Antiviral and Antioxidant Potential of Fungal Endophytes of Egyptian Medicinal Plants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Collected Medicinal Plants (Sampling)
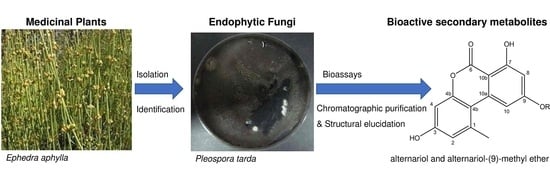
2.2. Isolation and Identification of Endophytic Fungi
2.3. Antioxidant and Antiviral Activities
2.4. Identification of the Secondary Metabolites Compounds of Pleospora tarda
3. Conclusions
4. Materials and Methods
4.1. Plant Material
4.2. Isolation of Endophytic Fungi
4.3. Identification of Endophytic Fungi
4.4. Fermentation and Extraction of Fungal Secondary Metabolites
4.5. Identification of Secondary Metabolites
4.6. Antioxidant Potential of Fungal Extracts
4.7. In Vitro Antiviral Assay of Endophytes Total Metabolites Using EPTT
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tan, R.; Zou, W. Endophytes: A rich source of functional metabolites. Nat. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.; Daisy, B.; Castillo, U.; Harper, J. Natural products from endophytic microorganisms. J. Nat. Prod. 2004, 67, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Selim, K.A.; El-Beih, A.A.; AbdEl-Rahman, T.M.; El-Diwany, A.I. Biology of Endophytic Fungi. Curr. Res. Environ. Appl. Mycol. 2012, 2, 31–82. [Google Scholar] [CrossRef]
- Selim, K.A.; Nagia, M.; El-Ghwas, D.E. Endophytic Fungi are Multifunctional Biosynthesizers: Ecological Role and Chemical Diversity. In Endophytic Fungi: Diversity, Characterization and Biocontrol, 1st ed.; NOVA Science Publishers: Hauppauge, NY, USA, 2016. [Google Scholar]
- Selim, K.A.; El-Ghwas, D.E.; Selim, R.M.; Hassan, M.I.A. Microbial volatile in defense. In Volatiles and Food Security: Role of Volatiles in Agro-Ecosystems, 1st ed.; Springer: Singapore, 2017. [Google Scholar]
- Bacon, C.; White, J. Microbial Endophytes; Marcel Dekker: New York, NY, USA, 2000. [Google Scholar]
- Soltan, M.M.; Zaki, A.K. Antiviral screening of forty-two Egyptian medicinal plants. J. Ethnopharmacol. 2009, 126, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Selim, K.A.; El-Beih, A.A.; AbdEl-Rahman, T.M.; El-Diwany, A.I. Biodiversity and antimicrobial activity of endophytes associated with Egyptian medicinal plants. Mycosphere 2011, 2, 669–678. [Google Scholar] [CrossRef]
- Abdel-Azeem, A.M.; Zaki, S.M.; Khalil, W.F.; Makhlouf, N.A.; Farghaly, L.M. Anti-rheumatoid Activity of Secondary Metabolites Produced by Endophytic Chaetomium globosum. Front. Microbiol. 2016, 7, 1477. [Google Scholar] [CrossRef] [PubMed]
- Selim, K.A.; El-Beih, A.A.; Abdel-Rahman, T.M.; El-Diwany, A.I. Biological evaluation of endophytic fungus, Chaetomium globosum JN711454, as potential candidate for improving drug discovery. Cell Biochem. Biophys. 2014, 68, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.K.E.J.; Ford, G.A.; Strobel, A.; Arif, D.M.; Grant, J.; Porco, D.P.; Tomer-Oneill, K. Pestacin: A 1,3-dihydro isobenzofuran from Pestalotiopsis microspora possessing antioxidant and antimycotic activities. Tetrahedron 2003, 59, 2471–2476. [Google Scholar] [CrossRef]
- Song, Y.C.; Huang, W.Y.; Sun, C.; Wang, F.W.; Tan, R.X. Characterization of graphislactone A as the antioxidant and free radical-scavenging substance from the culture of Cephalosporium sp. IFB-E001, an endophytic fungus in Trachelospermum jasminoides. Biol. Pharm. Bull. 2005, 28, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Münch, J.; Goerls, M.; Fiebig, H.H.; Lin, W.H.; Hertweck, C. Xiamycin, a pentacyclic indolosesquiterpene with selective anti-HIV activity from a bacterial mangrove endophyte. Bioorg. Med. Chem. Lett. 2010, 20, 6685–668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Sun, S.; Zhu, T.; Lin, Z.; Gu, J.; Li, D.; Gu, Q. Antiviral isoindolone derivatives from an endophytic fungus Emericella sp. associated with Aegiceras corniculatum. Phytochemistry 2011, 72, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Moubasher, A.H. Soil Fungi in Qatar and Other Arab Countries; Moreno, G., Diez, J., Manjon, J.L., Eds.; University of Qatar Press: Doha, Qatar, 1993; p. 566. [Google Scholar]
- Fröhlich, J.; Hyde, K.D.; Petrini, O. Endophytic fungi associated with palm. Mycol. Res. 2000, 104, 1202–1212. [Google Scholar] [CrossRef]
- Promputtha, I.; Jeewon, R.; Lumyong, S.; McKenzie, E.H.C.; Hyde, K.D. Ribosomal DNA fingerprinting in the identification of non sporulating endophytes from Magnolia liliifera (Magnoliaceae). Fungal Divers. 2005, 20, 167–186. [Google Scholar]
- Ravindran, C.; Naveenan, T. Adaptation of marine derived fungus Chaetomium globosum (NIOCC 36) to alkaline stress using antioxidant properties. Process Biochem. 2011, 46, 847–857. [Google Scholar] [CrossRef]
- Gu, W. Bioactive metabolites from Alternaria brassicicola ML-P08, an endophytic fungus residing in Malus halliana. World J. Microbiol. Biotechnol. 2009, 25, 1677–1683. [Google Scholar] [CrossRef]
- Lou, J.; Yu, R.; Wang, X.; Mao, Z.; Fu, L.; Liu, Y.; Zhou, L. Alternariol 9-methyl ether from the endophytic fungus Alternaria sp. Samif01 and its bioactivities. Braz. J. Microbiol. 2016, 47, 96–101. [Google Scholar] [CrossRef] [PubMed]
- He, J.W.; Chen, G.D.; Gao, H.; Yang, F.; Li, X.X.; Peng, T.; Guo, L.D.; Yao, X.S. Heptaketides with antiviral activity from three endolichenic fungal strain Nigrospora sp., Alternaria sp. and Phialophora sp. Fitoterapia 2012, 83, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, B.; Pfeiffer, E.; Metzler, M. Absorption and metabolism of the mycotoxins alternariol and alternariol-9-methyl ether in Caco-2 cells in vitro. Mycotoxin Res. 2009, 25, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Selim, K.A.; El-Beih, A.A.; Abdel-Rahman, T.M.; El-Diwany, A.I. High expression level of antioxidants and pharmaceutical bioactivities of endophytic fungus Chaetomium globosum JN711454. Prep. Biochem. Biotechnol. 2016, 46, 131–40. [Google Scholar] [CrossRef] [PubMed]
- Vlietinck, A.J.; Van, H.L.; Totte, J.; Lasure, A.; Berghe, D.V.; Rawangabo, P.C. Screening of hundred Rwandese medicinal plants for antimicrobial and antiviral properties. J. Ethnopharmacol. 1995, 46, 31–47. [Google Scholar] [CrossRef]
- El-Sayed, R.A. In Vitro Study on Cell–Virus Interaction under the Effect of Selected Ions Concentrations. Ph.D. Thesis, Medical Biophysics, Cairo University Egypt, Giza, Egypt, 2011. [Google Scholar]



| Plant Family | Host Medicinal Plants |
|---|---|
| Adiantaceae | Adiantum capillus-veneris |
| Asteraceae | Achillea fragrantissima |
| Artemisia herba alba | |
| Chiliadenus montanus | |
| Launaea spinosa | |
| Pulicaria undulate | |
| Tanacetum sinaicum | |
| Ephedraceae | Ephedra alata |
| Ephedra aphylla | |
| Euphorbiaceae | Euphorbia sanctae-catherine |
| Hypericaceae | Hypericum sinaicum |
| Lamiaceae | Lavandula coronopifolia |
| Phlomis aurea | |
| Stachys aegyptiaca | |
| Teucrium leucocladum | |
| Teucrium polium | |
| Thymus decussatus | |
| Rubiaceae | Galium sinaicum |
| Plant Family | Host Plant | Endophyte | % of Antioxidant Activity | % of CPE Inhibition of HSV | % of CPE Inhibition of VSV | Plant Family | Host Plant | Endophyte | % of Antioxidant Activity | % of CPE Inhibition of HSV | % of CPE Inhibition of VSV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adiantaceae | Adiantum capillus-veneris | Chaetomium globosum | 99% | 0% | −15.2 | Euphorbiaceae | Euphorbia sancte catherine | White sterile mycelia 3 | 23% | ND | ND |
| Penicillium sp. | 28% | ND | ND | White sterile mycelia 4 | 9% | ND | ND | ||||
| Asteraceae | Achillea fragrantissima | Dark sterile mycelia 1 | 4% | 14.8% | −6.8 | Dark sterile mycelia 3 | 8% | ND | ND | ||
| Dark sterile mycelia 2 | 15% | 0% | ND | Aspergillus sydowii | ---- | ND | ND | ||||
| White sterile mycelia 1 | 1% | 0% | 0 | Acremonium strictum | 1.5% | 2.2% | −6.8 | ||||
| White sterile mycelia 2 | 6% | 0% | −6.8 | Hypericaceae | Hypericum sinaicum | Ulocladium chartarum | 19% | 0% | ND | ||
| Penicillium corylophilum | ---- | ND | ND | Dark sterile mycelia | 14% | ND | ND | ||||
| Artimisia herba alba | Nigrospora sphaerica | 14% | ND | ND | Pleospora tarda | 16% | 0% | 8.4 | |||
| White sterile mycelia 1 | 15% | 0% | −6.8 | Chaetomium globosum | 12% | −14.8% | 6.8 | ||||
| Dark sterile mycelia | 14% | 0% | −15.2 | White sterile mycelia 1 | 12% | ND | ND | ||||
| White sterile mycelia 2 | 15% | 22.2% | 0 | Yeast | 7% | ND | ND | ||||
| Aspergillus flavus | 8% | ND | ND | White sterile mycelia 2 | 11% | 0% | 8.3 | ||||
| Chiliadenus montanus | Dark sterile mycelia | 10% | 14.8% | 0 | Lamiaceae | Lavandula coronopifolia | Dark sterile mycelia 1 | 9% | 17.0% | ND | |
| Nigrospora sphaerica | 13% | 14.8% | −15.2 | Dark sterile mycelia 2 | 21% | ND | ND | ||||
| Launea spinosa | Acremonium strictum | 6% | 14.8% | 0 | Dark sterile mycelia 3 | 5% | ND | ND | |||
| Acremonium sp. | 7% | ND | ND | Scopulariopsis sp. | ---- | ND | ND | ||||
| Penicillium chrysogenoum | 0.5% | −21.5% | −22.13 | Phlomis aurea | Dark sterile mycelia 1 | 2% | ND | ND | |||
| Aspergillus niger | 0.5% | 0% | −19.03 | White sterile mycelia | 8% | 0% | ND | ||||
| Pulicaria undulate | Dark sterile mycelia 1 | 21% | ND | ND | Dark sterile mycelia 2 | ---- | −18.5% | 0 | |||
| Dark sterile mycelia 2 | 15% | 0% | 8.4 | Chaetomium spirale | 2% | 0% | −15.26 | ||||
| Ulocladium chartarum | 8% | 0% | 8.4 | Penicillium sp. | ---- | −18.5% | 0 | ||||
| Penicillium sp. | 24% | 0% | −22.12 | Aspergillus flavus | 5.5% | ND | ND | ||||
| Dark sterile mycelia 3 | 14% | 26% | 8.4 | Stachys aegyptiaca | Mucor fuscus | ---- | 14.8% | 0% | |||
| Ulocladium atrum | 14% | ND | ND | White sterile mycelia 1 | 2% | ND | ND | ||||
| White sterile mycelia | 16% | ND | ND | Pleospora tarda | 1% | ND | ND | ||||
| Tanacetum sinaicum | White sterile mycelia 1 | 6% | −14.8% | 8.39 | White sterile mycelia 2 | ---- | ND | ND | |||
| White sterile mycelia 2 | 2% | ND | ND | Aspergillus flavus | 0.2% | 14.8% | −9.9% | ||||
| White sterile mycelia 3 | 1% | ND | ND | Teucrium leucocladum | White sterile mycelia 1 | 15% | 18.5% | ND | |||
| Penicillium chrysogenoum | ---- | ND | ND | White sterile mycelia 2 | 15% | 0% | 0 | ||||
| Penicillium sp. | 6% | 0% | 15.2 | Dark sterile mycelia 1 | 16% | −11.1% | −6.8 | ||||
| Aspergillus sydowii | 2% | ND | ND | Dark sterile mycelia 2 | 17% | ND | ND | ||||
| Ephedraceae | Ephedra alata | White sterile mycelia 1 | 9% | 40.7% | 15.2 | Dark sterile mycelia 3 | 14% | ND | ND | ||
| White sterile mycelia 2 | 15% | 0% | 8.3 | White sterile mycelia 3 | 15% | ND | ND | ||||
| Dark sterile mycelia 1 | 2% | ND | ND | Teucrium polium | Alternaria alternata | 11% | 0% | 15.2 | |||
| Dark sterile mycelia 2 | 7.5% | ND | ND | Nigrospora sphaerica | 1% | 0% | −15.4 | ||||
| Dark sterile mycelia 3 | 1% | −14.8% | 0 | White sterile mycelia 1 | 10% | 0% | 8.4 | ||||
| White sterile mycelia 3 | ---- | −11.1% | −6.8 | White sterile mycelia 2 | 18% | ND | ND | ||||
| Dark sterile mycelia 4 | 22% | ND | ND | Penicillium corylophilum | ---- | 0% | −15.2 | ||||
| Dark sterile mycelia5 | 4% | ND | ND | Penicillium chrysogenoum | ---- | ND | ND | ||||
| Unidentified bacteria | ---- | ND | ND | Aspergillus niger | 0.5% | 0% | 0 | ||||
| Ephedra aphylla | Pleospora tarda | 17% | 40.7% | 15.2% | Thymus decussates | Unidentified bacteria | 4% | ND | ND | ||
| Dark sterile mycelia 1 | ---- | 0% | ND | Rubiaceae | Galium sinaicum | Pleospora tarda 1 | 0 | 0% | −15.2% | ||
| Aspergillus versicolor | 11% | ND | ND | Fusarium oxysporum | ---- | ND | ND | ||||
| Dark sterile mycelia 2 | 6% | ND | ND | Ulocladium chartarum | ---- | ND | ND | ||||
| Aspergillus niger | ---- | ND | ND | Aspergillus sp. | ---- | −3.7% | 23.66% | ||||
| Euphorbiaceae | Euphorbia sancte catherine | Dark sterile mycelia 1 | 21% | 0% | 0 | Cochliobolus lunatus | 5% | ND | ND | ||
| White sterile mycelia 1 | 17% | 0% | ND | Pleospora tarda 2 | ---- | NA | ND | ||||
| Phoma leveillei | 19% | 14.8% | 15.2 | Absidia corymbifora | ---- | ND | ND | ||||
| White sterile mycelia 2 | 6% | ND | ND | Fusarium sp. | 1% | 11.1% | ND | ||||
| Dark sterile mycelia 2 | 8% | ND | ND |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selim, K.A.; Elkhateeb, W.A.; Tawila, A.M.; El-Beih, A.A.; Abdel-Rahman, T.M.; El-Diwany, A.I.; Ahmed, E.F. Antiviral and Antioxidant Potential of Fungal Endophytes of Egyptian Medicinal Plants. Fermentation 2018, 4, 49. https://doi.org/10.3390/fermentation4030049
Selim KA, Elkhateeb WA, Tawila AM, El-Beih AA, Abdel-Rahman TM, El-Diwany AI, Ahmed EF. Antiviral and Antioxidant Potential of Fungal Endophytes of Egyptian Medicinal Plants. Fermentation. 2018; 4(3):49. https://doi.org/10.3390/fermentation4030049
Chicago/Turabian StyleSelim, Khaled A., Waill A. Elkhateeb, Ahmed M. Tawila, Ahmed A. El-Beih, Tahany M. Abdel-Rahman, Ahmed I. El-Diwany, and Eman F. Ahmed. 2018. "Antiviral and Antioxidant Potential of Fungal Endophytes of Egyptian Medicinal Plants" Fermentation 4, no. 3: 49. https://doi.org/10.3390/fermentation4030049