Soaking in Aqueous Ammonia (SAA) Pretreatment of Whole Corn Kernels for Cellulosic Ethanol Production from the Fiber Fractions
Abstract
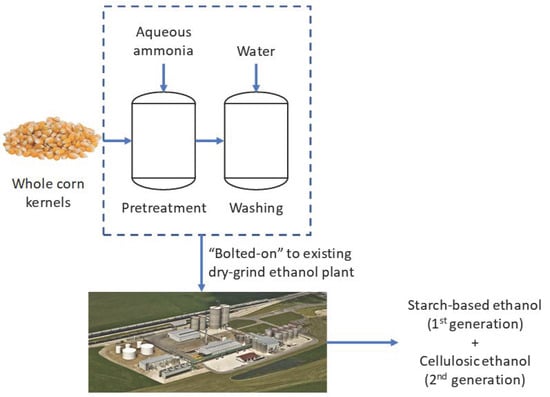
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Pretreatment of Corn Kernels
2.2.2. Ethanol Fermentation
2.2.3. Analytical Methods
3. Results
3.1. Aqueous Ammonia Treatment of Corn
3.2. Ethanol Fermentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- U.S. Energy Facts Explained. U.S. Energy Information Administration. Available online: https://www.eia.gov/energyexplained/?page=us_energy_home (accessed on 8 August 2018).
- U.S. Bioenergy Statistics. United States Department of Agriculture Economic Research Service. Available online: https://www.ers.usda.gov/data-products/us-bioenergy-statistics (accessed on 8 August 2018).
- Typical Composition of Yellow Dent Corn. Available online: https://www.bungeservices.com/irj/go/km/docs/documents/Public%20Documents/millingSite/Documents%20and%20Forms/Attachments/Corn%20Milling%20Process.pdf (accessed on 8 August 2018).
- Kim, D.; Orrego, D.; Ximenes, E.; Ladisch, M. Cellulose conversion of corn pericarp without pretreatment. Bioresour. Technol. 2017, 245, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, M.; Kalman, G.; Reczey, K. Corn fiber as a raw material for hemicellulose and ethanol production. Process Biochem. 2007, 42, 1135–1139. [Google Scholar] [CrossRef]
- Lynd, L. The grand challenge of cellulosic biofuels. Nat. Biotechnol. 2017, 35, 912–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- 2017 Ethanol Industry Outlook. Renewable Fuels Association. Available online: http://www.ethanolrfa.org/wp-content/uploads/2017/02/Ethanol-Industry-Outlook-2017.pdf (accessed on 9 August 2018).
- Biotechnology Industry Organization. The value proposition for cellulosic and advanced biofuels under the US federal renewable fuel standard. Ind. Biotechnol. 2011, 7, 111–117. [Google Scholar] [CrossRef]
- McPhail, L.; Westcott, P.; Lutman, H. The Renewable Identification Number System and U.S. Biofuel Mandates. United States Department of Agriculture. Available online: http://usda.mannlib.cornell.edu/usda/ers/BioEnergy/2010s/2011/BioEnergy-11-08-2011.pdf (accessed on 9 August 2018).
- Renewable Fuel Standard Program Regulatory Impact Analysis. United States Environmental Protection Agency. Available online: https://www.epa.gov/sites/production/files/2015-08/documents/420r07004.pdf (accessed on 9 August 2018).
- Rastogi, M.; Shrivastava, S. Recent advances in second generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes. Renew. Sustain. Energy Rev. 2017, 80, 330–340. [Google Scholar] [CrossRef]
- Maurya, D.; Singla, A.; Negi, S. An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 2015, 5, 597–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.; Kim, T.H.; Lee, Y.Y. Substrate dependency and effect of xylanase supplementation on enzymatic hydrolysis of ammonia-treated biomass. Appl. Biochem. Biotechnol. 2008, 148, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, N.P.; Montanti, J.; Johnston, D.; Drapcho, C. Fractionation of corn fiber treated by soaking in aqueous ammonia (SAA) for isolation of hemicellulose B and production of C5 sugars by enzyme hydrolysis. Appl. Biochem. Biotechnol. 2011, 164, 1390–1404. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, N.P.; Montanti, J.; Kim, T.H. Pretreatment of dried distillers grains with solubles by soaking in aqueous ammonia and subsequent enzymatic/dilute acid hydrolysis to produce fermentable sugars. Appl. Biochem. Biotechnol. 2016, 179, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.O.S.; Junqueria, T.L.; Cavalett, O.; Cunha, M.P.; Jesus, C.D.F.; Rossell, C.E.V.; Filho, R.M.; Bonomi, A. Integrated versus stand-alone second generation ethanol production from sugarcane bagasse and trash. Bioresour. Technol. 2012, 103, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Drapcho, C.M.; Nghiem, N.P.; Walker, T.H. Biofuels Engineering Process Technology, 1st ed.; McGraw-Hill: New York, NY, USA, 2008; pp. 105–174. ISBN 978-0-07-148749-8. [Google Scholar]
- A Guide to Distiller’s Dried Grains with Solubles. U.S. Grains Council. Available online: https://ethanolrfa.org/wp-content/uploads/2015/11/2012_DDGS_Handbook-1.pdf (accessed on 9 August 2018).
- Advantages of D3Max. Available online: https://www.d3maxllc.com/d3max-advantages (accessed on 24 August 2018).
- Quad County Corn Processors. Available online: https://ethanol.org/Delayne%20Johnson%20Quiet%20Ingenuity.pdf (accessed on 24 August 2018).
- Kim, T.H.; Nghiem, N.P.; Hicks, K.B. Pretreatment and fractionation of corn stover by soaking in ethanol and aqueous ammonia. Appl. Biochem. Biotechnol. 2009, 153, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Megazyme Total Starch Assay Procedure. Available online: https://secure.megazyme.com/files/Booklet/K-TSTA_DATA.pdf (accessed on 10 August 2018).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 10 August 2018).
- Srivastava, V.K.; Mowat, D.N. Preservation and processing of whole high moisture shelled corn with ammonia. Can. J. Anim. Sci. 1980, 60, 683–688. [Google Scholar] [CrossRef]
- Accellerase® 1500: Cellulase Enzyme Complex for Lignocellulosic Biomass Hydrolysis. Available online: http://www.shinshu-u.ac.jp/faculty/engineering/chair/chem010/manual/accellerase1500_Dupont.pdf (accessed on 16 August 2018).
- Sendich, E.N.; Laser, M.; Kim, S.; Alizadeh, H.; Laureano-Perez, L.; Dale, B.; Lynd, L. Recent process improvements for ammonia fiber expansion (AFEX) process and resulting reductions in minimum ethanol selling price. Bioresour. Technol. 2008, 99, 8429–8435. [Google Scholar] [CrossRef] [PubMed]



| Component | Content (wt%, Dry Basis) |
|---|---|
| Glucan | 17.51 ± 0.47 |
| Xylan | 14.00 ± 0.46 |
| Arabinan | 8.65 ± 0.32 |
| Lignin | 20.09 ± 0.15 |
| Ash | 0.16 ± 0.02 |
| Concentration of NH4OH Solution (wt%) | 2.5 | 5 | 7.5 | 10 |
|---|---|---|---|---|
| Mass of starting corn (g dry basis) | 263.43 | 260.40 | 260.66 | 260.48 |
| Mass of treated corn after washing (g dry basis) | 256.46 | 252.42 | 246.65 | 246.47 |
| Mass loss (% of original mass) | 2.65 | 3.06 | 5.37 | 5.38 |
| Experiment | Final Ethanol Concentration (g/L) | Yield (g Ethanol/kg Treated Corn) | Yield (g Ethanol/kg Raw Corn) |
|---|---|---|---|
| Corn treated with 2.5 wt% ammonia without cellulase | 103.91 ± 1.05 | 349.9 ± 3.1 | 340.7 ± 3.0 |
| Corn treated with 2.5 wt% ammonia with cellulase | 106.31 ± 0.76 | 359.3 ± 2.2 | 349.7 ± 2.2 |
| Corn treated with 5 wt% ammonia without cellulase | 108.79 ± 0.24 | 369.0 ± 0.7 | 357.7 ± 0.7 |
| Corn treated with 5 wt% ammonia with cellulase | 110.83 ± 0.58 | 377.0 ± 1.7 | 365.5 ± 1.6 |
| Corn treated with 7.5 wt% ammonia without cellulase | 112.16 ± 1.40 | 382.3 ± 4.1 | 361.7 ± 3.9 |
| Corn treated with 7.5 wt% ammonia with cellulase | 116.84 ± 0.36 | 401.0 ± 1.1 | 379.5 ± 1.0 |
| Corn treated with 7.5 wt% ammonia with cellulase plus urea | 114.69 ± 1.0 | 392.4 ± 3.7 | 371.3 ± 3.5 |
| Corn treated with 10 wt% ammonia without cellulase | 104.86 ± 0.36 | 353.6 ± 2.9 | 334.6 ± 2.8 |
| Corn treated with 10 wt% ammonia with cellulase | 112.98 ± 0.54 | 385.6 ± 1.1 | 364.8 ± 1.0 |
| Untreated corn without cellulase | 99.84 ± 1.67 | 334.2 ± 1.6 | |
| Untreated corn with cellulase | 102.64 ± 1.25 | 345.0 ± 4.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norvell, K.L.; Nghiem, N.P. Soaking in Aqueous Ammonia (SAA) Pretreatment of Whole Corn Kernels for Cellulosic Ethanol Production from the Fiber Fractions. Fermentation 2018, 4, 87. https://doi.org/10.3390/fermentation4040087
Norvell KL, Nghiem NP. Soaking in Aqueous Ammonia (SAA) Pretreatment of Whole Corn Kernels for Cellulosic Ethanol Production from the Fiber Fractions. Fermentation. 2018; 4(4):87. https://doi.org/10.3390/fermentation4040087
Chicago/Turabian StyleNorvell, Katherine L., and Nhuan P. Nghiem. 2018. "Soaking in Aqueous Ammonia (SAA) Pretreatment of Whole Corn Kernels for Cellulosic Ethanol Production from the Fiber Fractions" Fermentation 4, no. 4: 87. https://doi.org/10.3390/fermentation4040087