Response of Eustoma Leaf Phenotype and Photosynthetic Performance to LED Light Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Leaf Morphological Measurements
2.3. Leaf Gas Exchange and Photosynthetic Measurements
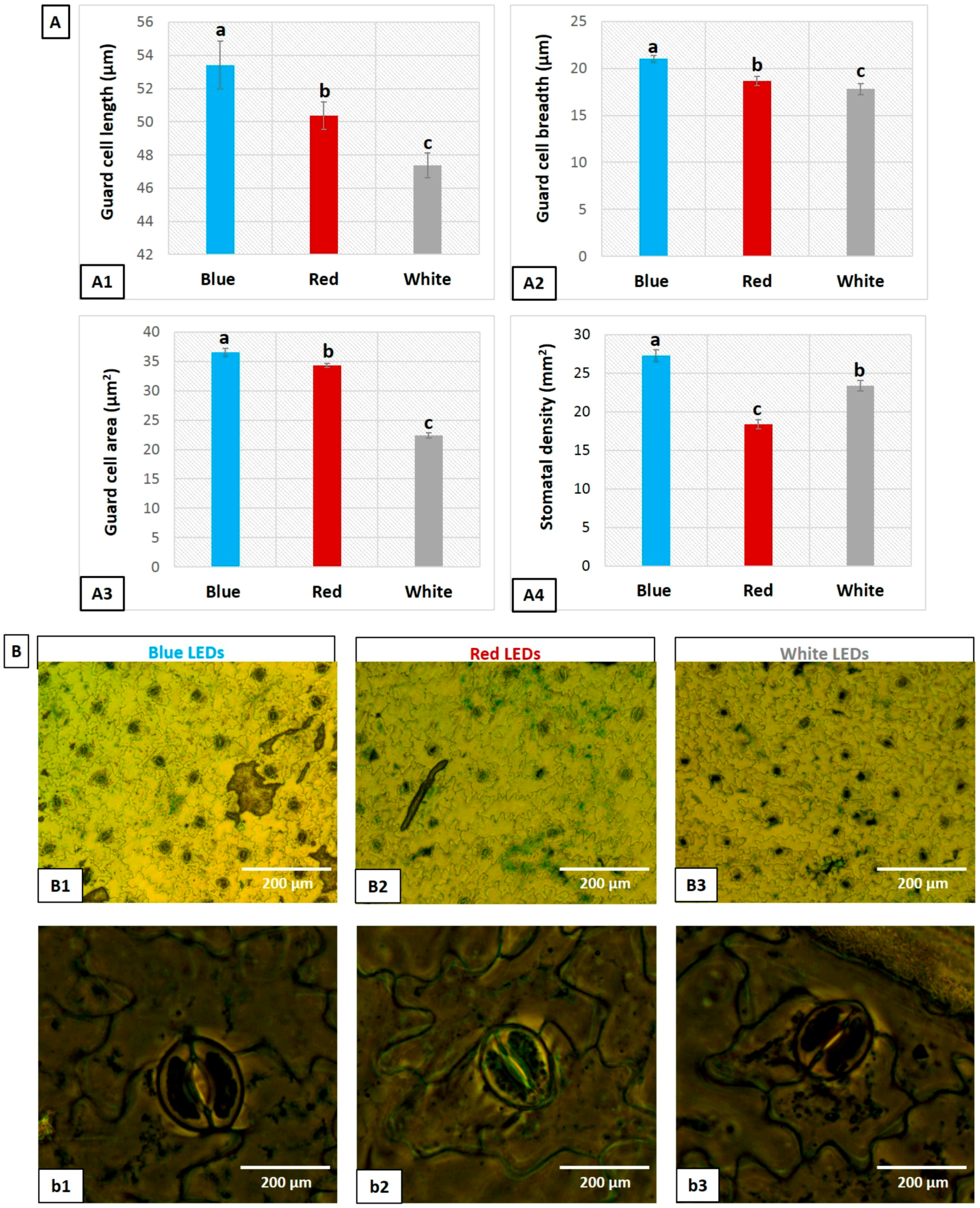
2.4. Leaf Anatomy and Stomatal Character Measurements
2.5. Statistical Analysis
3. Results
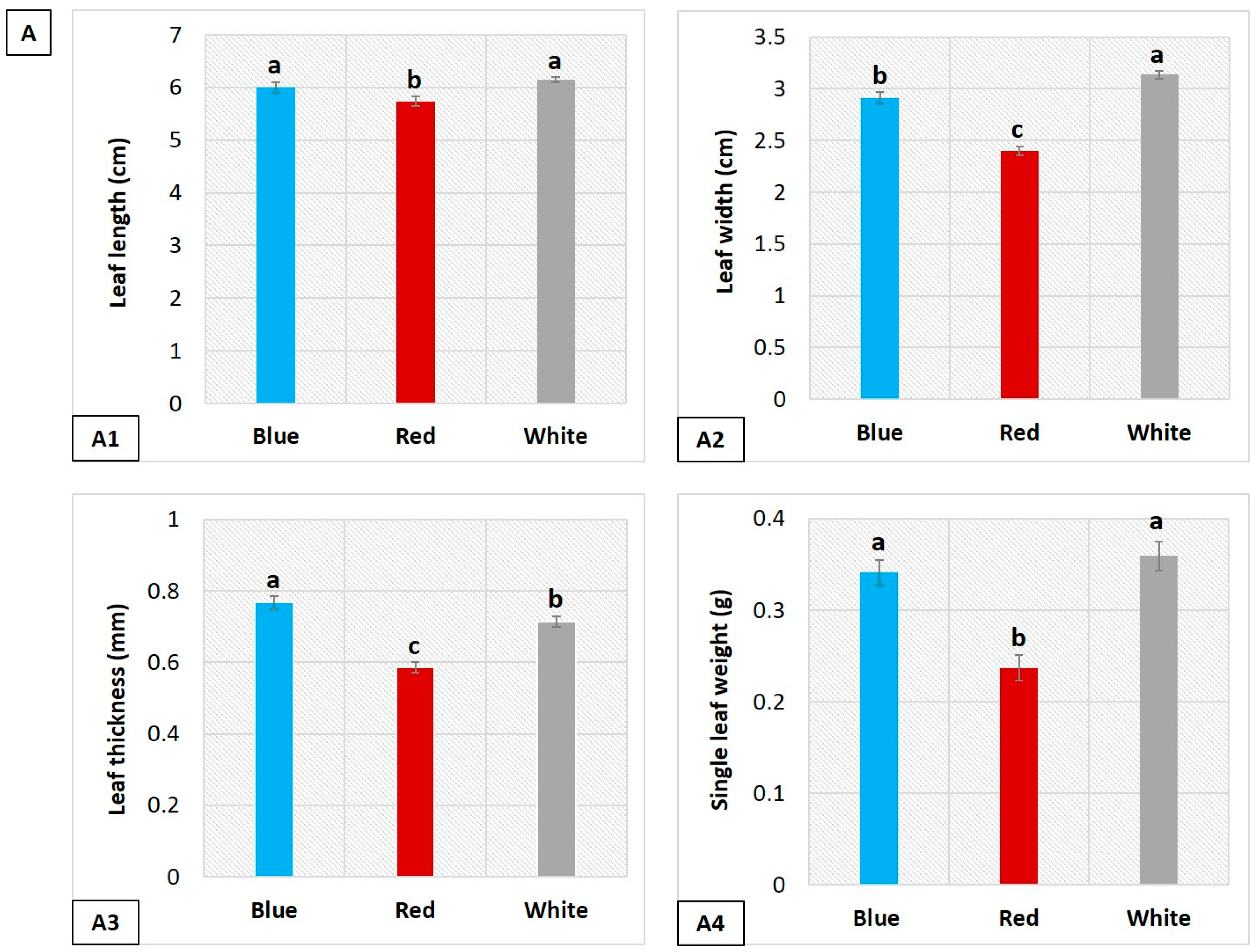
3.1. Influence of LEDs on Leaf Morphology
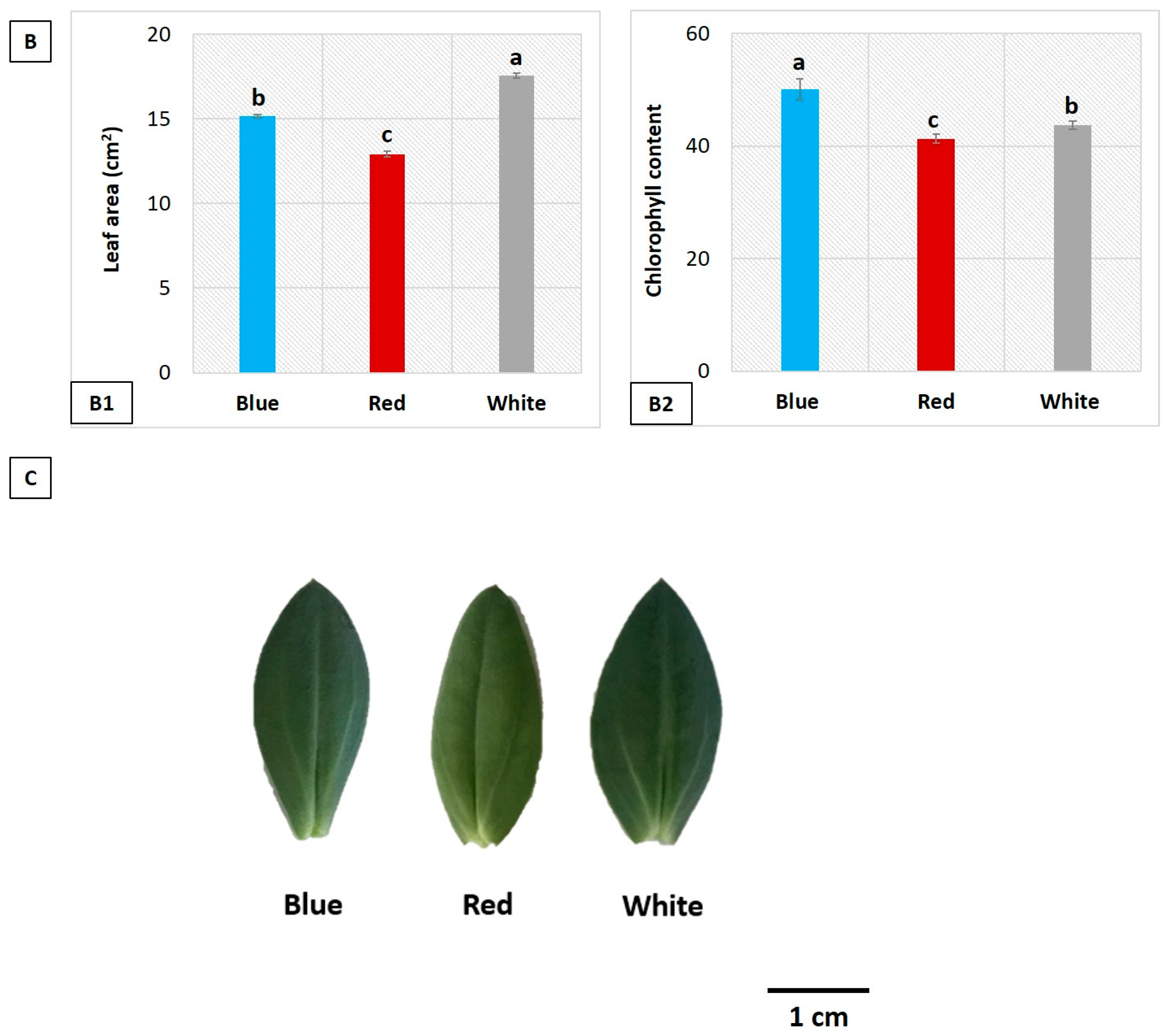
3.2. Influence of LEDs on Leaf Area and Chlorophyll Content
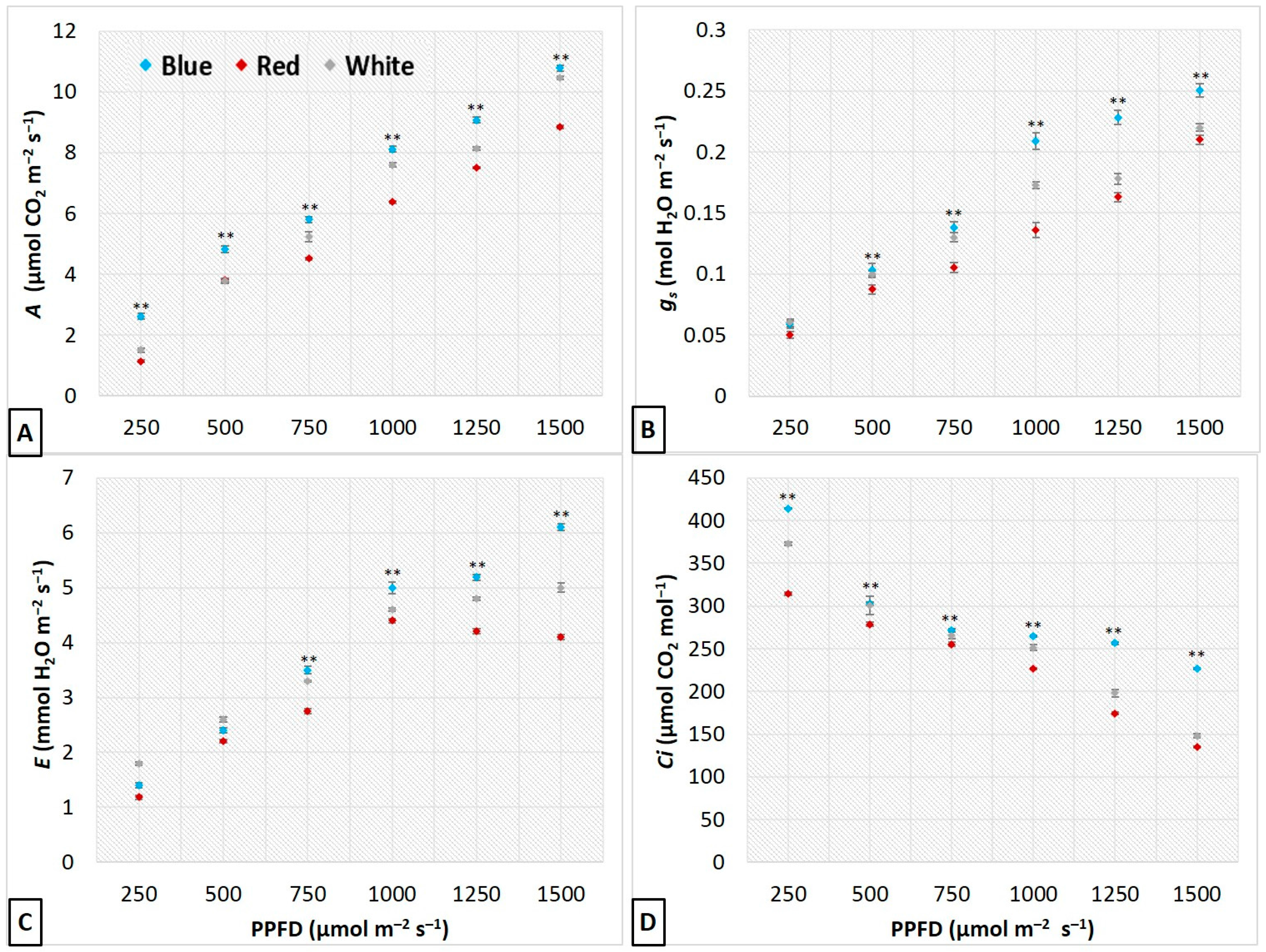
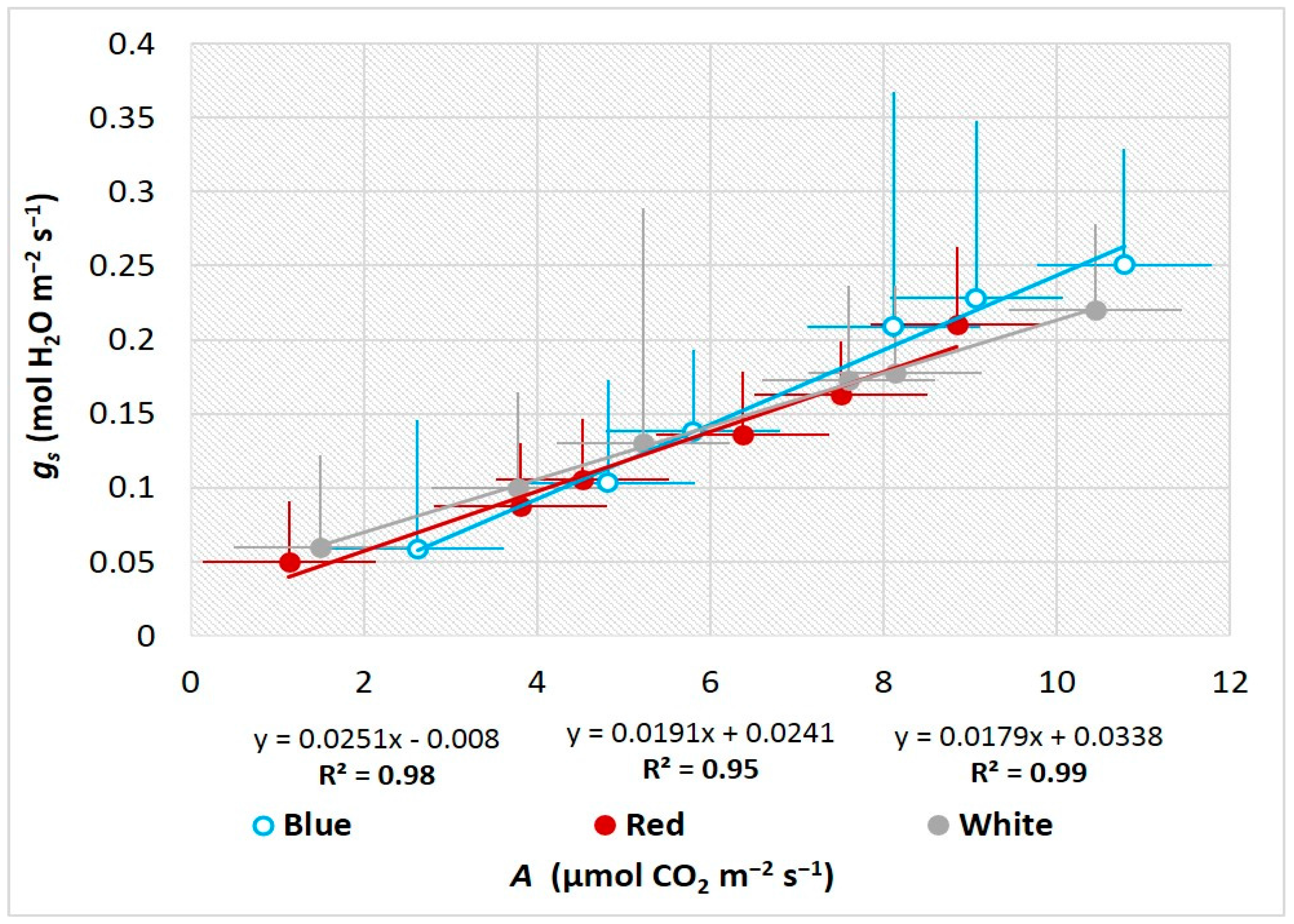
3.3. Influence of LEDs on Leaf Gas Exchange and Photosynthetic Performance
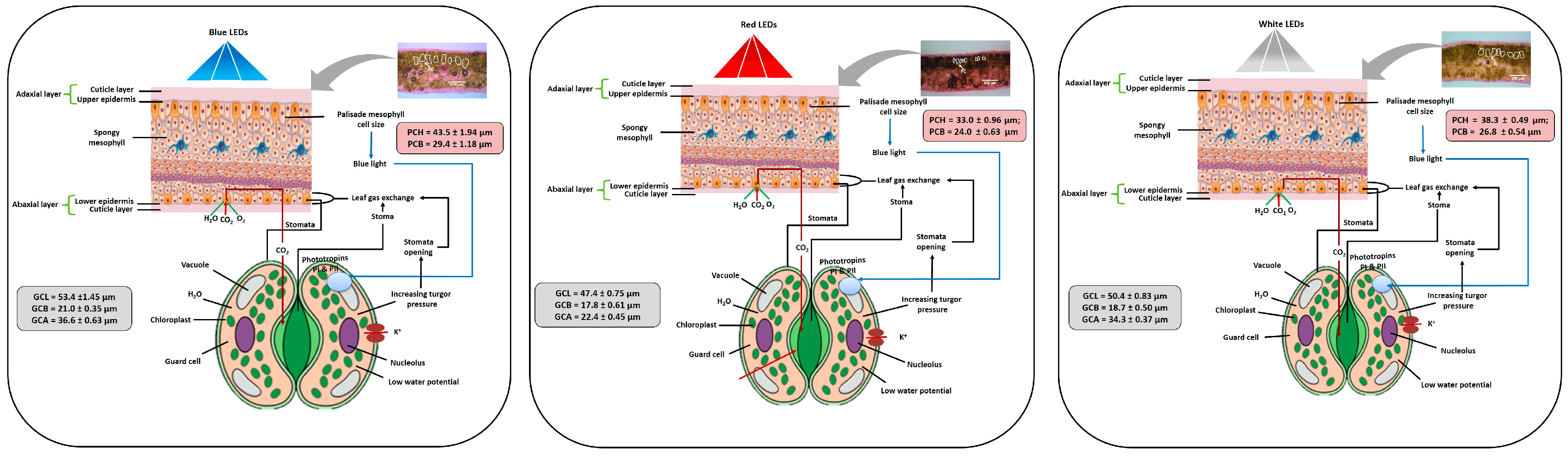
3.4. Influence of LEDs on Leaf Epidermal Layer
3.5. Influence of LEDs on Stomata
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| LED | Light-emitting diode |
| B | Blue |
| R | Red |
| W | White |
| A | Photosynthetic rate |
| gs | Stomatal conductance |
| E | Transpiration |
| Ci | Intercellular CO2 concentration |
| PPFD | Photosynthetic photon flux density |
| PSI and PSII | Photosystem I and II |
| NPQ | Non-photochemical quenching |
| RuBP | Ribulose 1,5-biphosphate |
| RuBisCO | Ribulose-1,5-bisphosphate carboxylase oxygenase |
References
- Massa, G.D.; Kim, H.-H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity in response to LED lighting. HortScience 2008, 43, 1951–1956. [Google Scholar]
- Marcelis, L.F.M.; Elings, A.; Bakker, M.; Brajeul, E.; Dieleman, J.A.; de Visser, P.H.B.; Heuvelink, E. Modelling dry matter production and partitioning in sweet pepper. Acta Hortic. 2006, 718, 121–128. [Google Scholar] [CrossRef]
- Mitchell, C.A. Plant lighting in controlled environments for space and earth applications. Acta Hortic. 2012, 956, 23–36. [Google Scholar] [CrossRef]
- Poulet, L.; Massa, G.D.; Morrow, R.C.; Bourget, C.M.; Wheeler, R.M.; Mitchell, C.A. Significant reduction in energy for plant-growth lighting in space using targeted LED lighting and spectral manipulation. Life Sci. Space Res. 2014, 2, 43–53. [Google Scholar] [CrossRef]
- Morrow, R.C. LED lighting in horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar]
- Pfündel, E.; Baake, E. A quantitative description of fluorescence excitation spectra in intact bean leaves greened under intermittent light. Photosynth. Res. 1990, 26, 19–28. [Google Scholar] [PubMed]
- Christie, J.M. Phototropin blue-light receptors. Annu. Rev. Plant Biol. 2007, 58, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.I.; Kinoshita, T.; Matsumoto, M.; Nakayama, K.I.; Doi, M.; Shimazaki, K. Blue light-induced autophosphorylation of phototropin is a primary step for signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 5626–5631. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gu, M.; Cui, J.; Shi, K.; Zhou, Y.; Yu, J. Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J. Photochem. Photobiol. B Biol. 2009, 96, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Kuwar, G.; Clarke, J.L.; Blystad, D.R.; Gislerød, H.R.; Olsen, J.E.; Torre, S. Artificial light from light emitting diodes (LEDs) with a high portion of blue light results in shorter poinsettias compared to high pressure sodium (HPS) lamps. Sci. Hortic. 2012, 147, 136–143. [Google Scholar] [CrossRef]
- Nascimento, L.; Leal-Costa, M.V.; Coutinho, M.A.; Moreira, N.D.S.; Lage, C.L.; Barbi, N.D.S.; Tavares, E.S. Increased antioxidant activity and changes in phenolic profile of Kalanchoe pinnata (Lamarck) Persoon (Crassulaceae) specimens grown under supplemental blue light. Photochem. Photobiol. 2013, 89, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.N.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar]
- Appelgren, M. Effects of light quality on stem elongation of Pelargonium in vitro. Sci Hortic. 1991, 45, 345–351. [Google Scholar] [CrossRef]
- Sæbø, A.; Krekling, T.; Appelgren, M. Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell Tissue Organ Cult. 1995, 41, 177–185. [Google Scholar] [CrossRef]
- Briggs, W.R.; Huala, E. Blue-light photoreceptors in higher plants. Annu. Rev. Cell Dev. Biol. 1999, 15, 33–62. [Google Scholar] [CrossRef] [PubMed]
- Ouzounis, T.; Parjikolaei, B.R.; Fretté, X.; Rosenqvist, E.; Ottosen, C.O. Predawn and high intensity application of supplemental blue light decreases the quantum yield of PSII and enhances the amount of phenolic acids, flavonoids, and pigments in Lactuca sativa. Front. Plant Sci. 2015, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.H.; Jung, S. Effects of light-emitting diode irradiation on growth characteristics and regulation of porphyrin biosynthesis in rice seedlings. Int. J. Mol. Sci. 2017, 18, 641. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, K.; Doi, M.; Assmann, S.M.; Kinoshita, T. Light regulation of stomatal movement. Annu. Rev. Plant Biol. 2007, 58, 219–247. [Google Scholar] [CrossRef] [PubMed]
- Mott, K.A.; Sibbernsen, E.D.; Shope, J.C. The role of the mesophyll in stomatal responses to light and CO2. Plant Cell Environ. 2008, 31, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Kim, J.S.; Hong, J.K.; Lee, Y.-H.; Choi, B.S.; Seol, Y.J.; Jeon, C.H. Comparative mapping, genomic structure, and expression analysis of eight pseudo-response regulator genes in Brassica rapa. Mol. Genet. Genom. 2012, 287, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Noguchi, K.; Terashima, I. Photosynthesis Dependent and -independent responses of stomata to blue, red and green monochromatic light: Differences between the normally oriented and inverted leaves of sunflower. Plant Cell Physiol. 2011, 52, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.; Niu, G. Chapter 27—Challenges for the next-generation PFAL. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production, 1st ed.; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press Elsevier: Amsterdam, The Netherlands, 2016; pp. 387–393. [Google Scholar]
- Monteith, J.; Unsworth, M. Principles of Environmental Physics; Academic Press: Elsevier, London, UK, 2007. [Google Scholar]
- Niinemets, Ü. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Yamori, W.; Takahashi, S.; Makino, A.; Price, G.D.; Badger, M.R.; Von Caemmerer, S. The roles of ATP synthase and the cytochrome b6/f complexes in limiting chloroplast electron transport and determining photosynthetic capacity. Plant Physiol. 2011, 155, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Marchese, J.A.; Mattana, R.S.; Ming, L.C.; Broetto, F.; Vendramini, P.F.; Moraes, R.M. Irradiance stress responses of gas exchange and antioxidant enzyme contents in pariparoba [Pothomorphe umbellata (L.) Miq.] plants. Photosynthetica 2005, 46, 501–505. [Google Scholar] [CrossRef]
- Li, T.; Heuvelink, E.; Dueck, T.A.; Janse, J.; Gort, G.; Marcelis, L.F.M. Enhancement of crop photosynthesis by diffuse light: Quantifying the contributing factors. Ann. Bot. 2014, 114, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: New York, NY, USA, 1999; Volume 198. [Google Scholar]
- Zeng, B.; Wang, Q.Y.; Tang, C.M. Anatomic analysis on heterosis in three transgenic bt pest-resistant hybrid cotton (G. hirsutum L.). Acta Agron. Sin. 2008, 34, 496–505. [Google Scholar] [CrossRef]
- Whitelam, G.; Halliday, K. Light and Plant Development; Blackwell Publishing: Oxford, UK, 2007. [Google Scholar]
- Chen, X.L.; Guo, W.Z.; Xue, X.Z.; Wang, L.C.; Qiao, X.J. Growth and quality responses of ‘Green Oak Leaf’lettuce as affected by monochromic or mixed radiation provided by fluorescent lamp (FL) and light-emitting diode (LED). Sci. Hortic. 2014, 172, 168–175. [Google Scholar] [CrossRef]
- Frąszczak, B.; Golcz, A.; Zawirska-Wojtasiak, R.; Janowska, B. Growth rate of sweet basil and lemon balm plants grown under fluorescent lamps and led modules. Acta Sci. Pol. Hortorum Cultus 2014, 13, 3–13. [Google Scholar]
- Tsukaya, H. The leaf index: Heteroblasty, natural variation and the genetic control of polar processes of leaf expansion. Plant Cell Physiol. 2002, 43, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Hirose, F.; Inagaki, N.; Hanada, A.; Yamaguchi, S.; Kamiya, Y.; Miyao, A.; Hirochika, H.; Takano, M. Cryptochrome and phytochrome cooperatively but independently reduce active gibberellin content in rice seedlings under light irradiation. Plant Cell Physiol. 2012, 53, 1570–1582. [Google Scholar] [CrossRef] [PubMed]
- Godo, T.; Fujiwara, K.; Guan, K.; Miyoshi, K. Effects of wavelength of LED-light on in vitro asymbiotic germination and seedling growth of Bletilla ochracea Schltr.(Orchidaceae). Plant Biotechnol. 2011, 28, 397–400. [Google Scholar] [CrossRef]
- Kozuka, T.; Suetsugu, N.; Wada, M.; Nagatani, A. Antagonistic regulation of leaf flattening by phytochrome B and phototropin in Arabidopsis thaliana. Plant Cell Physiol. 2013, 54, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kook, H.S.; Jang, Y.J.; Lee, W.H.; Kamala-Kannan, S.; Chae, J.C.; Lee, K.J. The effect of blue-light-emitting diodes on antioxidant properties and resistance to Botrytis cinerea in tomato. J. Plant Pathol. Microb. 2013, 4, 203. [Google Scholar]
- Wilson, D.; Cooper, J.P. Effect of light intensity during growth on leaf anatomy and subsequent light-saturated photosynthesis among contrasting lolium genotypes. New Phytol. 1969, 68, 1125–1135. [Google Scholar] [CrossRef]
- Ye, Y.M.; Tong, J.; Shi, X.P.; Yuan, W.; Li, G.R. Morphological and cytological studies of diploid and colchicine-induced tetraploid lines of crape myrtle (Lagerstroemia indica L.). Sci. Hortic. 2010, 124, 95–101. [Google Scholar] [CrossRef]
- Mortensen, L.M.; Strømme, E. Effects of light quality on some greenhouse crops. Sci. Hortic. 1987, 33, 27–36. [Google Scholar] [CrossRef]
- Zhang, G.; Shen, S.; Takagaki, M.; Kozai, T.; Yamori, W. Supplemental upward lighting from underneath to obtain higher marketable lettuce (Lactuca sativa) leaf fresh weight by retarding senescence of outer leaves. Front. Plant Sci. 2015, 6, 1110. [Google Scholar] [CrossRef] [PubMed]
- Dougher, T.A.; Bugbee, B. Differences in the response of wheat, soybean and lettuce to reduced blue radiation. Photochem. Photobiol. 2001, 73, 199–207. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, X.M.; Cui, J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 2015, 53, 213–222. [Google Scholar] [CrossRef]
- Davies, K. (Ed.) Plant pigments and their manipulation. Annu. Plant Rev. 2004, 14, 1–342. [Google Scholar]
- Buschmann, C.; Meier, D.; Kleudgen, H.K.; Lichtenthaler, H.K. Regulation of chloroplast development by red and blue light. Photochem. Photobiol. 1978, 27, 195–198. [Google Scholar] [CrossRef]
- Lamb, J.J.; Eaton-Rye, J.J.; Hohmann-Marriott, M.F. An LED-based fluorometer for chlorophyll quantification in the laboratory and in the field. Photosynth. Res. 2012, 114, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Hsu, M.H.; Yang, Z.W.; Yang, C.M. The effects of red blue and white light-emitting diodes on the growth development and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. Capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Muneer, S.; Kim, E.J.; Park, J.S.; Lee, J.H. Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). Int. J. Mol. Sci. 2014, 15, 4657–4670. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, R.; Ohashi-Kaneko, K.; Fujiwara, K.; Goto, E.; Kurata, K. Photosynthetic characteristics of rice leaves grown under red light with or without supplemental blue light. Plant Cell Physiol. 2004, 45, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Yano, A.; Fujiwara, K. Plant lighting system with five wavelength-band light-emitting diodes providing photon flux density and mixing ratio control. Plant Methods. 2012, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Lanoue, J.; Leonardos, E.D.; Ma, X.; Grodzinski, B. The effect of spectral quality on daily patterns of gas exchange, biomass gain, and water-use-efficiency in tomatoes and lisianthus: An assessment of whole plant measurements. Front. Plant Sci. 2017, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-light supplementation for enhanced lettuce growth under red- and blue-light-emitting diodes. HortScience 2004, 39, 1617–1622. [Google Scholar]
- Sabir, A.; Yazar, K. Diurnal dynamics of stomatal conductance and leaf temperature of grapevines (Vitis vinifera L.) in response to daily climatic variables. Acta Sci. Pol. Hortorum Cultus 2015, 14, 3–15. [Google Scholar]
- Savvides, A.; Fanourakis, D.; van Ieperen, W. Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves. J. Expt. Bot. 2012, 63, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Whiteman, P.C.; Koller, D. Interactions of carbon dioxide concentration, light intensity and temperature on plant resistance to water vapour and carbon dioxide diffusion. New Phytol. 1967, 66, 463–473. [Google Scholar] [CrossRef]
- Lawson, T.; Oxborough, K.; Morison, J.I.; Baker, N.R. The responses of guard and mesophyll cell photosynthesis to CO2, O2, light, and water stress in a range of species are similar. J. Exp. Bot. 2003, 54, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Morison, J.I.; Gallouët, E.; Lawson, T.; Cornic, G.; Herbin, R.; Baker, N.R. Lateral diffusion of CO2 in leaves is not sufficient to support photosynthesis. Plant Physiol. 2005, 139, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Wang, F.; Coe, R.A.; Karki, S.; Wanchana, S.; Thakur, V.; Henry, A.; Lin, H.C.; Huang, J.; Peng, S.; Quick, W.P. Overexpression of Ossap16 regulates photosynthesis and the expression of a broad range of stress response genes in rice (Oryza sativa L.). PLoS ONE 2016, 11, e0157244. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Wheeler, R.M.; Sager, J.C.; Goins, G.D.; Norikane, J.H. Evaluation of lettuce growth using supplemental green light with red and blue light-emitting diodes in a controlled environment—A review of research at Kennedy Space Center. Acta Hortic. 2006, 711, 111–119. [Google Scholar] [CrossRef]
- Yarkhunova, Y.; Edwards, C.E.; Ewers, B.E.; Baker, R.L.; Aston, T.L.; McClung, C.R.; Lou, P.; Weinig, C. Selection during crop diversification involves correlated evolution of the circadian clock and ecophysiological traits in Brassica rapa. New Phytol. 2016, 210, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Kozuka, T.; Kong, S.G.; Doi, M.; Shimazaki, K.I.; Nagatani, A. Tissue-autonomous promotion of palisade cell development by phototropin 2 in Arabidopsis. Plant Cell 2011, 23, 3684–3695. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Fujita, M.; Ohta, Y.; Sase, S.; Nishimura, S.; Ezura, H. Directional blue light irradiation triggers epidermal cell elongation of abaxial side resulting in inhibition of leaf epinasty in geranium under red light condition. Sci. Hortic. 2008, 115, 176–182. [Google Scholar] [CrossRef]
- Macedo, A.F.; Leal-Costa, M.V.; Tavares, E.S.; Lage, C.L.S.; Esquibel, M.A. The effect of light quality on leaf production and development of in vitro-cultured plants of Alternanthera brasiliana Kuntze. Environ. Exp. Bot. 2011, 70, 43–50. [Google Scholar] [CrossRef]
- Lee, S.H.; Tewari, R.K.; Hahn, E.J.; Paek, K.Y. Photon flux density and light quality induce changes in growth, stomatal development, photosynthesis and transpiration of Withania somnifera (L.) Dunal. plantlets. Plant Cell Tissue Organ Cult. 2007, 90, 141–151. [Google Scholar] [CrossRef]
- Hattori, T.; Sonobe, K.; Inanaga, S.; An, P.; Tsuji, W.; Araki, H.; Eneji, A.E.; Morita, S. Short term stomatal responses to light intensity changes and osmotic stress in sorghum seedlings raised with and without silicon. Environ. Exp. Bot. 2007, 60, 177–182. [Google Scholar] [CrossRef]
- Talbott, L.D.; Zhu, J.; Han, S.W.; Zeiger, E. Phytochrome and blue light-mediated stomatal opening in the orchid, paphiopedilum. Plant Cell Physiol. 2002, 43, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kollist, H.; Nuhkat, M.; Roelfsema, M. Closing gaps: Linking elements that control stomatal element. New Phytol. 2014, 203, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Sakhonwasee, S.; Tummachai, K.; Nimnoy, N. Influences of LED light quality and intensity on stomatal behavior of three petunia cultivars grown in a semi-closed system. Environ. Control Biol. 2017, 55, 93–103. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roni, M.Z.K.; Islam, M.S.; Shimasaki, K. Response of Eustoma Leaf Phenotype and Photosynthetic Performance to LED Light Quality. Horticulturae 2017, 3, 50. https://doi.org/10.3390/horticulturae3040050
Roni MZK, Islam MS, Shimasaki K. Response of Eustoma Leaf Phenotype and Photosynthetic Performance to LED Light Quality. Horticulturae. 2017; 3(4):50. https://doi.org/10.3390/horticulturae3040050
Chicago/Turabian StyleRoni, Md Zohurul Kadir, Md Saiful Islam, and Kazuhiko Shimasaki. 2017. "Response of Eustoma Leaf Phenotype and Photosynthetic Performance to LED Light Quality" Horticulturae 3, no. 4: 50. https://doi.org/10.3390/horticulturae3040050






