Two Novel Strains of Torulaspora delbrueckii Isolated from the Honey Bee Microbiome and Their Use in Honey Fermentation
Abstract
:1. Introduction
2. Results and Discussion
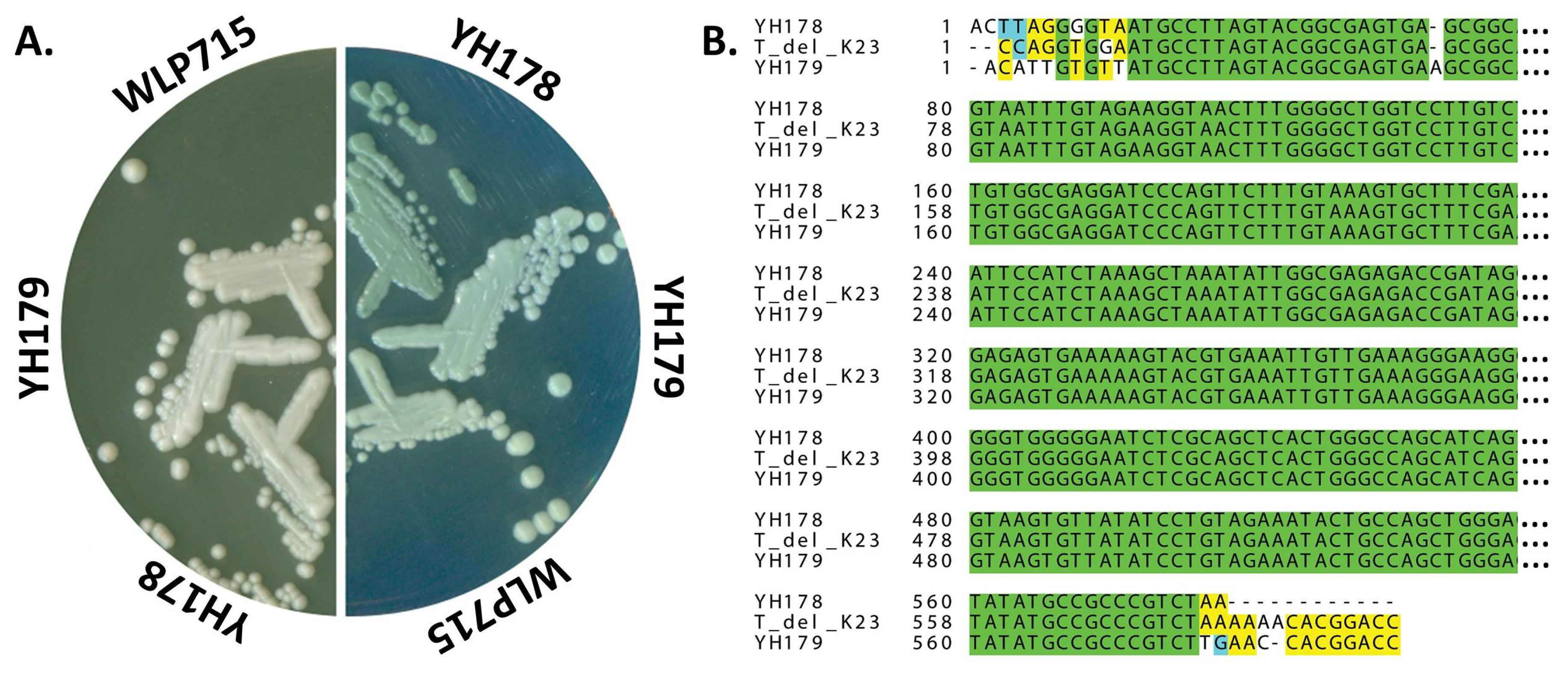
2.1. Isolation of T. delbrueckii Strains YH178 and YH179
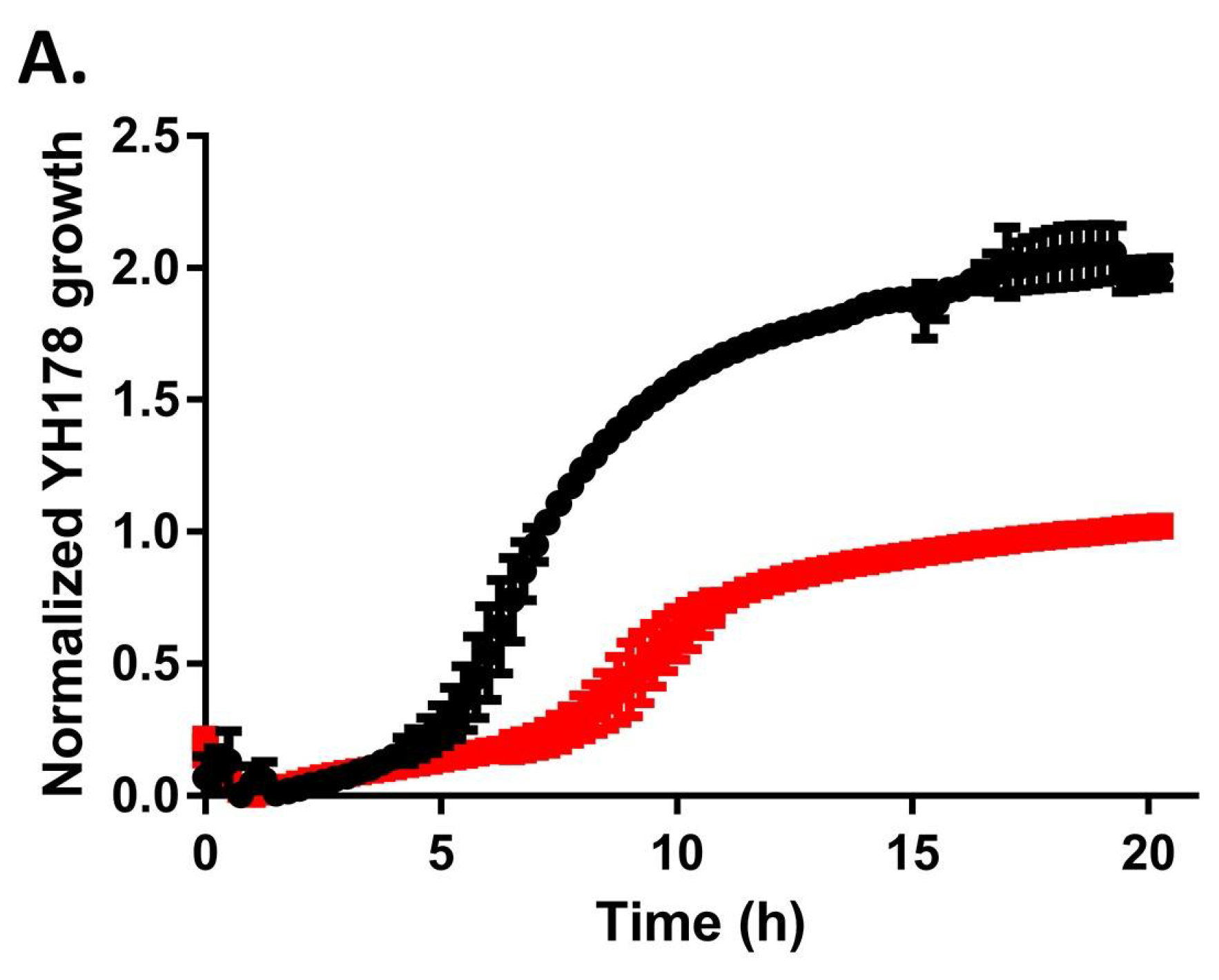
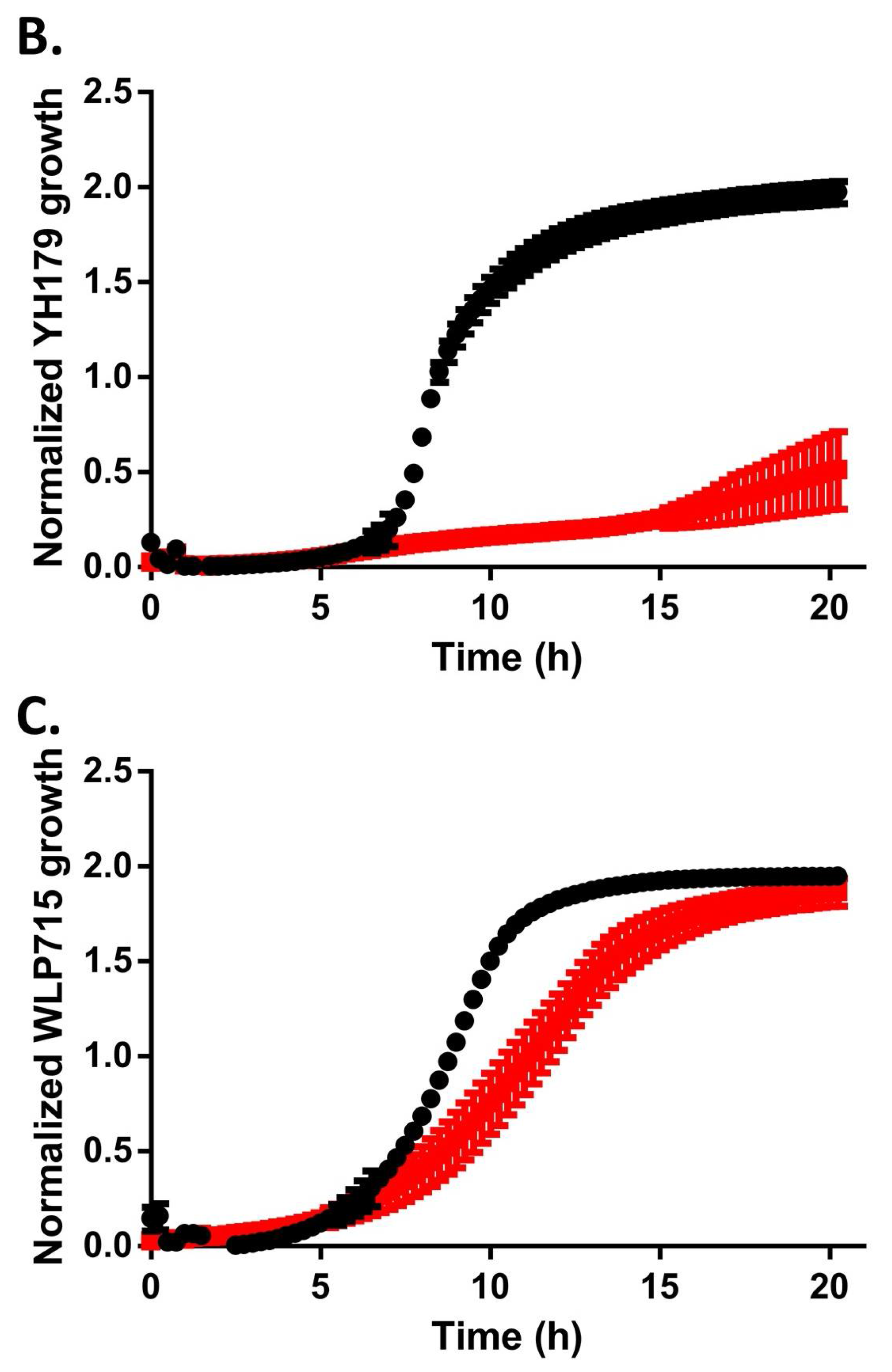
2.2. YH178 and YH179 Can Use Honey as a Carbon Source during Fermentation
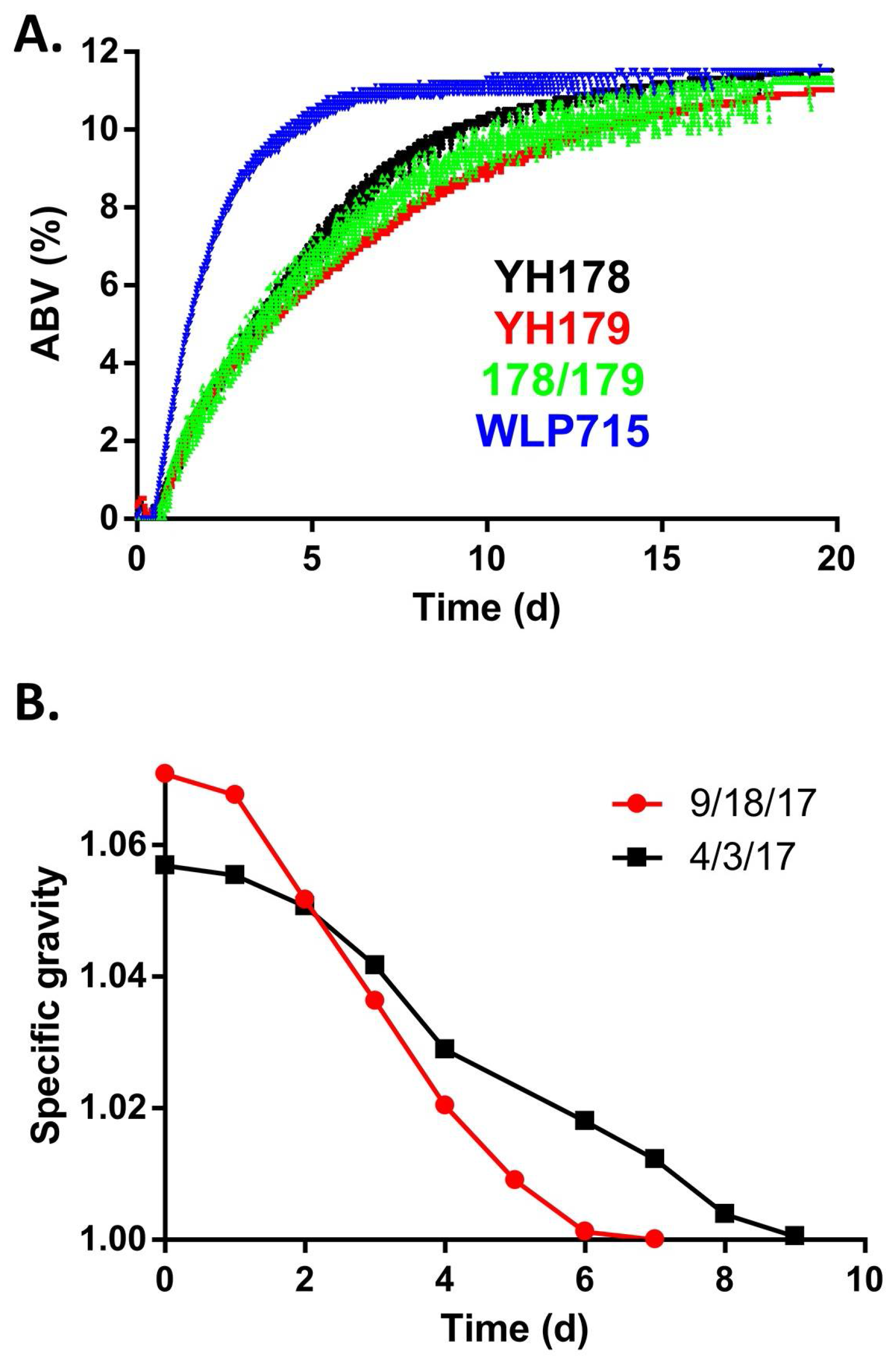
2.3. YH178 and YH179 Produced Superior Meads Compared to WLP715
2.4. YH178 and YH179 Were Used in the Creation of Honey Schnapps
3. Materials and Methods
3.1. Strains and Culture Conditions
3.2. Yeast Isolation
3.3. Strain Identification
3.4. Growth Curves
3.5. Laboratory-Scale Honey Fermentation
3.6. Laboratory-Scale Wort Fermentation
3.7. Mead Sensory Analysis
3.8. Production-Scale Honey Fermentation and Distillation
3.9. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McGovern, P.E.; Mirzoian, A.; Hall, G.R. Ancient Egyptian herbal wines. Proc. Natl. Acad. Sci. USA 2009, 106, 7361–7366. [Google Scholar] [CrossRef] [PubMed]
- McGovern, P.E.; Zhang, J.; Tang, J.; Zhang, Z.; Hall, G.R.; Moreau, R.A.; Nunez, A.; Butrym, E.D.; Richards, M.P.; Wang, C.S.; et al. Fermented beverages of pre- and proto-historic China. Proc. Natl. Acad. Sci. USA 2004, 101, 17593–17598. [Google Scholar] [CrossRef] [PubMed]
- Crewe, L.; Hill, I. Finding Beer in the Archaeological Record: A Case Study from Kissonerga-Skalia on Bronze Age Cyprus. Levant 2012, 44, 205–237. [Google Scholar] [CrossRef]
- Rogers, A. Proof: The Science of Booze; Mariner Books: Wilmington, DE, USA, 2015. [Google Scholar]
- Iglesias, A.; Pascoal, A.; Choupina, A.B.; Carvalho, C.A.; Feas, X.; Estevinho, L.M. Developments in the fermentation process and quality improvement strategies for mead production. Molecules 2014, 19, 12577–12590. [Google Scholar] [CrossRef] [PubMed]
- American Mead Makers Association. What’s the Buzz? 2017 Mead Industry Report; American Mead Makers Association: Seattle, WA, USA, 2017. [Google Scholar]
- Osburn, K.; Ahmad, N.N.; Bochman, M.L. Bio-prospecting, selection, and analysis of wild yeasts for ethanol fermentation. Zymurgy 2016, 39, 81–88. [Google Scholar]
- Osburn, K.; Amaral, J.; Metcalf, S.R.; Nickens, D.M.; Rogers, C.M.; Sausen, C.; Caputo, R.; Miller, J.; Li, H.; Tennessen, J.M.; et al. Primary souring: A novel bacteria-free method for sour beer production. Food Microbiol. 2017, 70, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.M.; Veatch, D.; Covey, A.; Staton, C.; Bochman, M.L. Terminal acidic shock inhibits sour beer bottle conditioning by Saccharomyces cerevisiae. Food Microbiol. 2016, 57, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Independent We Stand. Craft Beer Goes Even More Local with the Farm-to-Glass Movement. 2017. Available online: https://www.independentwestand.org/craft-beer-and-the-farm-to-glass-movement/ (accessed on 28 February 2018).
- Kallenberger, M. Local Sourcing from the Craft Beer Drinker’s Perspective. New Brew. 2016, 34, 84–88. [Google Scholar]
- Olaitan, P.B.; Adeleke, O.E.; Ola, I.O. Honey: A reservoir for microorganisms and an inhibitory agent for microbes. Afr. Health Sci. 2007, 7, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.S.; Rabadzhiev, Y.; Eller, M.R.; Iliev, I.; Ivanova, I.; Santana, W.C. Microorganisms in Honey. In Honey Analysis; de Toledo, V.A.A., Ed.; InTech: Rijeka, Croatia, 2017. [Google Scholar]
- Loira, I.; Vejarano, R.; Banuelos, M.A.; Morata, A.; Tesfye, W.; Uthurry, C.; Villa, A.; Cintora, I.; Suarez-Lepe, J.A. Influence of sequential fermentation with Torulaspora delbrueckii and Saccharomyces cerevisiae on wine quality. LWT Food Sci. Technol. 2014, 59, 915–922. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Torulaspora delbrueckii contribution in mixed brewing fermentations with different Saccharomyces cerevisiae strains. Int. J. Food Microbiol. 2017, 259, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Torulaspora delbrueckii in the brewing process: A new approach to enhance bioflavour and to reduce ethanol content. Food Microbiol. 2016, 56, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Kopecka, J.; Meier-Dornberg, T.; Zarnkow, M.; Jacob, F.; Hutzler, M. Screening for new brewing yeasts in the non-Saccharomyces sector with Torulaspora delbrueckii as model. Yeast 2016, 33, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Pires, E.J.; Teixeira, J.A.; Branyik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etschmann, M.M.; Huth, I.; Walisko, R.; Schuster, J.; Krull, R.; Holtmann, D.; Wittmann, C.; Schrader, J. Improving 2-phenylethanol and 6-pentyl-α-pyrone production with fungi by microparticle-enhanced cultivation (MPEC). Yeast 2015, 32, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Al Toufailia, H.M.; Amiri, E.; Scandian, L.; Kryger, P.; Ratnieks, F.L.W. Towards integrated control of varroa: Effect of variation in hygienic behaviour among honey bee colonies on mite population increase and deformed wing virus incidence. J. Apic. Res. 2015, 53, 555–562. [Google Scholar] [CrossRef]
- Bigio, G.; Al Toufailia, H.M.; Hughes, W.O.H.; Ratnieks, F.L.W. The effect of one generation of controlled mating on the expression of hygienic behaviour in honey bees. J. Apic. Res. 2014, 53, 563–568. [Google Scholar] [CrossRef]
- Wilson-Rich, N.; Spivak, M.; Fefferman, N.H.; Starks, P.T. Genetic, individual, and group facilitation of disease resistance in insect societies. Annu. Rev. Entomol. 2009, 54, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Schoning, C.; Gisder, S.; Geiselhardt, S.; Kretschmann, I.; Bienefeld, K.; Hilker, M.; Genersch, E. Evidence for damage-dependent hygienic behaviour towards Varroa destructor-parasitised brood in the western honey bee, Apis mellifera. J. Exp. Biol. 2012, 215, 264–271. [Google Scholar] [CrossRef] [PubMed]
- WLP715 Champagne Yeast; White Labs: San Diego, CA, USA, 2016; Available online: https://www.whitelabs.com/yeast-bank/wlp715-champagne-yeast (accessed on 7 February 2018).
- Zimmermann, F.K.; Entian, K.-D. Yeast Sugar Metabolism: Biochemistry, Genetics, Biotechnology, and Applications; Technomic Publishing Company, Inc.: Lancaster, PA, USA, 1997. [Google Scholar]
- Fujita, I. Determination of Maltose in Honey. Int. J. Food Sci. Nutr. Diet. 2012, 1, 1–2. [Google Scholar]
- Briggs, D.E.; Hough, J.S.; Stevens, R.; Young, T.W. Malting and Brewing Science, 2nd ed.; St. Edmundsbury Press: Great Britain, UK, 1981. [Google Scholar]
- Smith, L. Walk on the Wild Side With Beer Made From Wasp Yeast. In The Plate; National Geographic: Washington, DC, USA, 2015; Available online: http://theplate.nationalgeographic.com/2015/09/29/walk-on-the-wild-side-with-beer-made-from-wasp-yeast/ (accessed on 28 February 2018).
- Hernandez, C.Y.; Serrato, J.C.; Quicazan, M.C. Evaluation of Physicochemical and Sensory Aspects of Mead, Produced by Different Nitrogen Sources and Commercial Yeast. Chem. Eng. Trans. 2015, 43, 1–6. [Google Scholar] [CrossRef]
- Gomes, T.; Dias, T.; Cadavez, V.; Verdial, J.; Sa Morais, J.; Ramalhosa, E.; Estevinho, L.M. Influence of Sweetness and Ethanol Content on Mead Acceptability. Pol. J. Food Nutr. Sci. 2015, 65, 137–142. [Google Scholar] [CrossRef]
- Sagon, E. Honey Schnapps: How We Made It; Cardinal Spirits: Bloomington, IN, USA, 2017; Available online: http://cardinalspirits.com/thedrop/honey-schnapps-how-we-made-it (accessed on 2 March 2018).
- Lee, Y.J.; Choi, Y.R.; Lee, S.Y.; Park, J.T.; Shim, J.H.; Park, K.H.; Kim, J.W. Screening wild yeast strains for alcohol fermentation from various fruits. Mycobiology 2011, 39, 33–39. [Google Scholar] [CrossRef] [PubMed]
| Strain | Honey Fermentation 1 | Beer Fermentation | ||||
|---|---|---|---|---|---|---|
| Final Gravity | ABV 2 | Final pH 3 | Final Gravity | ABV | Final pH | |
| S. cerevisiae WLP001 | N.D. | N.D. | N.D. | 1.011 | 4.33% | 4.40 |
| S. cerevisiae WLP715 | 0.992 | 11.5% | 4.25 | N.D. | N.D. | N.D. |
| T. delbrueckii YH178 | 0.992 | 11.5% | 4.15 | 1.041 | 0.39% | 5.40 |
| T. delbrueckii YH179 | 0.996 | 11.0% | 4.23 | 1.042 | 0.26% | 5.40 |
| YH178 + YH179 | 0.994 | 11.2% | 4.20 | N.D. | N.D. | N.D. |
| H. vineae YH72 | 1.018 | 8.2% | 3.31 | 1.016 | 3.68% | 3.26 |
| Yeast 1 | Appearance | Aroma | Acidity | Balance | Body | Flavor | Finish | Overall Quality | Total |
|---|---|---|---|---|---|---|---|---|---|
| YH178 | 2 ± 0 2 | 3.7 ± 0.9 | 1 ± 0.5 | 1 ± 0.5 | 0.9 ± 0.7 | 1.5 ± 0.3 | 1.3 ± 0.5 | 1.4 ± 0.2 | 12.8 ± 2.4 |
| YH179 | 2 ± 0 | 3.7 ± 1.2 | 1.1 ± 0.7 | 1.7 ± 0.4 | 1.4 ± 0.5 | 1.8 ± 0.4 | 1.8 ± 0.4 | 1.8 ± 0.4 | 15.3 ± 2.4 |
| 178/179 | 2 ± 0 | 4.5 ± 1.2 | 0.8 ± 0.6 | 0.9 ± 0.2 | 0.8 ± 0.6 | 1 ± 0.5 | 1.1 ± 0.2 | 1.2 ± 0.2 | 12.3 ± 1.9 |
| WLP715 | 2 ± 0 | 4.3 ± 0.8 | 0.8 ± 0.6 | 1.3 ± 0.4 | 0.6 ± 0.2 | 1.2 ± 0.6 | 1.1 ± 0.4 | 1.4 ± 0.3 | 12.7 ± 2.2 |
| YH72 | 2 ± 0 | 5.2 ± 0.7 | 1.5 ± 0 | 2 ± 0 | 0.7 ± 0.2 | 2 ± 0 | 2 ± 0 | 2 ± 0 | 17.4 ± 0.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barry, J.P.; Metz, M.S.; Hughey, J.; Quirk, A.; Bochman, M.L. Two Novel Strains of Torulaspora delbrueckii Isolated from the Honey Bee Microbiome and Their Use in Honey Fermentation. Fermentation 2018, 4, 22. https://doi.org/10.3390/fermentation4020022
Barry JP, Metz MS, Hughey J, Quirk A, Bochman ML. Two Novel Strains of Torulaspora delbrueckii Isolated from the Honey Bee Microbiome and Their Use in Honey Fermentation. Fermentation. 2018; 4(2):22. https://doi.org/10.3390/fermentation4020022
Chicago/Turabian StyleBarry, Joseph P., Mindy S. Metz, Justin Hughey, Adam Quirk, and Matthew L. Bochman. 2018. "Two Novel Strains of Torulaspora delbrueckii Isolated from the Honey Bee Microbiome and Their Use in Honey Fermentation" Fermentation 4, no. 2: 22. https://doi.org/10.3390/fermentation4020022






