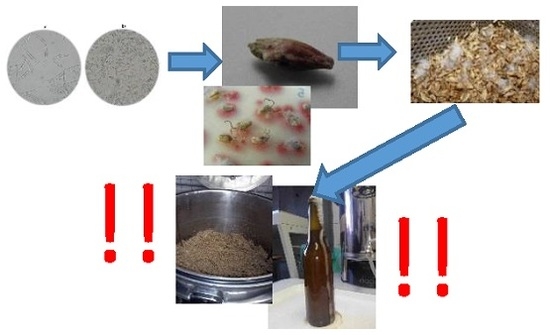
Malting and Brewing Industries Encounter Fusarium spp. Related Problems
Abstract
:1. Introduction
2. Fusarium Fungi and Its Effect on Barley Grains
3. Fusarium Proliferation during Malting
4. Fungal Toxins in Malting and Brewing
5. Gushing—An Important Economic Factor
6. Malting and Brewing By-Products and Toxic Metabolites
7. What to Do with Contaminated Cereals and By-Products?
8. Analytical Methods for Mycotoxin Detection
9. Conclusions
Author Contributions
Conflicts of Interest
References
- Krstanović, V.; Lalić, A.; Kosović, I.; Velić, N.; Mastanjević, K.; Mastanjević, K. A survey of total β-glucan content in Croatian barley varieties. Cereal Res. Commun. 2016, 44, 650–657. [Google Scholar] [CrossRef]
- Narziss, L.; Back, W. Die Bierbrauerei: Band 1-Die Technologie der Malzbereitung, 8th ed.; Wiley: Weinheim, Germany, 2012; ISBN 978-3-527-32532-0. [Google Scholar]
- Bishop, L.R. The nitrogen content and quality of barley. J. Inst. Brew. 1930, 36, 352–369. [Google Scholar] [CrossRef]
- Havlova, P.; Lancova, K.; Váňová, M.; Havel, J.; Hajšlová, J. The effect of fungicidal treatment on selected quality parameters of barley and malt. J. Agric. Food Chem. 2006, 54, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Malachová, A.; Sulyok, M.; Beltrán, E.; Berthiller, F.; Krska, R. Optimization and validation of a quantitative liquid chromatography-tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J. Chromatogr. A 2014, 1362, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, M.; Berthiller, F.; Krska, R.; Schumacher, R. Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun. Mass Spectrom. 2006, 20, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Klapec, T.; Šarkanj, B.; Banjari, I.; Strelec, I. Urinary ochratoxin A and ochratoxin alpha in pregnant women. Food Chem. Toxicol. 2012, 50, 4487–4492. [Google Scholar] [CrossRef] [PubMed]
- Šarkanj, B.; Warth, B.; Uhlig, S.; Abiab, W.A.; Sulyok, M.; Klapec, T.; Krska, R.; Banjari, I. Urinary analysis reveals high deoxynivalenol exposure in pregnant women from Croatia. Food Chem. Toxicol. 2013, 62, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Bolechová, M.; Benešová, K.; Běláková, S.; Čáslavský, J.; Pospíchalová, M.; Mikulíková, R. Determination of seventeen mycotoxins in barley and malt in the Czech Republic. Food Control 2014, 47, 108–113. [Google Scholar] [CrossRef]
- Juan, C.; Berrada, H.; Mañes, J.; Oueslati, S. Multi-mycotoxin determination in barley and derived products from Tunisia and estimation of their dietary intake. Food Chem. Toxicol. 2017, 103, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No 1881/2006. Off. J. Eur. Union 2006, L 364/5, 5–24.
- Mastanjević, K.; Mastanjević, K.; Krstanović, V. The Gushing Experience—A Quick Overview. Beverages 2017, 3, 25. [Google Scholar] [CrossRef]
- Jurković, D.; Culek, M.; Ćosić, J. Mycopopulation of the treated winter wheat seed in eastern Croatia. Cereal Res. Commun. 1998, 26, 67–72. [Google Scholar]
- Chelkowski, J. Formation of mycotoxins produced by Fusaria in heads of wheat, triticale and rye. In Fusarium: Mycotoxins, Taxonomy, Pathogenicity (Topics in Secondary Metabolism, Taxonomy and Pathogenicity); Chelkowski, J., Ed.; Elsevier Science: Amsterdam, The Netherlands, 1989; p. 72. ISBN 0-444-87468-2. [Google Scholar]
- Bottalico, A. Fusarium disease of cereals: Species complex and related mycotoxin profiles in Europe. J. Plant Pathol. 1998, 80, 85–103. [Google Scholar] [CrossRef]
- Krstanović, V.; Klapec, T.; Velić, N.; Milaković, Z. Infection of malt barley and wheat by Fusarium graminearum and Fusarium culmorum from the crop years 2001–2003 in Eastern Croatia. Microbiol. Res. 2005, 160, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Spanic, V.; Lemmens, M.; Drezner, G. Morphological and molecular identification of Fusarium species associated with head blight on wheat in East Croatia. Eur. J. Plant Pathol. 2010, 128, 511–516. [Google Scholar] [CrossRef]
- Parikka, P.; Hakala, K.; Tiilikkala, K. Expected shifts in Fusarium species’ composition on cereal grain in Northern Europe due to climatic change. Food Addit. Contam. A 2012, 29, 1543–1555. [Google Scholar] [CrossRef] [PubMed]
- Tomasović, S. Fuzarijska oboljenja pšenice (Fusarium spp.). Glasnik Zaštite Bilja 1993, 7–8, 230–238. [Google Scholar]
- Ivić, D.; Domijan, A.-M.; Peraica, M.; Miličević, T.; Cvjetković, B. Fusarium spp. contamination of wheat, maize, soybean, and pea in Croatia. Arh. Hig. Rada. Toksikol. 2009, 60, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Poštić, J.; Ćosić, J.; Jurković, D.; Vrandečić, K.; Saleh, A.A.; Leslie, J.F. Diversity of Fusarium Species Isolated from Weeds and Plant Debris in Croatia. J. Phytopathol. 2012, 160, 76–81. [Google Scholar] [CrossRef]
- Snijders, C.H.A. Systemic fungal growth of Fusarium culmorum in stems of winter wheat. J. Phytopathol. 1990, 129, 133–140. [Google Scholar] [CrossRef]
- Ward, T.J.; Clear, R.M.; Rooney, A.P.; O’Donnell, K.; Gaba, D.; Patrick, S.; Starkey, D.E.; Gilbert, J.; Geiser, D.M.; Nowicki, T.W. An adaptive evolutionary shift in Fusarium head blight pathogen populations is driving the rapid spread of more toxigenic Fusarium graminearum in North America. Fungal Genet. Biol. 2008, 45, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Van der Lee, T.; Waalwijk, C.; Chen, W.; Xu, J.; Xu, J.; Zhang, Y.; Feng, L. Population Analysis of the Fusarium graminearum Species Complex from Wheat in China Show a Shift to More Aggressive Isolates. PLoS ONE 2012, 7, e31722. [Google Scholar] [CrossRef] [PubMed]
- MEBAK®. Methodensammlung der Mitteleuropäischen Analysenkommission. In Raw Materials: Barley, Adjuncts, Malt, Hops and Hop Products; Jacob, F., Ed.; Selbstverlag der MEBAK®: Freising-Weihenstephan, Germany, 2011. [Google Scholar]
- Analytica Microbiologica European Brewery Convention; Fachverlag Hans Carl: Nürnberg, Germany, 2005.
- Schwarz, P.B.; Casper, H.H.; Barr, J.; Musial, M. Impact of Fusarium head blight on the malting and brewing quality of barley. Cereal Res. Commun. 1997, 25, 813–814. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Professional: Ames, IA, USA, 2006. [Google Scholar]
- Teich, A.H. Epidemiology of Wheat Scab Caused by Fusarium spp. In Fusarium: Mycotoxins, Taxonomy, Pathogenicity (Topics in Secondary Metabolism, Taxonomy and Pathogenicity); Chelkowski, J., Ed.; Elsevier Science: Amsterdam, The Netherlands, 1989; pp. 269–282. ISBN 0-444-87468-2. [Google Scholar]
- Španic, V.; Drezner, G.; Horvat, D. Changes of agronomic and quality traits in Fusarium-inoculated wheat genotypes. Croat. J. Food Technol. Biotechnol. Nutr. 2012, 7, 85–89. [Google Scholar]
- Bai, G.; Shaner, G. Scab in wheat: Prospects for control. Plant Dis. 1994, 78, 760–766. [Google Scholar] [CrossRef]
- McMullen, M.; Jones, R.; Gallenberg, D. Scab of wheat and barley: A re-emerging disease of devastating impact. Plant Dis. 1997, 81, 1340–1348. [Google Scholar] [CrossRef]
- Siranidou, E.; Kang, Z.; Buchenauer, H. Studies on symptom development, phenolic compounds and morphological defence response in wheat cultivars differing in resistance to Fusarium head blight. J. Phytopathol. 2001, 150, 200–2008. [Google Scholar] [CrossRef]
- Vanne, L.; Haikara, A. Mycotoxins in the total chain from barley to beer. In Proceedings of the 28th European Brewery Convention Congress, Hungary, Budapest, 12–17 May 2001; pp. 838–848. [Google Scholar]
- Sarlin, T.; Laitila, A.; Pekkarinen, A.; Haikara, A. Effects of three Fusarium species on the quality of barley and malt. J. Am. Soc. Brew. Chem. 2005, 63, 43–49. [Google Scholar] [CrossRef]
- Van Nierop, S.N.E.; Rautenbach, M. The impact of microorganisms on barley and malt quality—A review. J. Am. Soc. Brew. Chem. 2006, 64, 69–78. [Google Scholar] [CrossRef]
- De la Pena, R.C.; Smith, K.P.; Capettini, F.; Muehlbauer, G.J.; Gallo-Meagher, M.; Dill-Macky, R.; Somers, D.A.; Rasmusson, D.C. Quantitative trait loci associated with resistance to Fusarium head blight and kernel discoloration in barley. Theor. Appl. Genet. 1999, 99, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Franckowiak, J.D. Notes on plant height in sixrowed barley and FHB resistance. In Proceedings of the 8th International Barley Genetics Symposium, Adelaide, Australia, 22–27 October 2000; pp. 107–109. [Google Scholar]
- Ma, Z.; Steffenson, B.J.; Prom, L.K.; Lapitan, N.L. Mapping of quantitative trait loci for Fusarium head blight resistance in barley. Phytopathology 2000, 90, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Magan, N.; Hope, R.; Colleate, A.; Baxter, E.S. Relationship between growth and mycotoxin production by Fusarium species, biocides and environment. Eur. J. Plant Pathol. 2002, 108, 685–690. [Google Scholar] [CrossRef]
- Niessen, L.; Donhauser, S.; Weideneder, A.; Geiger, E. Möglichkeiten einer verbesserten isuellen Beurteilung des mikrobiologishen Status von Malz. Brauwelt 1991, 131, 1556–1562. [Google Scholar]
- Schwarz, P.B.; Schwarz, J.G.; Zhou, A.; Prom, L.K.; Steffenson, B.J. Effect of Fusarium graminearum and Fusarium poae infection on barley and malt quality. Monatsschrift für Brauwissenschaft 2001, 54, 55–63. [Google Scholar]
- Schwarz, P.B.; Jones, L.J.; Steffenson, B.J. Enzymes associated with Fusarium infection of barley. J. Am. Soc. Brew. Chem. 2002, 60, 130–134. [Google Scholar] [CrossRef]
- Vaughan, A.; O’Sullivan, T.; van Sinderen, D. Enhancing the microbiological stability of malt and beer—A review. J. Inst. Brew. 2005, 111, 355–371. [Google Scholar] [CrossRef]
- Flannigan, B. The microflora of barley and malt. In Brewing Microbiology, 1st ed.; Priest, F.G., Campbell, I., Eds.; Elsevier Applied Science: London, UK; New York, NY, USA, 1987; pp. 83–120. ISBN 978-1-4757-4679-2. [Google Scholar]
- Berthiller, F.; Sulyok, M.; Krska, R.; Schuhmacher, R. Chromatographic methods for the simultaneous determination of mycotoxins and their conjugates in cereals. Int. J. Food Microbiol. 2007, 119, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Varga, E.; Wiesenberger, G.; Hametner, C.; Ward, T.J.; Dong, Y.; Schöfbeck, D.; McCormick, S.; Broz, K.; Stückler, R.; Schuhmacher, R.; et al. New tricks of an old enemy: Isolates of Fusarium graminearum produce a type A trichothecene mycotoxin. Environ. Microbiol. 2015, 17, 2588–2600. [Google Scholar] [CrossRef]
- Bullerman, L.B.; Bianchini, A. Stability of mycotoxins during food processing. Int. J. Food Microbiol. 2007, 19, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Krstanović, V.; Mastanjević, K.; Velić, N.; Pleadin, J.; Perši, N.; Španić, V. The influence of Fusarium culmorum contamination level on deoxynivalenol content in wheat, malt and beer. Rom. Biotech. Lett. 2015, 20, 10901–10910. [Google Scholar]
- Nathanail, A.V.; Gibson, B.; Han, L.; Peltonen, K.; Ollilainen, V.; Jestoi, M.; Laitila, A. The lager yeast Saccharomyces pastorianus removes and transforms Fusarium trichothecene mycotoxins during fermentation of brewer’s wort. Food Chem. 2016, 203, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Dupire, S. Mycotoxins and other contaminants in the malting and brewing industries. In Proceedings of the European Brewery Convention Congress, Dublin, Ireland, 17–22 May 2003. [Google Scholar]
- Melotte, L. Survey on the analysis of mycotoxins. Analysis Committee of the European Brewery Convention, European Brewery Association. J. Inst. Brew. 2004, 110, 235–239. [Google Scholar] [CrossRef]
- Boivin, P. Emergent mycotoxins in malting barley. In Proceedings of the Congress of the European Brewery Convention, Prauge, Czech Republic, 14–19 May 2005. [Google Scholar]
- Council for Agricultural Science and Technology (CAST). Mycotoxins: Risk in Plant, Animal and Human Systems; Task Force Report No. 139; Council for Agricultural Science and Technology: Ames, IA, USA, 2003. [Google Scholar]
- Krska, R.; Schubert-Ullrich, P.; Molinelli, A.; Sulyok, M.; Macdonald, S.; Crews, C. Mycotoxin analysis: An update. Food Addit. Contam. 2008, 25, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Nagatomi, Y.; Uyama, A.; Mochizuki, N. Fate of mycotoxins during beer brewing and fermentation. Biosci. Biotechnol. Biochem. 2013, 77, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Pfliegler, W.P.; Pusztahelyi, T.; Pócsi, I. Mycotoxins-prevention and decontamination by yeasts. J. Basic Microb. 2015, 55, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; Saeger, S.D.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rychlik, M.; Humpf, H.-U.; Marko, D.; Dänicke, S.; Mally, A.; Berthiller, F.; Klaffke, H.; Lorenz, N. Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxin. Res. 2014, 30, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, P.B. Fusarium head blight and deoxynivalenol in malting and brewing: Successes and future challenges. Trop. Plant Pathol. 2017, 3, 153–164. [Google Scholar] [CrossRef]
- Warth, B.; Sulyok, M.; Fruhmann, P.; Berthiller, F.; Schuhmacher, R.; Hametner, C.; Adam, G.; Fröhlich, J.; Krska, R. Assessment of human deoxynivalenol exposure using an LC–MS/MS based biomarker method. Toxicol. Lett. 2012, 211, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, K.C.; Dagostim Savi, G.; Olivo, G.; Scussel, V.M. Quality and ccurrence of deoxynivalenol and fumonisins in craft beer. Food Control 2015, 50, 925–929. [Google Scholar] [CrossRef]
- Varga, E.; Malachova, A.; Schwartz, H.; Krska, R.; Berthiller, F. Survey of deoxynivalenol and its conjugates deoxynivalenol-3-glucoside and 3-acetyl-deoxynivalenol in 374 beer samples. Food Addit. Contam. A 2013, 30, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Benešová, K.; Běláková, S.; Mikulíková, R.; Svoboda, Z. Monitoring of selected aflatoxins in brewing materials and beer by liquid chromatography/mass spectrometry. Food Control 2012, 25, 626–630. [Google Scholar] [CrossRef]
- Laitila, A. Toxigenic fungi and mycotoxins in the barley-to-beer chain. In Brewing Microbiology: Managing Microbes, Ensuring Quality and Valorising Waste; Hill, A.E., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 107–139. ISBN 9781782423492. [Google Scholar]
- Bauer, J.I.; Gross, M.; Gottschalk, C.; Usleber, E. Investigations on the occurence of mycotoxins in beer. Food Control 2016, 63, 135–139. [Google Scholar] [CrossRef]
- Peters, J.; van Dam, R.; van Doorn, R.; Katerere, D.; Berthiller, F.; Haasnoot, W.; Nielen, M.W.F. Mycotoxin profiling of 1000 beer samples with special focus on craft beer. PLoS ONE 2017, 12, e0185887. [Google Scholar] [CrossRef] [PubMed]
- Lancova, K.; Hajslova, J.; Poustka, J.; Krplova, A.; Zachariasova, M.; Dostalek, P.; Sachambula, L. Transfer of Fusarium mycotoxins and ‘masked’ deoxynivalenol (deoxynivalenol-3-glucoside) from field barley through malt to beer. Food Addit. Contam. Part A 2008, 25, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Kostelanska, M.; Hajslova, J.; Zachariasova, M.; Malachova, A.; Kalachova, K.; Poustka, J.; Fiala, J.; Scott, P.M.; Berthiller, F.; Krska, R. Occurrence of deoxynivalenol and its major conjugate, deoxynivalenol-3-glucoside, in beer and some brewing intermediates. J. Agric. Food Chem. 2009, 57, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Boutigny, A.-L.; Richard-Forget, F.; Barreau, C. Natural mechanisms for cereal resistance to the accumulation of Fusarium trichothecenes. Eur. J. Plant Pathol. 2008, 121, 411–423. [Google Scholar] [CrossRef]
- Nakagawa, H.; Ohmichi, K.; Sakamoto, S.; Sago, Y.; Kushiro, M.; Nagashima, H.; Yoshida, M.; Nakajima, T. Detection of a new Fusarium masked mycotoxin in wheat grain by high-resolution LC-Orbitrap MS. Food Addit. Contam. A 2011, 28, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zhou, B.; Gillespie, J.; Gross, T.; Barr, J.; Simsek, S.; Brueggeman, R.; Schwarz, P. Production of deoxynivalenol (DON) and DON-3-glucoside during the malting of Fusarium infected hard red spring wheat. Food Control 2018, 85, 6–10. [Google Scholar] [CrossRef]
- Solarska, E. Study on cause of Fusarium cone tip blight. In Proceedings of the Scientific Commission CICH–IHB–IHGC, International Hop Growers’ Convention, Tettnang, Germany, 24–28 June 2007; pp. 95–97. [Google Scholar]
- Bienapfl, J.C.; Ocamb, C.M.; Klein, R.; Nelson, M. Fusarium cone tip blight: A new disease of Humulus lupulus. In Proceedings of the Scientific Commission IHGC, Canterbury, UK, 5–7 August 2001; p. 113. [Google Scholar]
- Hazel, C.M.; Patel, S. Influence of processing on trichothecene levels. Toxicol. Lett. 2004, 153, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Gjertsen, P.; Trolle, B.; Andersen, K. Gushing caused by microorganisms, specially Fusarium species. In Proceedings of the 10th European Brewery Convention, Stockholm, Sweden, 1965; Elsevier: Amsterdam, The Netherlands, 1965; pp. 428–438. [Google Scholar]
- Haikara, A. Relationship between Malt and Beer. In Proceedings of the European Brewery Convention, Helsinki, Finland, 4–5 November 1980; Monograph VI. pp. 251–258. [Google Scholar]
- Niessen, L.; Donhauscr, S.; Wcidendcr, A.; Geiger, E.; Vogel, H. Mycologische Untersuchungen an Cerealien und Malzen im Zusammenhang mit Wildwerden (Gushing) des Bieres. Brauwelt 1992, 132, 702–714. [Google Scholar]
- Sarlin, T.; Vilpola, A.; Kotaviita, E.; Olkku, J.; Haikara, A. Fungal hydrophobins in the barley-to-beer chain. J. Inst. Brew. 2007, 113, 147–153. [Google Scholar] [CrossRef]
- Sarlin, T.; Nakari-Setälä, T.; Linder, M.; Penttilä, M.; Haikara, A. Fungal hydrophobins as predictors of the gushing activity of malt. J. Inst. Brew. 2005, 111, 105–111. [Google Scholar] [CrossRef]
- Christian, M.; Titze, J.; Ilberg, V.; Jacob, F. Novel Perspectives in Gushing Analysis: A Review. J. Inst. Brew. 2011, 117, 295–313. [Google Scholar] [CrossRef]
- Blechová, P.; Havlová, P.; Havel, J. The study of premature yeast flocculation and its relationship with gushing of beer. Monatsschrift für Brauwissenschaft 2005, 58, 64–78. [Google Scholar]
- Christian, M.; Titze, J.; Ilberg, V.; Jacob, F. Combined particle analysis as a new tool to predict gushing shown with alcohol-free beverage products. Brew. Sci. 2010, 63, 72–79. [Google Scholar]
- Schumacher, T. Gushing in Fruchtsaftschorlen—Ursachen und Gegenmaßnahmen. Flüssiges Obst. 2002, 69, 304–310. [Google Scholar]
- Stübner, M.; Lutterschmid, G.; Vogel, R.F.; Niessen, L. Heterologous expression of the hydrophobin FcHyd5p from Fusarium culmorum in Pichia pastoris and evaluation of its surface activity and contribution to gushing of carbonated beverages. Int. J. Food Microbiol. 2010, 141, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Gjertsen, P.; Trolle, B.; Andersen, K. Weath-microflora of possible importance to malting and ered barley as a contributory of gushing in beer. In Proceedings of the European Brewery Convention Congress, Brussels, Belgium; 1963; pp. 320–341. [Google Scholar]
- Gjertsen, P. Gushing in beer; its nature, cause and prevention. Brew. Dig. 1967, 42, 80–84. [Google Scholar]
- Zepf, M. Gushing-Ursachenfindung anhand von Modellversuchen; Diss. TU München; NA Publishing: Ann Arbor, MI, USA, 1998. [Google Scholar]
- Sarlin, T.; Kivioja, T.; Kalkkinen, N.; Linder, M.B.; Nakari-Setälä, T. Identification and characterization of gushing-active hydrophobins from Fusarium graminearum and related species. J. Basic Microbiol. 2012, 52, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Wessels, J.G.H. Fungal hydrophobins: Proteins that function at an interface. Trends Plant Sci. 1996, 1, 9–15. [Google Scholar] [CrossRef]
- Haikara, A.; Sarlin, T.; Nakari-Setälä, T.; Penttilä, M. Method for Determining a Gushing Factor for a Beverage. Patent EP 1071949, 9 May 2000. [Google Scholar]
- Kleemola, T.; Nakari-Setälä, T.; Linder, M.; Penttilä, M.; Kotaviita, E.; Olkku, J.; Haikara, A. Characterisation and detection of the gushing factors produced by fungi. In Proceedings of the European Brewery Convention Congress, Budapest, Hungary, 12–17 May 2001; Fachverlag Hans Carl: Nürnberg, Germany, 2001; pp. 129–138. [Google Scholar]
- Wolf-Hall, C.E. Mold and mycotoxin problems encountered during malting and brewing. Int. J. Food Microbiol. 2007, 199, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Christian, M.; Illberg, V.; Aydin, A.A.; Titze, J.; Jacob, F.; Parlar, H. New gushing mechanism proposed by applying particle size analysis and several surfactants. Brew. Sci. 2009, 62, 100–107. [Google Scholar]
- Gorjanović, S. A Review: The Role of Barley Seed Pathogenesis-Related Proteins (PRs) in Beer Production. J. Inst. Brew. 2010, 116, 111–124. [Google Scholar] [CrossRef]
- Lutterschmid, G.; Stübner, M.; Vogel, R.F.; Niessen, L. Induction of gushing with recombinant class II hydrophobin FcHyd5p from Fusarium culmorum and the impact of hop compounds on its gushing potential. J. Inst. Brew. 2010, 116, 339–347. [Google Scholar] [CrossRef]
- Műlller, M.P.; Schmid, F.; Becker, T.; Gastl, M. Impact of different hop compounds on the overfoaming volume of beer caused by primary gushing. J. Inst. Brew. 2010, 116, 459–463. [Google Scholar] [CrossRef]
- Zapf, M.W.; Theisen, S.; Vogel, R.F.; Niessen, L. Cloning of wheat LTP1500 and two Fusarium culmorum hydrophobins in Saccharomyces cerevisiae and assessment of their gushing inducing potential in experimental wort fermentation. J. Inst. Brew. 2006, 112, 237–245. [Google Scholar] [CrossRef]
- Zapf, M.W.; Theisen, S.; Rohde, S.; Rabenstein, F.; Vogel, R.F.; Niessen, L. Characterization of AfpA, an alkaline foam protein from cultures of Fusarium culmorum and its identification in infected malt. J. Appl. Microbiol. 2007, 103, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, P.B.; Beattie, S.; Casper, H.H. Relationship between Fusarium infestation of barley and the gushing potential of malt. J. Inst. Brew. 1996, 102, 93–96. [Google Scholar] [CrossRef]
- Pellaud, J. Gushing: State of the art. In Proceedings of the Xth Belgian Brewing Conference Jean de Cleck Chair, Leuven, Belgium, 6–8 September 2002; pp. 1–21. [Google Scholar]
- Linder, M.B. Hydrophobins: Proteins that self-assemble at interfaces. Curr. Opin. Colloid Interface Sci. 2009, 14, 356–363. [Google Scholar] [CrossRef]
- Müller, V.; Besier, A.; Pätz, R.; Fröhlich, J. Method for the Treatment of the Phenomenon Gushing in Beer and Malt Beverages; Erbslöh Geisenheim AG-AnGus1516® Brauindustrie; Erbslöh Geisenheim: Geisenheim, Germany, 2013; pp. 1–9. [Google Scholar]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Habschied, K.; Šarkanj, B.; Krstanović, V.; Velić, N.; Novak, M.; Mastanjević, K. Fusarium mycotoxins in malting and brewing by-products. In Proceedings of the World Mycotoxin Forum 8th Conference; Mycotoxin Control: The System Approach, Vienna, Austria, 10–12-November 2014; p. 85. [Google Scholar]
- Zechner-Krpan, V. Potential Application of Yeast β-Glucans in Food Industry. Agric. Conspec. Sci. 2009, 74, 277–282. [Google Scholar]
- Cavaglieri, L.R.; Keller, K.M.; Pereya, C.M.; Gonzalez Pereya, M.; Alonso, V.A.; Rojo, F.G.; Dalcero, A.M.; Rosa, C.A.R. Fungi and natural incidence of selected mycotoxins in barley rootlets. J. Stored Prod. Res. 2009, 45, 147–150. [Google Scholar] [CrossRef]
- Caupert, J.; Zhang, Y.; Imerman, P.; Richard, J.J.; Shurson, G.C. Mycotoxin Occurrence in DDGS. In Distiller’s Grain—Production, Properties, and Utilization; Liu, K., Rosentrater, K.A., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2011; pp. 215–229. [Google Scholar]
- A Guide to Distiller’s Dried Grains with Solubles (DDGS), 3rd ed.; U.S. Grain Council: Washington, DC, USA, 2012.
- Directive 2002/32/EC. Off. J. Eur. Communities 2002, L140/10, 1–22.
- Recommendation 2006/576/EC. Off. J. Eur. Communities 2006, L229/7.
- Recommendation 2013/165/EC. Off. J. Eur. Communities 2013, L91/12.
- Scott, T. Feed and Processing Options for Heavily Downgraded Wheat. 2014. Available online: https://cigi.ca/wp-content/uploads/2014/11/Tom-Scott-Feed-and-Processing-Options-for-Heavily-Downgraded-Wheat.pdf (accessed on 12 December 2017).
- Zachariasova, M.; Hajslova, J.; Kostelanska, M.; Poustka, J.; Krplova, A.; Cuhra, P.; Hochel, I. Deoxynivalenol and its conjugates in beer: A critical assessment of data obtained by enzyme-linked immunosorbent assay and liquid chromatography coupled to tandem mass spectrometry. Anal. Chim. Acta 2008, 625, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wong, J.W.; Krynitsky, A.J.; Trucksess, M.W. Perspective on advancing FDA regulatory monitoring for mycotoxins in foods using liquid chromatography and mass spectrometry. J. AOAC Int. 2016, 99, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Brera, C.; Iha, M.H.; Krska, R.; Lattanzio, V.M.T.; MacDonald, S.; Malone, R.J.; Maragos, C.; Solfrizzo, M.; Stranska-Zachariasova, M.; et al. Developments in mycotoxin analysis: An update for 2015–2016. World Mycotoxin. J. 2017, 10, 5–29. [Google Scholar] [CrossRef]
- Habler, K.; Gotthard, M.; Schüler, J.; Rychlik, M. Multi-mycotoxin stable isotope dilution LC–MS/MS method for Fusarium toxins in beer. Food Chem. 2017, 218, 447–454. [Google Scholar] [CrossRef] [PubMed]
- De Girolamo, A.; Ciasca, B.; Stroka, J.; Bratinova, S.; Pascale, M.; Visconti, A.; Lattanzio, V.M.T. Performance evaluation of LC–MS/MS methods for multi-mycotoxin determination in maize and wheat by means of international Proficiency Testing. TrAC Trends Anal. Chem. 2017, 86, 222–234. [Google Scholar] [CrossRef]
- De Santis, B.; Debegnach, F.; Gregori, E.; Russo, E.; Marchegiani, F.; Moracci, G.; Brera, C. Development of a LC-MS/MS Method for the Multi-Mycotoxin Determination in Composite Cereal-Based Samples. Toxins 2017, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Hong, S.-H.; Kang, J.W.; Cho, S.M.; Lee, K.R.; An, T.-K.; Lee, C.; Chung, S.H. Simultaneous Determination of Multi-Mycotoxins in Cereal Grains Collected from South Korea by LC/MS/MS. Toxins 2017, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Freire, L.; Sant’Ana, A.S. Modified mycotoxins: An updated review on their formation, detection, occurrence, and toxic effects. Food Chem. Toxicol. 2018, 111, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.; Liang, G.; Li, A.; Pan, L. Recent advances in mycotoxin determination for food monitoring via microchip. Toxins 2017, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Rosenau, A.; Jecu, L.; Badea, M.; Evans, R.W. Mycotoxins: An overview on their quantification methods. Rom. J. Biochem. 2010, 47, 79–86. [Google Scholar]
- Turner, N.W.; Bramhmbhatt, H.; Szabo-Vezse, M.; Poma, A.; Coker, R.; Piletsky, S.A. Analytical methods for determination of mycotoxins: An update (2009–2014). Anal. Chim. Acta 2015, 901, 12–33. [Google Scholar] [CrossRef] [PubMed]
- Homdork, S.; Fehrmann, H.; Beck, R. Effect of field application of tebuconazole on yield, yield components and the mycotoxin content of Fusarium-infected wheat grain. J. Phytopathol. 2000, 148, 1–6. [Google Scholar] [CrossRef]
- Španić, V.; Drezner, G. The importance of fungicide Prosaro protection against Fusarium Head Blight (FHB) of wheat. Agronomski Glasnik 2011, 1–2, 17–26. [Google Scholar]
- Mesterházy, Á.; Bartók, T.; Lamper, C. Influence of cultivar resistance, epidemic severity, and Fusarium species on the efficacy of fungicide control of Fusarium head blight in wheat and deoxynivalenol (DON) contamination of grain. Plant Dis. 2003, 87, 1107–1115. [Google Scholar] [CrossRef]



| Species | Weak Pathogens | Aggressive Pathogens |
|---|---|---|
| F. graminearum | + | − |
| F. culmorum | + | − |
| F. cerealis | + | − |
| F. avanceum | − | + |
| F. tricintum | − | + |
| F. sporotrichoides | − | + |
| F. poea | − | + |
| F. acuminatum | − | + |
| F. oxysporum | − | + |
| F. equiseti | − | + |
| F. sambucinum | − | + |
| Method | Advantage | Disadvantage | Source | |
|---|---|---|---|---|
| Chromatographic Techniques | TLC | low cost; simple; rapid; | lack of automation | [123] |
| GC | for volatile compounds; | [123] | ||
| HPLC | high resolution; low limit of detection; can be coupled with a multiple detection automated system; specific; | expensive; time-consuming; expensive equipment and clean-up procedures; | [123] | |
| LC-MS/MS | high selectivity; high sensitivity; relatively easy sample clean up; multi-mycotoxin determination; | costly; expensive; time-consuming; expensive equipment and clean-up procedures; | [123] | |
| Immunological | ELISA | screening method for different matrices; sensitive, specific, rapid, relatively low cost and simple; low detection limit; | due to the cross-reactivity with masked mycotoxins, ELISA results usually show an overestimation of results; enzyme stability; | [49,114,115,123,124] |
| Biological | Biosensors | rapid; sensitive; practical; | regeneration of the receptor surface; specificity; sensitivity; reproducibility; stability; | [123,124] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastanjević, K.; Krstanović, V.; Mastanjević, K.; Šarkanj, B. Malting and Brewing Industries Encounter Fusarium spp. Related Problems. Fermentation 2018, 4, 3. https://doi.org/10.3390/fermentation4010003
Mastanjević K, Krstanović V, Mastanjević K, Šarkanj B. Malting and Brewing Industries Encounter Fusarium spp. Related Problems. Fermentation. 2018; 4(1):3. https://doi.org/10.3390/fermentation4010003
Chicago/Turabian StyleMastanjević, Kristina, Vinko Krstanović, Krešimir Mastanjević, and Bojan Šarkanj. 2018. "Malting and Brewing Industries Encounter Fusarium spp. Related Problems" Fermentation 4, no. 1: 3. https://doi.org/10.3390/fermentation4010003