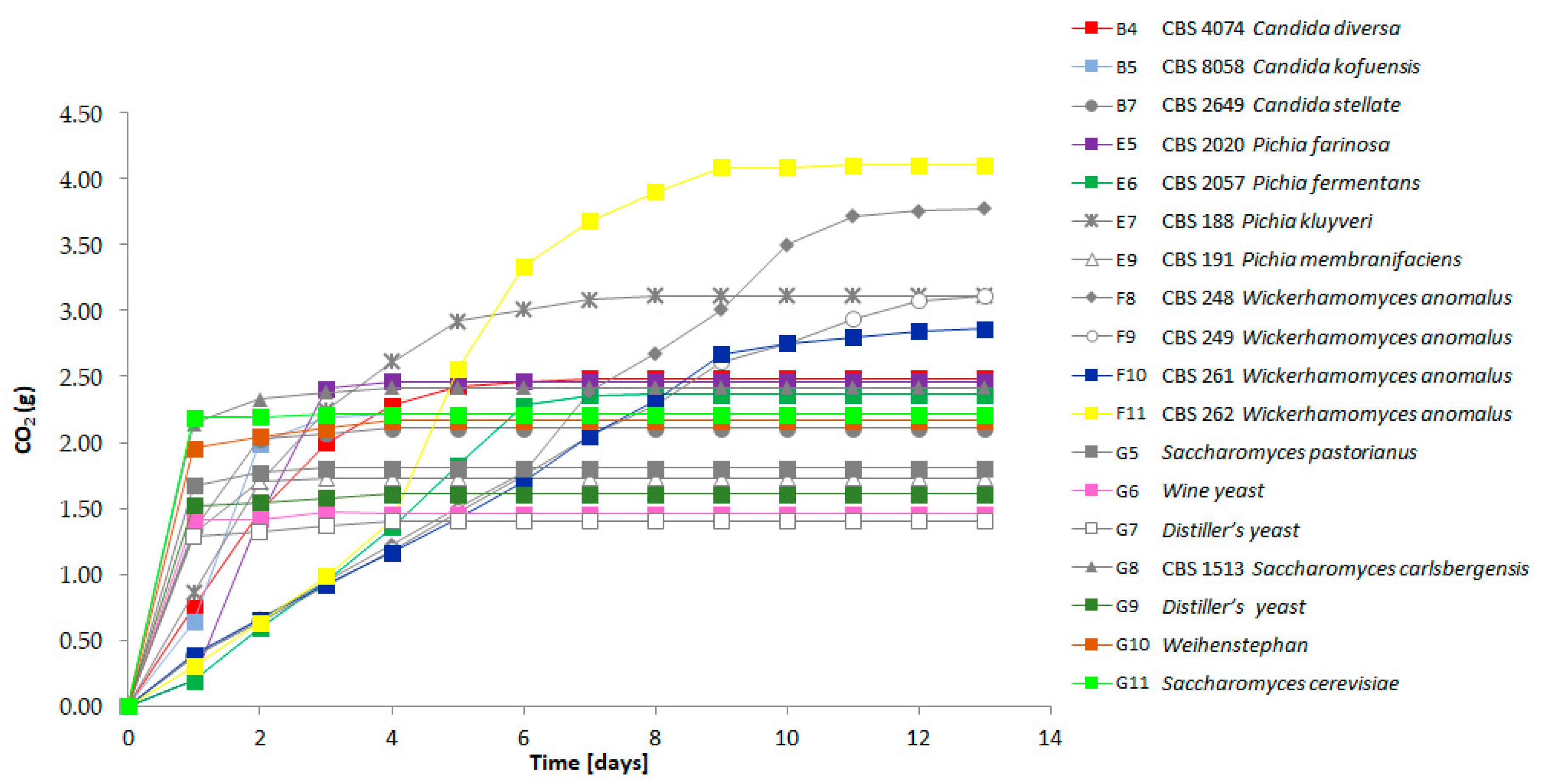
3.3. Fermentation Performance
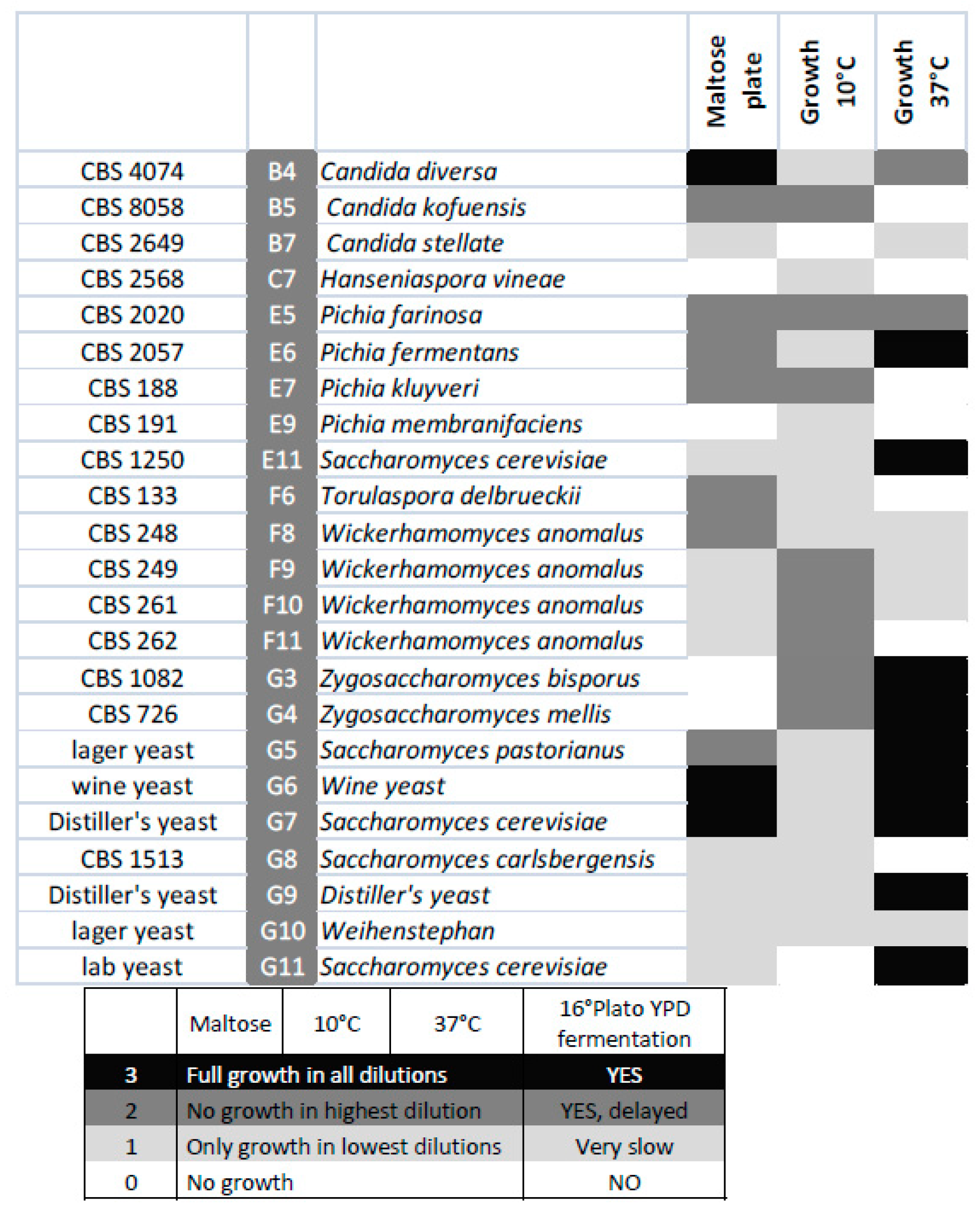
3.4. Growth on Other Carbon Sources and Temperatures
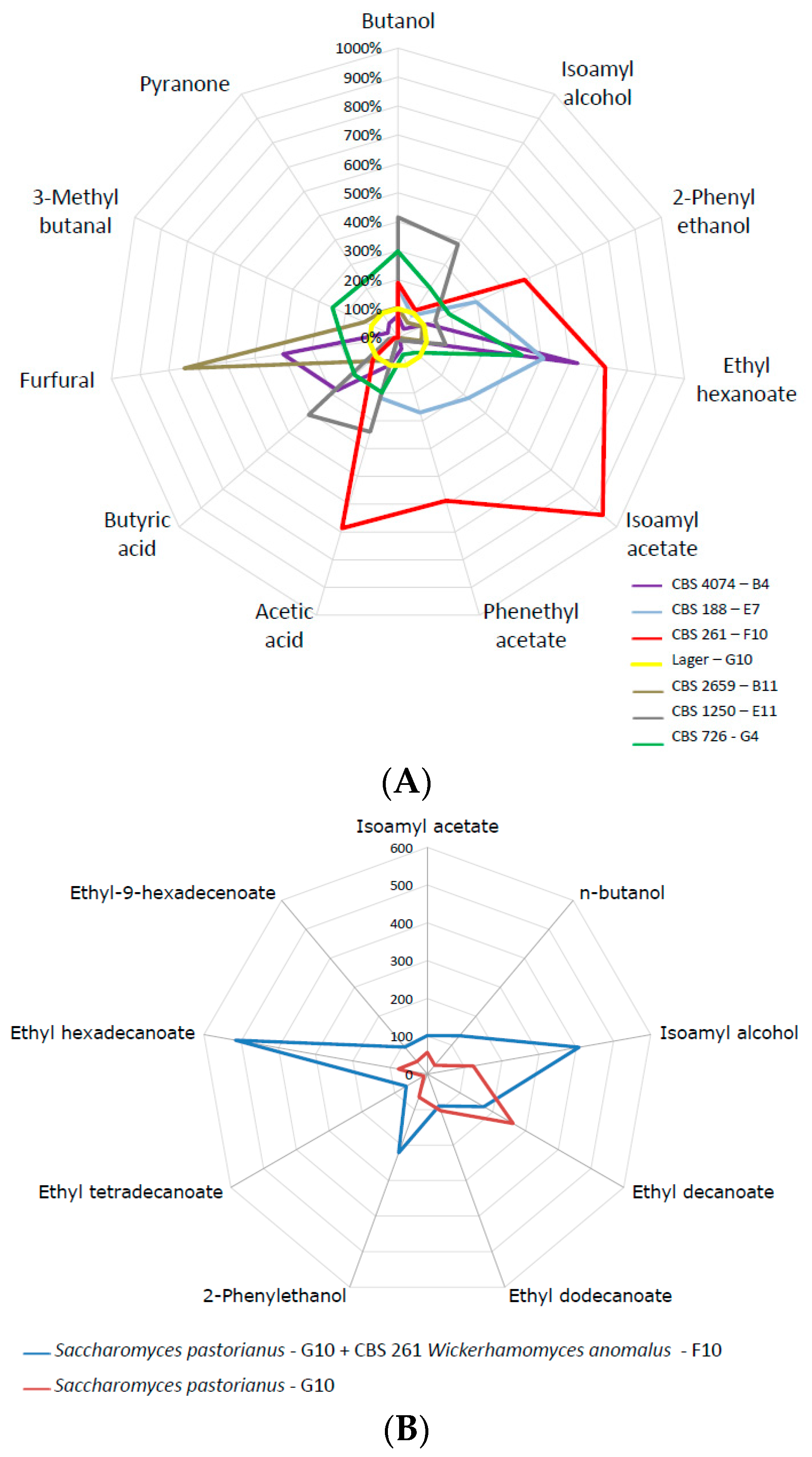
3.5. Utilization of Mixed Fermentations for Flavor Improvement
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dunn, B.; Sherlock, G. Reconstruction of the genome origins and evolution of the hybrid lager yeast Saccharomyces pastorianus. Genome Res. 2008, 18, 1610–1623. [Google Scholar] [CrossRef] [PubMed]
- Libkind, D.; Hittinger, C.T.; Valerio, E.; Goncalves, C.; Dover, J.; Johnston, M.; Goncalves, P.; Sampaio, J.P. Microbe domestication and the identification of the wild genetic stock of lager-brewing yeast. Proc. Natl. Acad. Sci. USA 2011, 108, 14539–14544. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Kanamori, T.; Itoh, T.; Kodama, Y.; Rainieri, S.; Nakamura, N.; Shimonaga, T.; Hattori, M.; Ashikari, T. Genome sequence of the lager brewing yeast, an interspecies hybrid. DNA Res. 2009, 16, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Hesselbart, A.; Wendland, J. Genome sequence of Saccharomyces carlsbergensis, the world's first pure culture lager yeast. G3 (Bethesda) 2014, 4, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Spitaels, F.; Wieme, A.D.; Janssens, M.; Aerts, M.; Daniel, H.M.; Van Landschoot, A.; De Vuyst, L.; Vandamme, P. The microbial diversity of traditional spontaneously fermented lambic beer. PLoS ONE 2014, 9, e95384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mertens, S.; Steensels, J.; Saels, V.; De Rouck, G.; Aerts, G.; Verstrepen, K.J. A large set of newly created interspecific Saccharomyces hybrids increases aromatic diversity in lager beers. Appl. Environ. Microbiol. 2015, 81, 8202–8214. [Google Scholar] [CrossRef] [PubMed]
- Verstrepen, K.J.; Derdelinckx, G.; Dufour, J.P.; Winderickx, J.; Thevelein, J.M.; Pretorius, I.S.; Delvaux, F.R. Flavor-active esters: Adding fruitiness to beer. J. Biosci. Bioeng. 2003, 96, 110–118. [Google Scholar] [CrossRef]
- Pires, E.J.; Teixeira, J.A.; Branyik, T.; Vicente, A.A. Yeast: The soul of beer's aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderhaegen, B.; Neven, H.; Coghe, S.; Verstrepen, K.J.; Verachtert, H.; Derdelinckx, G. Evolution of chemical and sensory properties during aging of top-fermented beer. J. Agric. Food Chem. 2003, 51, 6782–6790. [Google Scholar] [CrossRef] [PubMed]
- Duong, C.T.; Strack, L.; Futschik, M.; Katou, Y.; Nakao, Y.; Fujimura, T.; Shirahige, K.; Kodama, Y.; Nevoigt, E. Identification of sc-type ilv6 as a target to reduce diacetyl formation in lager brewers' yeast. Metab. Eng. 2011, 13, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Gamero, A.; Quintilla, R.; Groenewald, M.; Alkema, W.; Boekhout, T.; Hazelwood, L. High-throughput screening of a large collection of non-conventional yeasts reveals their potential for aroma formation in food fermentation. Food Microbiol. 2016, 60, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Varela, C. The impact of non-saccharomyces yeasts in the production of alcoholic beverages. Appl. Microbiol. Biotechnol. 2016, 100, 9861–9874. [Google Scholar] [CrossRef] [PubMed]
- Steensels, J.; Verstrepen, K.J. Taming wild yeast: Potential of conventional and nonconventional yeasts in industrial fermentations. Annu. Rev. Microbiol. 2014, 68, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Viana, F.; Gil, J.V.; Genoves, S.; Valles, S.; Manzanares, P. Rational selection of non-saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol. 2008, 25, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Sadoudi, M.; Tourdot-Marechal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacon, J.J.; Ballester, J.; Vichi, S.; Guerin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast-yeast interactions revealed by aromatic profile analysis of sauvignon blanc wine fermented by single or co-culture of non-saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Comitini, F.; Ciani, M. Torulaspora delbrueckii contribution in mixed brewing fermentations with different saccharomyces cerevisiae strains. Int. J. Food Microbiol. 2017, 259, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ravasio, D.; Wendland, J.; Walther, A. Major contribution of the Ehrlich pathway for 2-phenylethanol/rose flavor production in Ashbya gossypii. FEMS Yeast Res. 2014, 14, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Hazelwood, L.A.; Daran, J.M.; van Maris, A.J.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, H. Flor yeasts of Saccharomyces cerevisiae—Their ecology, genetics and metabolism. Int. J. Food Microbiol. 2013, 167, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Viana, F.; Belloch, C.; Valles, S.; Manzanares, P. Monitoring a mixed starter of Hanseniaspora vineae-Saccharomyces cerevisiae in natural must: Impact on 2-phenylethyl acetate production. Int. J. Food Microbiol. 2011, 151, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H.M.; Moons, M.C.; Huret, S.; Vrancken, G.; De Vuyst, L. Wickerhamomyces anomalus in the sourdough microbial ecosystem. Antonie Van Leeuwenhoek 2011, 99, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Di Cagno, R.; Rizzello, C.G.; Nionelli, L.; Edema, M.O.; Gobbetti, M. Utilization of African grains for sourdough bread making. J. Food Sci. 2011, 76, M329–M335. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.T.; Lim, S.L.; Tan, H.W. Growth inhibition of Candida species by Wickerhamomyces anomalus mycocin and a lactone compound of Aureobasidium pullulans. BMC Complement. Altern. Med. 2014, 14, 439. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Schifferdecker, A.J.; Gamero, A.; Compagno, C.; Boekhout, T.; Piskur, J.; Knecht, W. Kazachstania gamospora and Wickerhamomyces subpelliculosus: Two alternative baker's yeasts in the modern bakery. Int. J. Food Microbiol. 2017, 250, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Crafack, M.; Mikkelsen, M.B.; Saerens, S.; Knudsen, M.; Blennow, A.; Lowor, S.; Takrama, J.; Swiegers, J.H.; Petersen, G.B.; Heimdal, H.; et al. Influencing cocoa flavour using Pichia kluyveri and Kluyveromyces marxianus in a defined mixed starter culture for cocoa fermentation. Int. J. Food Microbiol. 2013, 167, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.P.; Etschmann, M.M.; Schrader, J.; de Billerbeck, G.M. Cell factory applications of the yeast Kluyveromyces marxianus for the biotechnological production of natural flavour and fragrance molecules. Yeast 2015, 32, 3–16. [Google Scholar] [PubMed]
- Urit, T.; Li, M.; Bley, T.; Loser, C. Growth of Kluyveromyces marxianus and formation of ethyl acetate depending on temperature. Appl. Microbiol. Biotechnol. 2013, 97, 10359–10371. [Google Scholar] [CrossRef] [PubMed]
- Lobs, A.K.; Lin, J.L.; Cook, M.; Wheeldon, I. High throughput, colorimetric screening of microbial ester biosynthesis reveals high ethyl acetate production from Kluyveromyces marxianus on c5, c6, and c12 carbon sources. Biotechnol. J. 2016, 11, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Masneuf-Pomarede, I.; Bely, M.; Marullo, P.; Albertin, W. The genetics of non-conventional wine yeasts: Current knowledge and future challenges. Front. Microbiol. 2015, 6, 1563. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Terenius, O.; Rajarao, G.K.; Nagahama, K.; Nordenhem, H.; Nordlander, G.; Borg-Karlson, A.K. Chemodiversity and biodiversity of fungi associated with the pine weevil Hylobius abietis. Fungal Biol. 2015, 119, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, K.; Konstanzer, V.; Rajarao, G.K.; Terenius, O.; Seriot, L.; Nordenhem, H.; Nordlander, G.; Borg-Karlson, A.K. Antifeedants produced by bacteria associated with the gut of the pine weevil Hylobius abietis. Microb. Ecol. 2017, 74, 177–184. [Google Scholar] [CrossRef] [PubMed]
- James, S.A.; Barriga, E.J.; Barahona, P.P.; Harrington, T.C.; Lee, C.F.; Bond, C.J.; Roberts, I.N. Wickerhamomyces arborarius f.A.; sp. Nov., an ascomycetous yeast species found in arboreal habitats on three different continents. Int. J. Syst. Evol. Microbiol. 2014, 64, 1057–1061. [Google Scholar] [CrossRef] [PubMed]


| Position | Strain Number | Taxon Name | Substrate of Isolation | Origin |
|---|---|---|---|---|
| B2 | CBS 10151 | Candida alimentaria | Cured ham | Norway |
| B3 | CBS 12367 | Candida alimentaria | Brie Régalou cheese | |
| B4 | CBS 4074 | Candida diversa | Grape must | Japan |
| B5 | CBS 8058 | Candida kofuensis | Berries of Vitis coignetiae | Japan |
| B6 | CBS 1760 | Candida versatilis | Pickling vat with 22% brine | USA |
| B7 | CBS 2649 | Candida stellate | Grape juice | France |
| B8 | CBS 6936 | Clavispora lusitaniae | Citrus essence | Israel |
| B9 | CBS 4373 | Debaryomyces fabryi | Dry white wine | South Africa |
| B10 | CBS 767 | Debaryomyces hansenii | ||
| B11 | CBS 2659 | Debaryomyces subglobosus | Apple | Italy |
| C2 | CBS 8139 | Dekkera anomala | Netherlands | |
| C3 | CBS 615.84 | Geotrichum candidum | Brie cheese | France |
| C4 | CBS 95 | Hanseniaspora guilliermondii | Fermenting bottled tomatoes | Netherlands |
| C5 | CBS 6783 | Hanseniaspora occidentalis var. citrica | Orange juice | Italy |
| C6 | CBS 2585 | Hanseniaspora uvarum | Sour dough | Portugal |
| C7 | CBS 2568 | Hanseniaspora vineae | Drosophila persimilis (fruit fly) | |
| C8 | CBS 2352 | Hyphopichia burtonii | Pollen, carried by wild bees | |
| C9 | CBS 4311 | Kazachstania servazii | Soil | Finland |
| C10 | CBS 3019 | Kazachstania spencerorum | Soil | South Africa |
| C11 | CBS 2186 | Kazachstania transvaalensis | Soil | South Africa |
| D2 | CBS 398 | Kazachstania unispora | ||
| D3 | CBS 7775 | Kluyveromyces aestuarii | Neotredo reynei (shipworm) | Brazil |
| D4 | CBS 8530 | Kluyveromyces dobzhanskii | Drosophila sp. | Canada |
| D5 | CBS 1557 | Kluyveromyces marxianus | Stracchino cheese | Italy |
| D6 | CBS 7005 | Lachancea fermentati | Alpechín | Spain |
| D7 | CBS 3082 | Lachancea kluyveri | Drosophila pinicola (fruit fly) | |
| D8 | CBS 7703 | Lachancea waltii | Either fruit or leaf of fruit tree | |
| D9 | CBS 5833 | Metschnikowia pulcherrima | Berries of Vitis labrusca (Concord grapes) | USA |
| D10 | CBS 2030 | Meyerozyma guilliermondii | Insect frass on Ulmus americana (elm tree) | USA |
| D11 | CBS 8417 | Meyerozyma guilliermondii | Brine bath in cheese factory | Netherlands |
| E2 | CBS 7720 | Nakaseomyces bacillisporus | Exudate of Quercus emoryi (Emory oak) | USA |
| E3 | CBS 2170 | Nakaseomyces delphensis | Sugary deposit on dried figs | South Africa |
| E4 | CBS 8255 | Pichia | Kefyr | |
| E5 | CBS 2020 | Pichia farinosa | Fermenting cacao | Trinidad and Tobago |
| E6 | CBS 2057 | Pichia fermentans | Brewers yeast | |
| E7 | CBS 188 | Pichia kluyveri | Olives | |
| E8 | CBS 5147 | Pichia kudriavzevii | Fruit juice | |
| E9 | CBS 191 | Pichia membranifaciens | Wine | Italy |
| E10 | CBS 429 | Saccharomyces cerevisiae | Fermenting must of champagne grapes | |
| E11 | CBS 1250 | Saccharomyces cerevisiae | Sherry | Spain |
| F2 | CBS 1782 | Saccharomyces cerevisiae | Super-attenuated beer | |
| F3 | CBS 820 | Saccharomycodes ludwigii | Grape must | Germany |
| F4 | CBS 2863 | Schwanniomyces occidentalis | Soil of vineyard | Spain |
| F5 | CBS 6741 | Schwanniomyces polymorphus var. africanus | Soil | South Africa |
| F6 | CBS 133 | Torulaspora delbrueckii | Ragi | Indonesia |
| F7 | CBS 427 | Torulaspora microellipsoides | Apple juice | Germany |
| F8 | CBS 248 | Wickerhamomyces anomalus | Red currants | Netherlands |
| F9 | CBS 249 | Wickerhamomyces anomalus | Berries | |
| F10 | CBS 261 | Wickerhamomyces anomalus | Ragi | Indonesia |
| F11 | CBS 262 | Wickerhamomyces anomalus | Beer | |
| G2 | CBS 4689 | Zygosaccharomyces bailii var. bailii | Grape must | Italy |
| G3 | CBS 1082 | Zygosaccharomyces bisporus | Tea-beer fungus | Indonesia |
| G4 | CBS 726 | Zygosaccharomyces mellis | Wine grapes | Germany |
| G5 | C1030 | Saccharomyces pastorianus | Brewers’ yeast | |
| G6 | C1039 | Saccharomyces cerevisiae | Wine yeast | |
| G7 | C746 | Saccharomyces cerevisiae | Brewers’ yeast | |
| G8 | CBS 1513 | Saccharomyces carlsbergensis | Brewers’ yeast | |
| G9 | C482 | Saccharomyces cerevisiae | Brewers’ yeast | |
| G10 | WS34/70 | Saccharomyces pastorianus | Brewers’ yeast | |
| G11 | C598 | Saccharomyces cerevisiae | Laboratory strain |
| Number | Alcohols |
|---|---|
| 1 | Benzyl alcohol |
| 2 | Butanol |
| 3 | Dodecanol |
| 4 | Fenchyl alcohol |
| 5 | Furaneol |
| 6 | Isoamyl alcohol |
| 7 | Propanol |
| 8 | 2-Ethyl-1-hexanol |
| 9 | 2-Furanmethanol |
| 10 | 2-Methyl propanol |
| 11 | 2-Nonanol |
| 12 | 2-Phenyl ethanol |
| 13 | 3 Ethoxy-1-Propanol |
| 14 | 3-(Methylthio)-1-propanol |
| Esters | |
| 15 | Butyl acetate |
| 16 | Ethyl (4E)-4-decenoate |
| 17 | Ethyl 2-methylbutyrate |
| 18 | Ethyl acetate |
| 19 | Ethyl butanoate |
| 20 | Ethyl decanoate |
| 21 | Ethyl dodecanoate |
| 22 | Ethyl heptanoate |
| 23 | Ethyl hexadecanoate |
| 24 | Ethyl hexanoate |
| 25 | Ethyl isobutyrate |
| 26 | Ethyl isovalerate |
| 27 | Ethyl octanoate |
| 28 | Ethyl propanoate |
| 29 | Ethyl tetradecanoate |
| 30 | Isoamyl acetate |
| 31 | Isoamyl butyrate |
| 32 | Isobutyl acetate |
| 33 | Isobutyl butanoate |
| 34 | Phenethyl acetate |
| 35 | S-methyl thioacetate |
| 36 | 2-Methyl propanoate |
| Acids | |
| 37 | Acetic acid |
| 38 | Butyric acid |
| 39 | Decanoic acid |
| 40 | Hexanoic acid |
| 41 | Isovaleric acid |
| 42 | Octanoic acid |
| Aldehydes | |
| 43 | Acetaldehyde |
| 44 | Benzaldehyde |
| 45 | Furfural |
| 46 | Phenyl acetaldehyde |
| 47 | 1-Decanal |
| 48 | 1-Nonanal |
| 49 | 3-Methyl butanal |
| 50 | 4-Methyl benzaldehyde |
| 51 | 5 Methyl furfural |
| 52 | 5-Hydroxymethylfurfural |
| Ketons | |
| 53 | Acetoin |
| 54 | Diacetyl |
| 55 | Pyranone |
| 56 | 2-Cyclopentene-1,4-dione |
| 57 | 2-Dodecanone |
| 58 | 2-Methyltetrahydrothiophen-3-one |
| 59 | 2-Nonanone |
| 60 | 2-Undecanone |
| Pyrazines | |
| 61 | 2,5-Dimethyl-3-ethylpyrazine |
| 62 | 2,6-Dimethylpyrazine |
| Strain Identifier | CBS1250 | CBS262 | CBS1082 | CBS726 | CBS261 | CBS191 | CBS188 | CBS2568 | CBS2649 |
|---|---|---|---|---|---|---|---|---|---|
| Position | E11 | F11 | G3 | G4 | F10 | E9 | E7 | C7 | B7 |
| Species | S. cerevisiae | W. anomalus | Z. bisporus | Z. mellis | W. anomalus | P. membrani-faciens | P. kluyveri | H. vineae | C. stellata |
| Butanol | 4.15 | 3.23 | 4.57 | 2.98 | 1.89 | 1.80 | 1.75 | 1.27 | 1.49 |
| Furaneol | 1.81 | 0.00 | 3.49 | 2.07 | 0.00 | 0.00 | 0.00 | 0.00 | 1.82 |
| Isoamyl alcohol | 3.83 | 2.45 | 1.86 | 2.04 | 1.12 | 1.91 | 0.90 | 0.79 | 0.76 |
| Propanol | 4.28 | 1.18 | 2.19 | 2.37 | 1.68 | 0.00 | 2.04 | 1.87 | 0.00 |
| 2-Furanmethanol | 0.00 | 0.00 | 3.11 | 2.10 | 0.00 | 0.00 | 0.00 | 0.00 | 9.13 |
| 2-Methyl propanol | 9.10 | 2.92 | 2.78 | 3.62 | 2.31 | 3.61 | 1.24 | 0.59 | 2.08 |
| 2-Phenyl ethanol | 1.42 | 4.26 | 1.98 | 1.94 | 4.81 | 2.19 | 2.97 | 1.53 | 1.45 |
| 3-(Methylthio)-1-propanol | 2.38 | 0.00 | 0.00 | 2.98 | 0.00 | 1.83 | 0.00 | 12.58 | 1.98 |
| Strain Identifier | CBS261 | CBS262 | CBS188 | CBS249 | CBS248 | CBS1082 | CBS133 |
|---|---|---|---|---|---|---|---|
| Position | F10 | F11 | E7 | F9 | F8 | G3 | F6 |
| Species | W. anomalus | W. anomalus | P. kluyveri | W. anomalus | W. anomalus | Z. bisporus | T. delbrueckii |
| Butyl acetate | 23.64 | 16.84 | 9.18 | 8.76 | 11.73 | 3.84 | 3.56 |
| Ethyl (4E)-4-decenoate | 0.00 | 0.00 | 0.17 | 0.00 | 0.04 | 0.56 | 0.07 |
| Ethyl 2-methylbutyrate | 50.89 | 368.00 | 36.00 | 62.34 | 39.06 | 2.94 | 10.63 |
| Ethyl acetate | 31.81 | 29.17 | 15.88 | 13.09 | 10.02 | 8.72 | 5.70 |
| Ethyl butanoate | 16.59 | 26.92 | 8.00 | 9.26 | 10.75 | 2.58 | 2.71 |
| Ethyl decanoate | 0.14 | 0.00 | 2.07 | 0.46 | 0.45 | 2.67 | 0.72 |
| Ethyl dodecanoate | 0.91 | 0.00 | 2.60 | 0.88 | 1.44 | 2.69 | 0.98 |
| Ethyl heptanoate | 1.17 | 2.19 | 6.96 | 3.14 | 1.77 | 1.58 | 2.82 |
| Ethyl hexadecanoate | 12.19 | 13.62 | 16.05 | 9.10 | 8.22 | 11.00 | 2.36 |
| Ethyl hexanoate | 7.23 | 12.89 | 5.07 | 6.90 | 7.47 | 4.33 | 2.72 |
| Ethyl isovalerate | 11.84 | 120.99 | 33.97 | 14.43 | 15.00 | 0.00 | 10.79 |
| Ethyl octanoate | 0.56 | 0.26 | 1.59 | 0.59 | 0.32 | 0.64 | 0.55 |
| Isoamyl acetate | 9.36 | 5.84 | 3.21 | 2.92 | 3.10 | 1.27 | 0.99 |
| Isobutyl acetate | 152.96 | 59.59 | 24.70 | 40.69 | 26.57 | 6.75 | 9.93 |
| Isobutyl butanoate | 209.13 | 167.38 | 29.44 | 88.87 | 52.26 | 10.77 | 13.62 |
| 2-Phenylethyl acetate | 5.88 | 4.07 | 2.70 | 2.68 | 2.90 | 0.44 | 0.91 |
| methyl thioacetate | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.21 | 0.00 |
| 2-Methyl propanoate | 0,89 | 0,00 | 1,16 | 1,13 | 0,46 | 0,00 | 0,44 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravasio, D.; Carlin, S.; Boekhout, T.; Groenewald, M.; Vrhovsek, U.; Walther, A.; Wendland, J. Adding Flavor to Beverages with Non-Conventional Yeasts. Fermentation 2018, 4, 15. https://doi.org/10.3390/fermentation4010015
Ravasio D, Carlin S, Boekhout T, Groenewald M, Vrhovsek U, Walther A, Wendland J. Adding Flavor to Beverages with Non-Conventional Yeasts. Fermentation. 2018; 4(1):15. https://doi.org/10.3390/fermentation4010015
Chicago/Turabian StyleRavasio, Davide, Silvia Carlin, Teun Boekhout, Marizeth Groenewald, Urska Vrhovsek, Andrea Walther, and Jürgen Wendland. 2018. "Adding Flavor to Beverages with Non-Conventional Yeasts" Fermentation 4, no. 1: 15. https://doi.org/10.3390/fermentation4010015





