Recent Advances in Organic Radicals and Their Magnetism
Abstract
:1. Introduction
2. Phenalenyl-Based π-Radical
3. Nitronylnitroxides
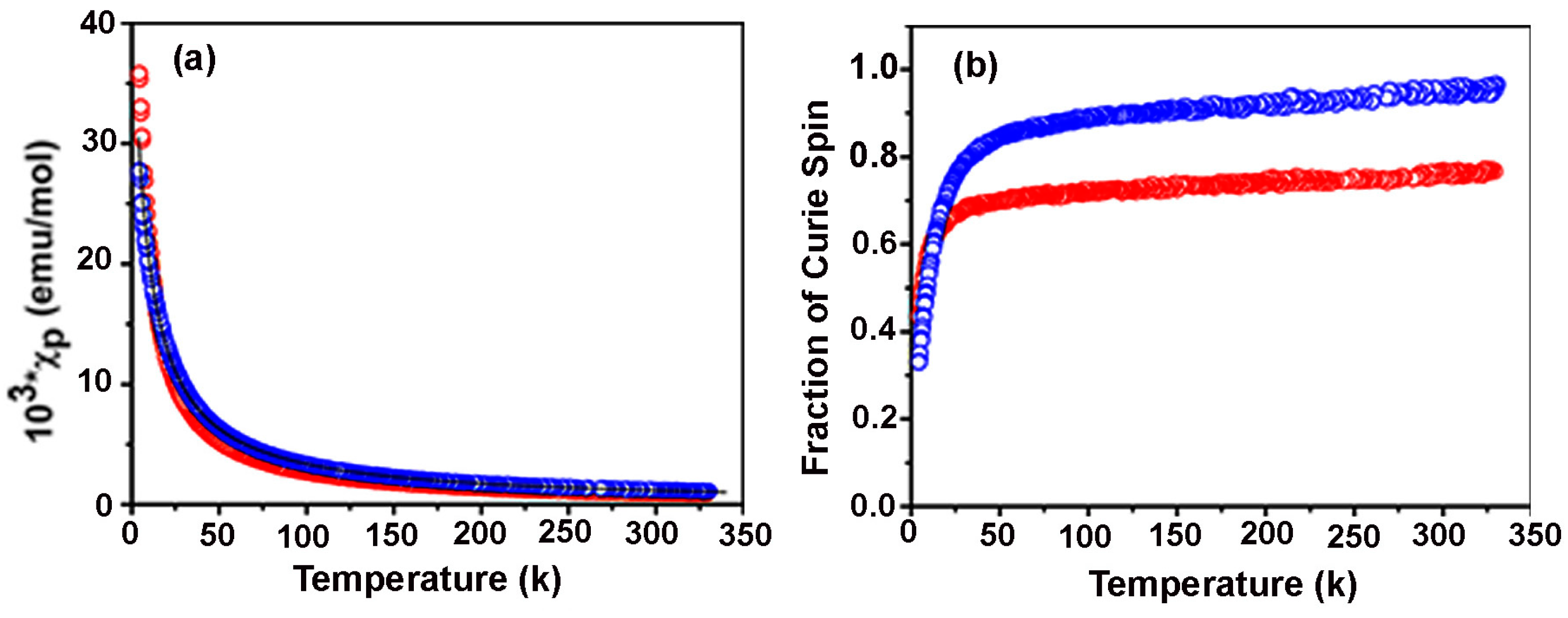
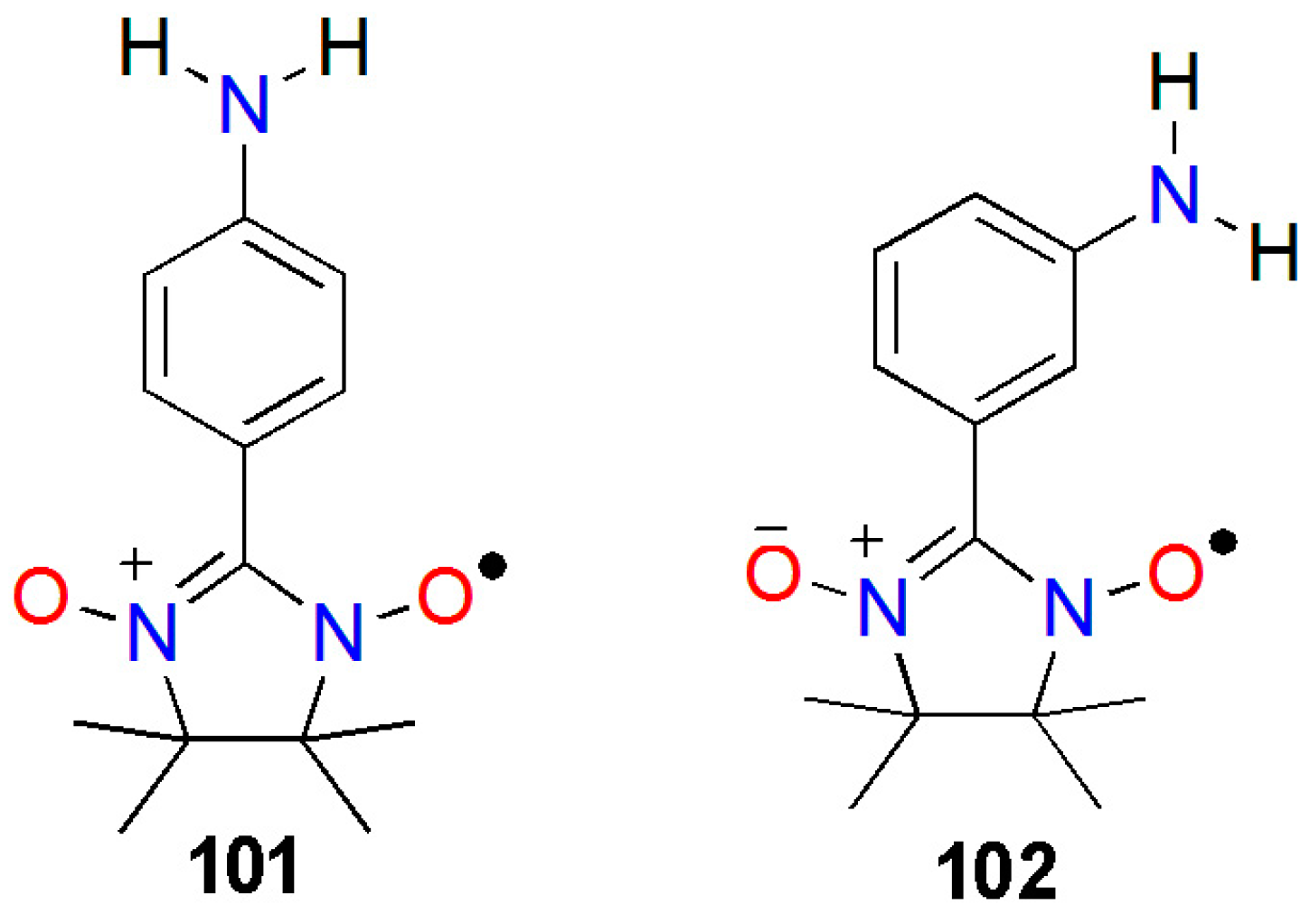
3.1. Aminophenylnitronylnitroxides
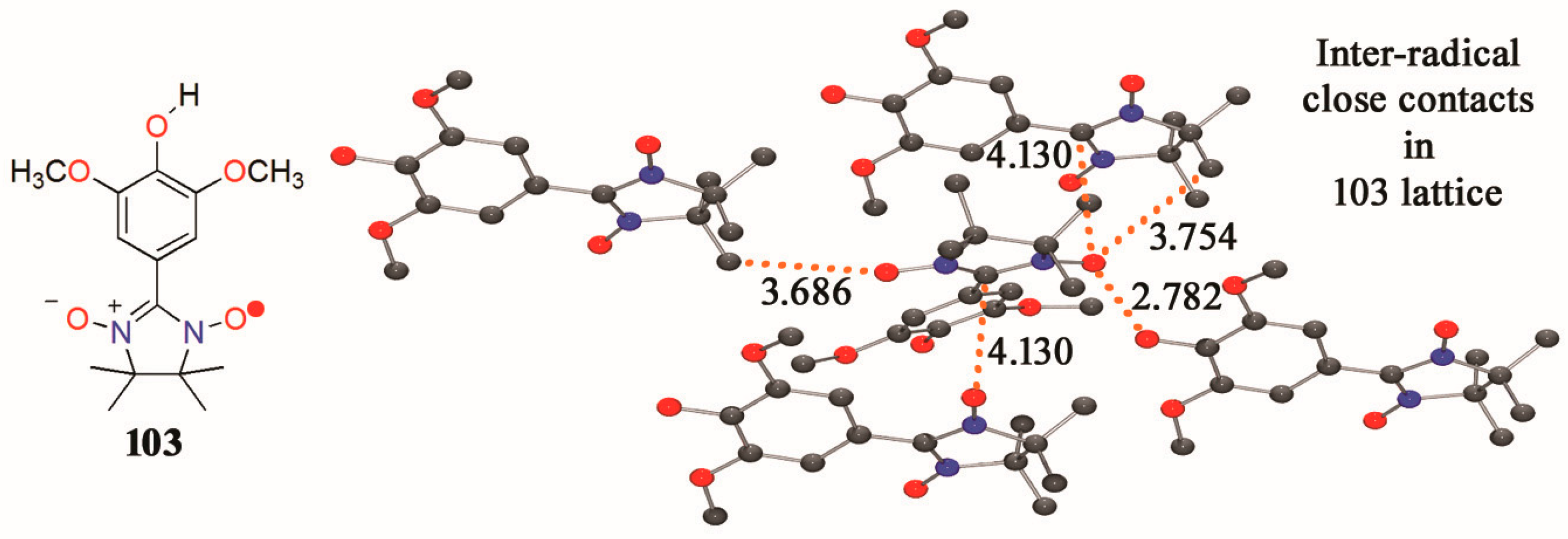
3.2. Hydroxyphenylnitronylnitroxides
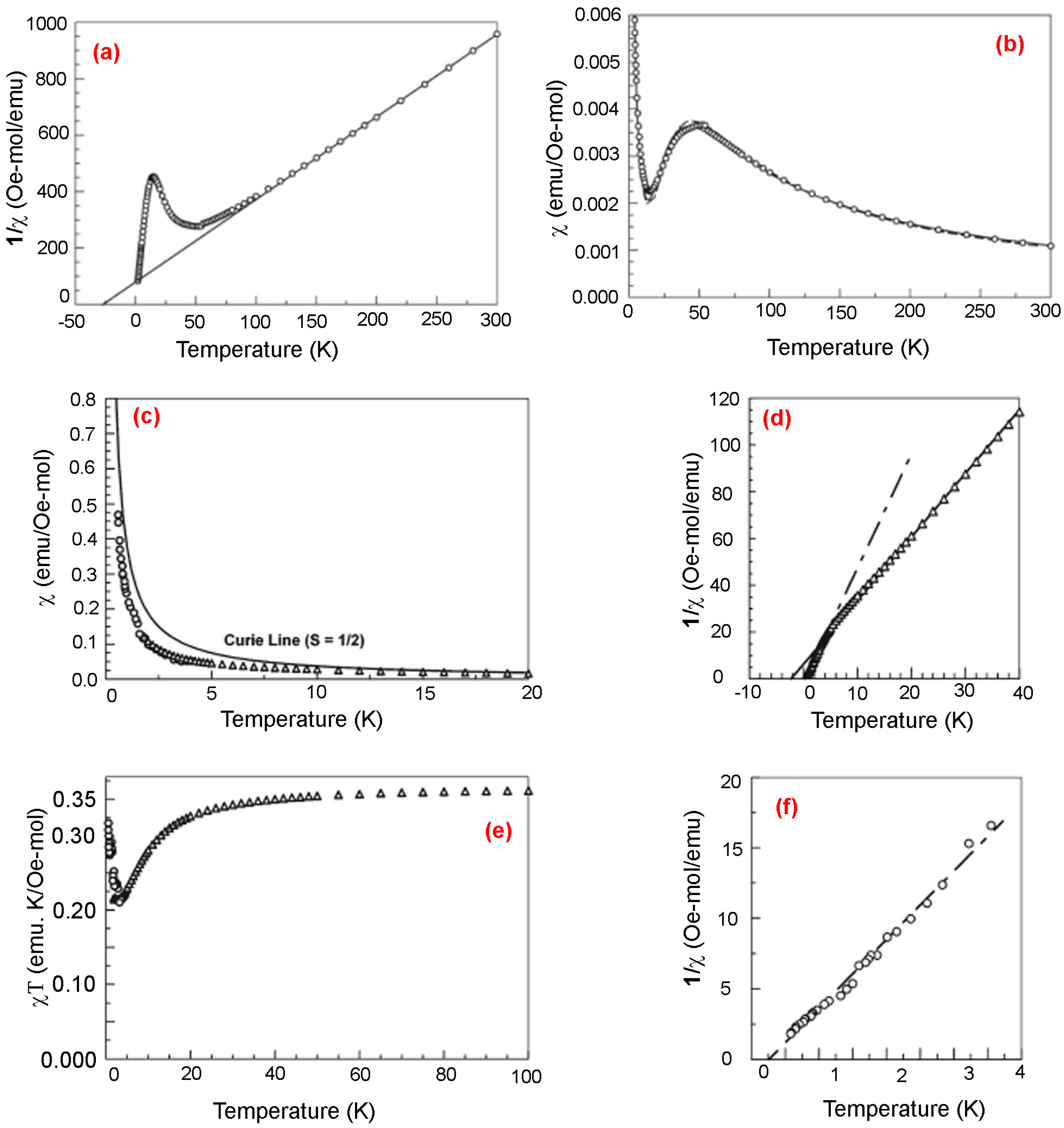
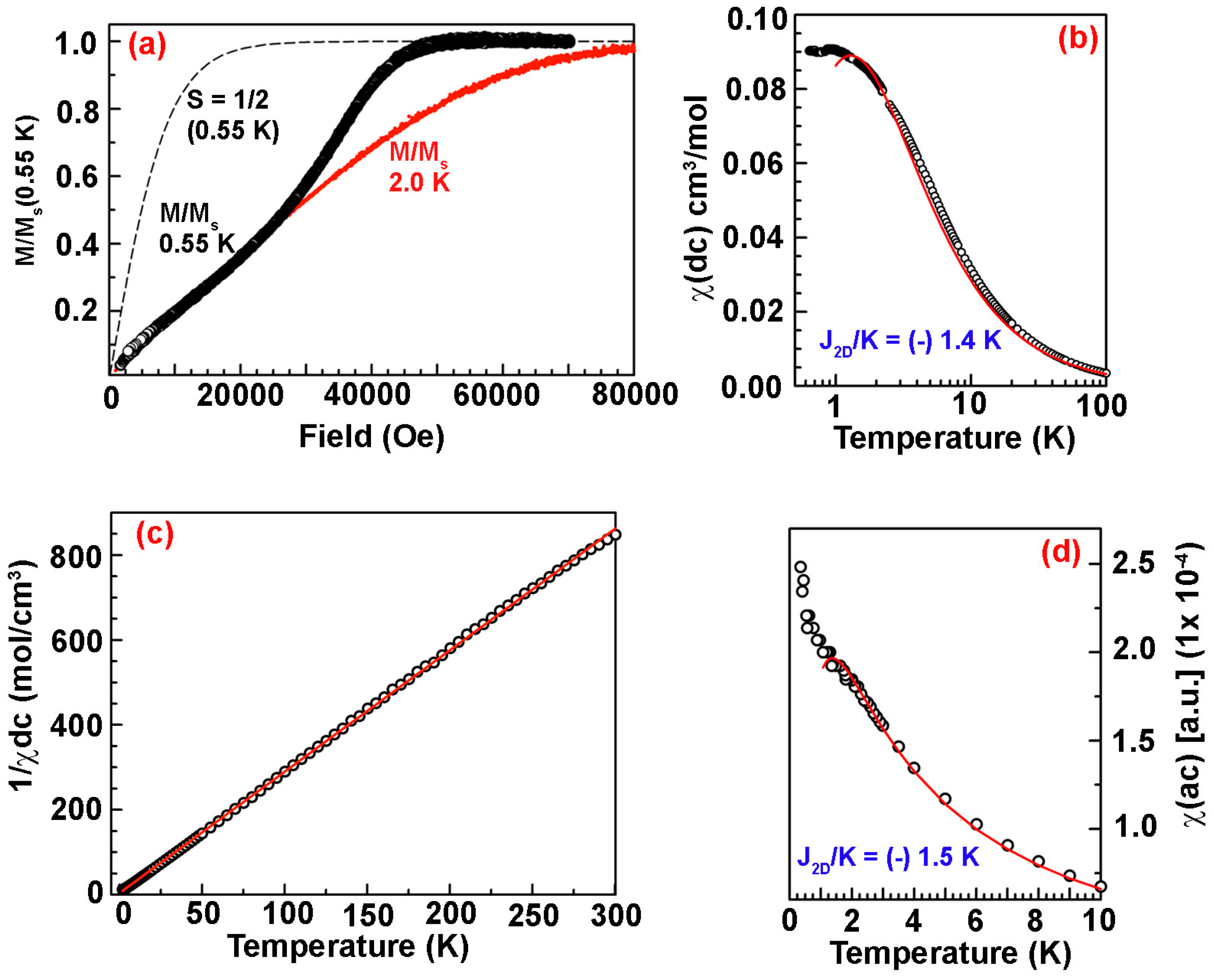
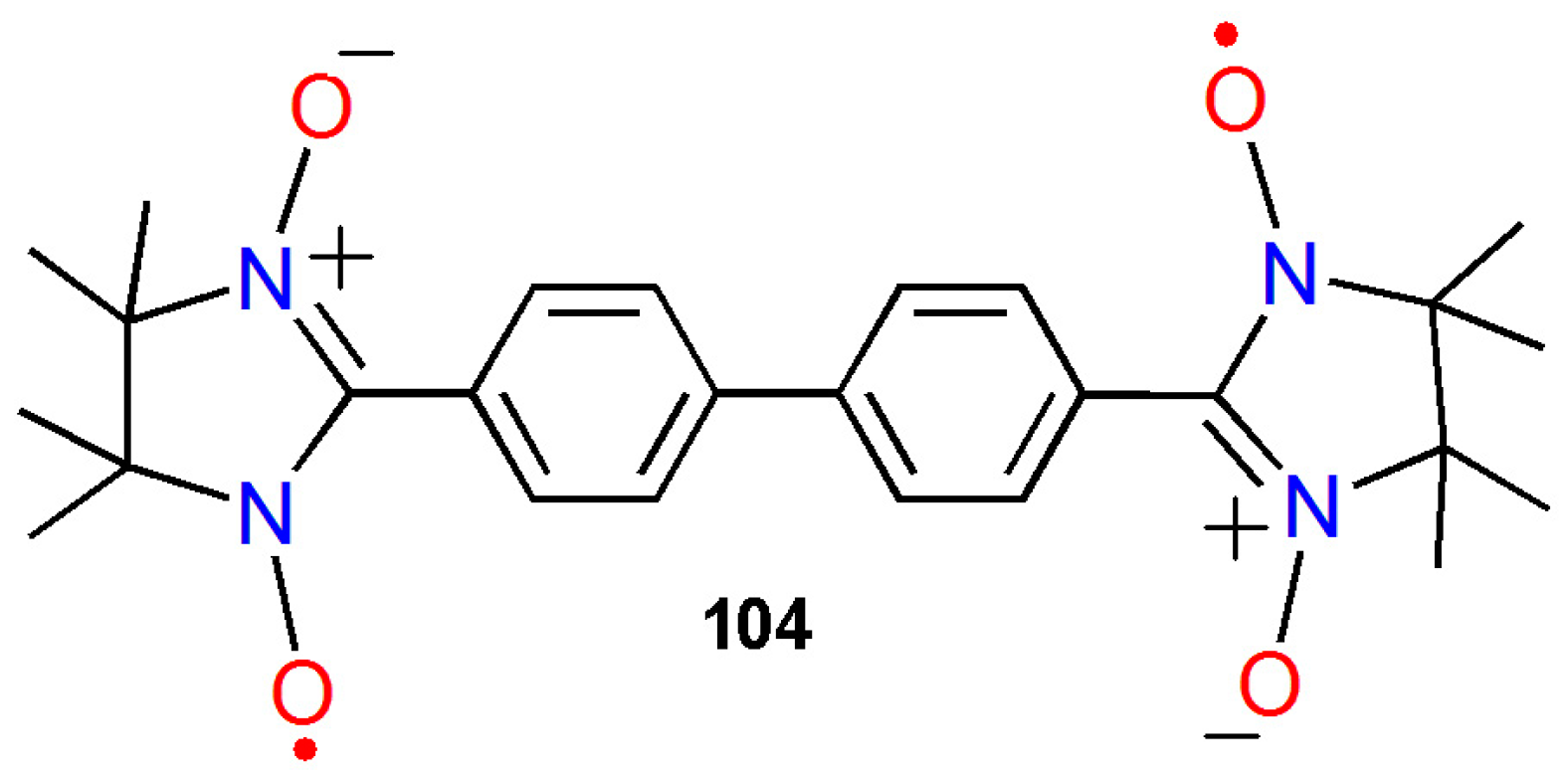
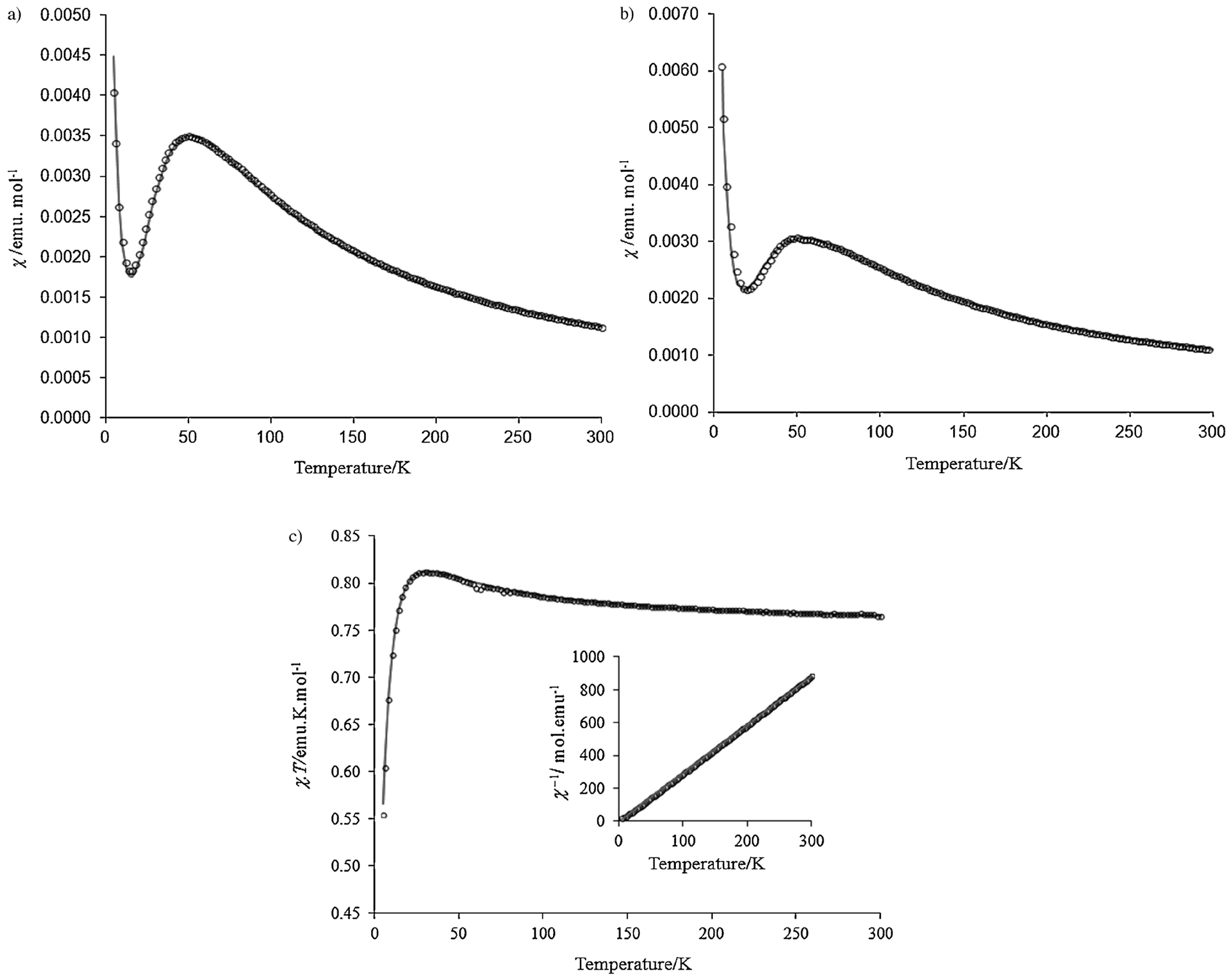
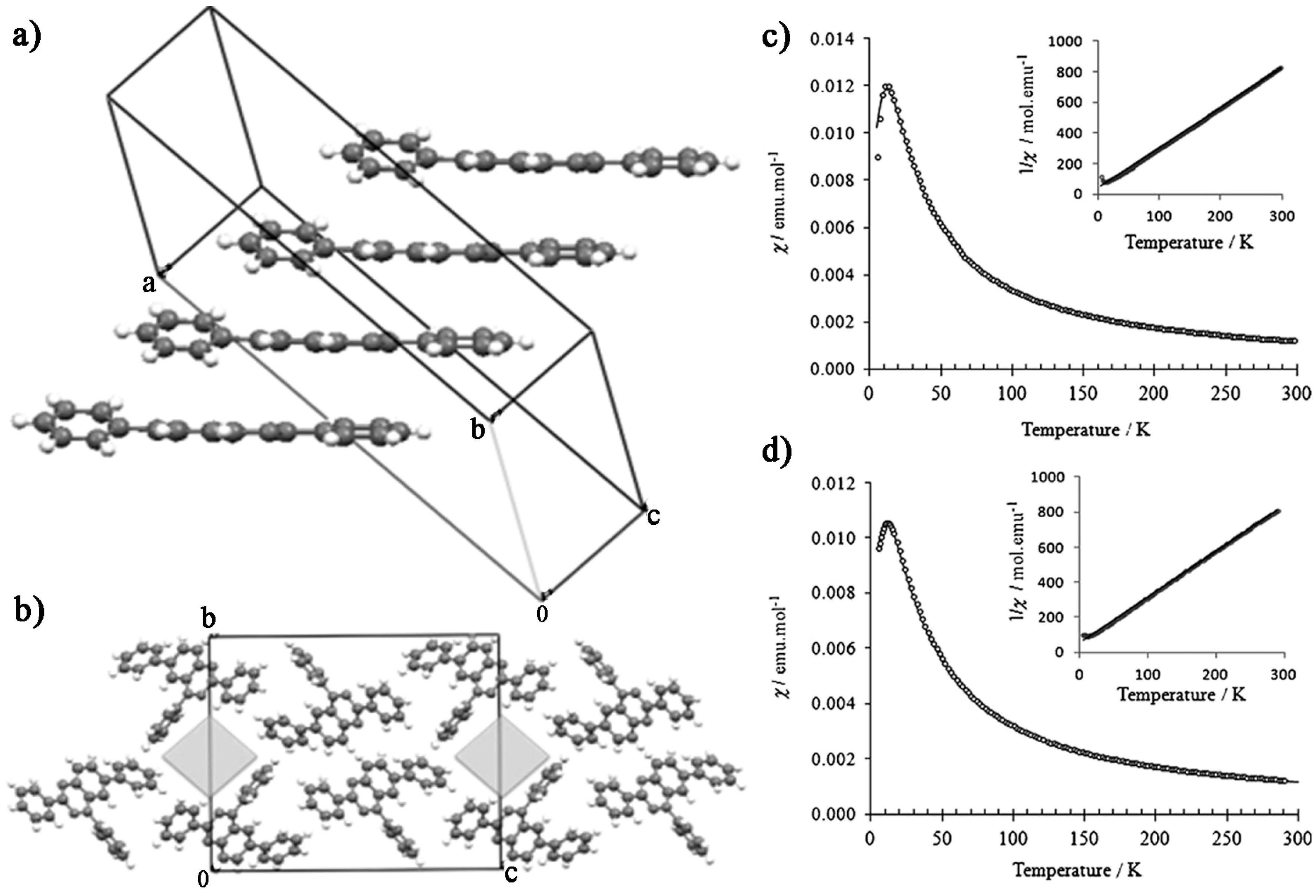
3.3. Biphenyl-4,4’-bis(nitronylnitroxide)
4. Benzotriazinyl Radicals
5. Bis-Dithiazolyl Radicals
6. Aminyl Radicals
6.1. Aminyl Diradicals
6.2. Aminyl Tetraradicals
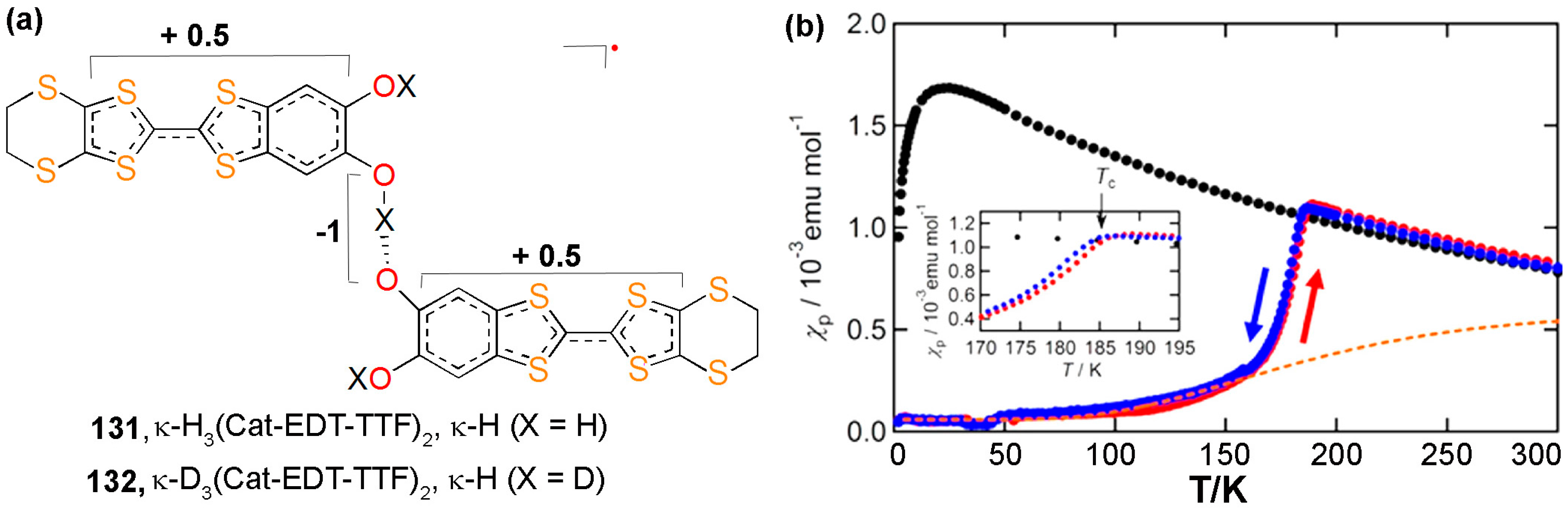
7. H-Bonded TTF-Based Radicals
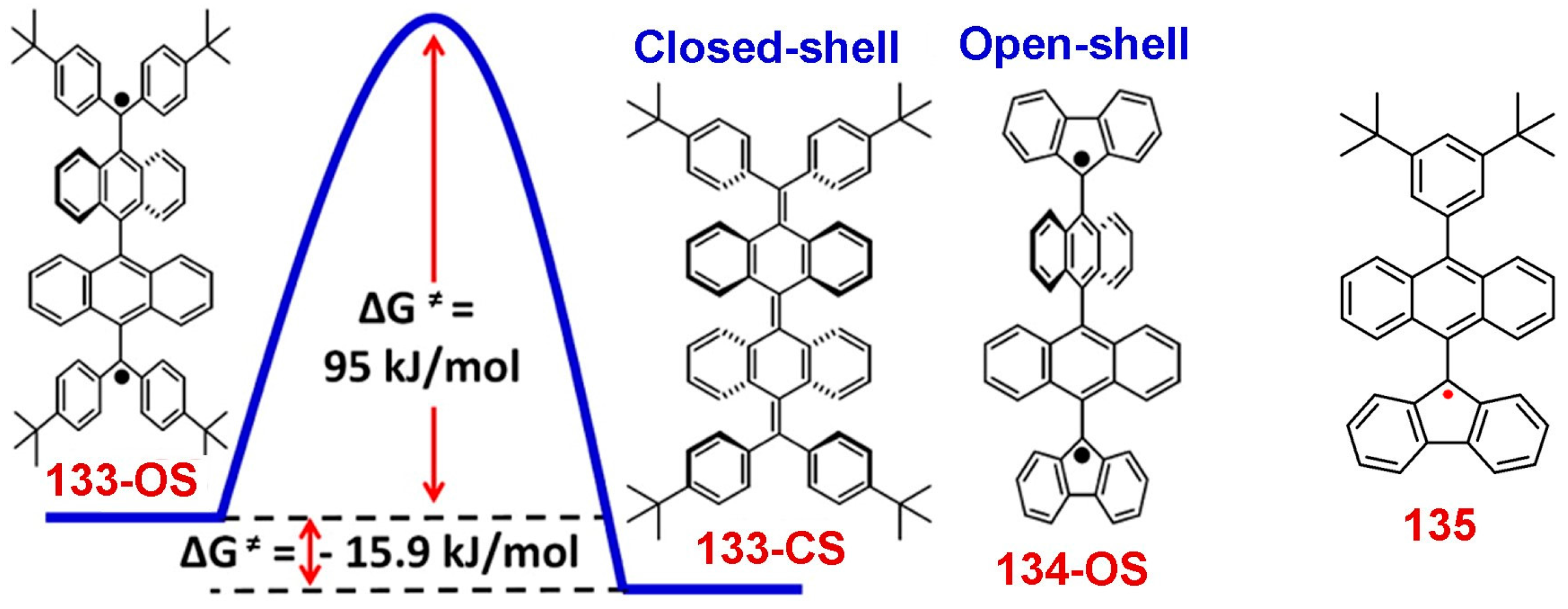
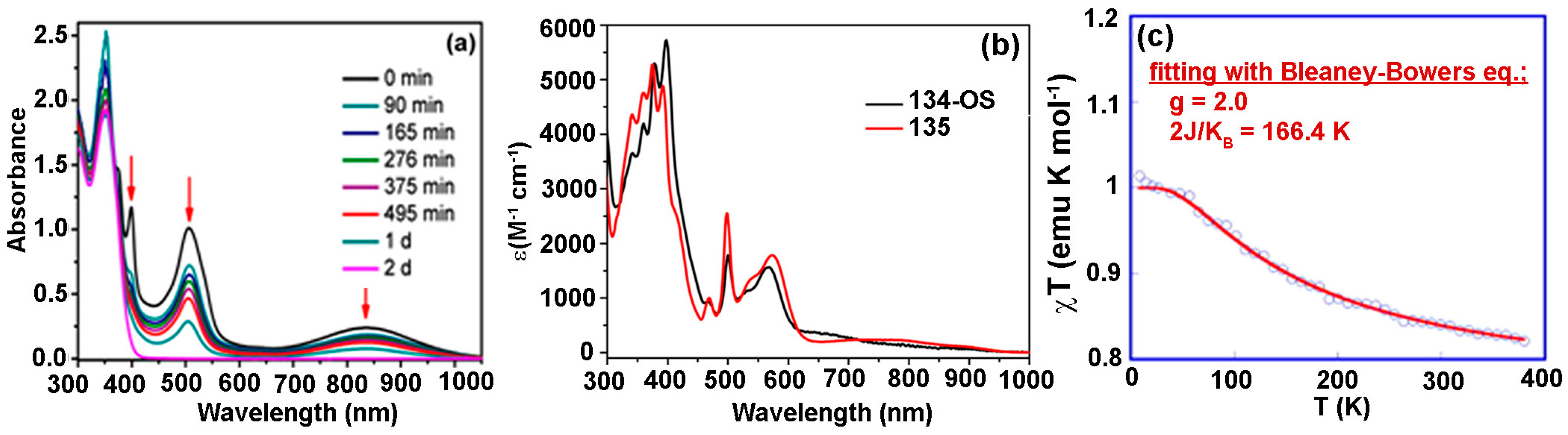
8. Polycyclic Aromatic Hydrocarbon Radicals
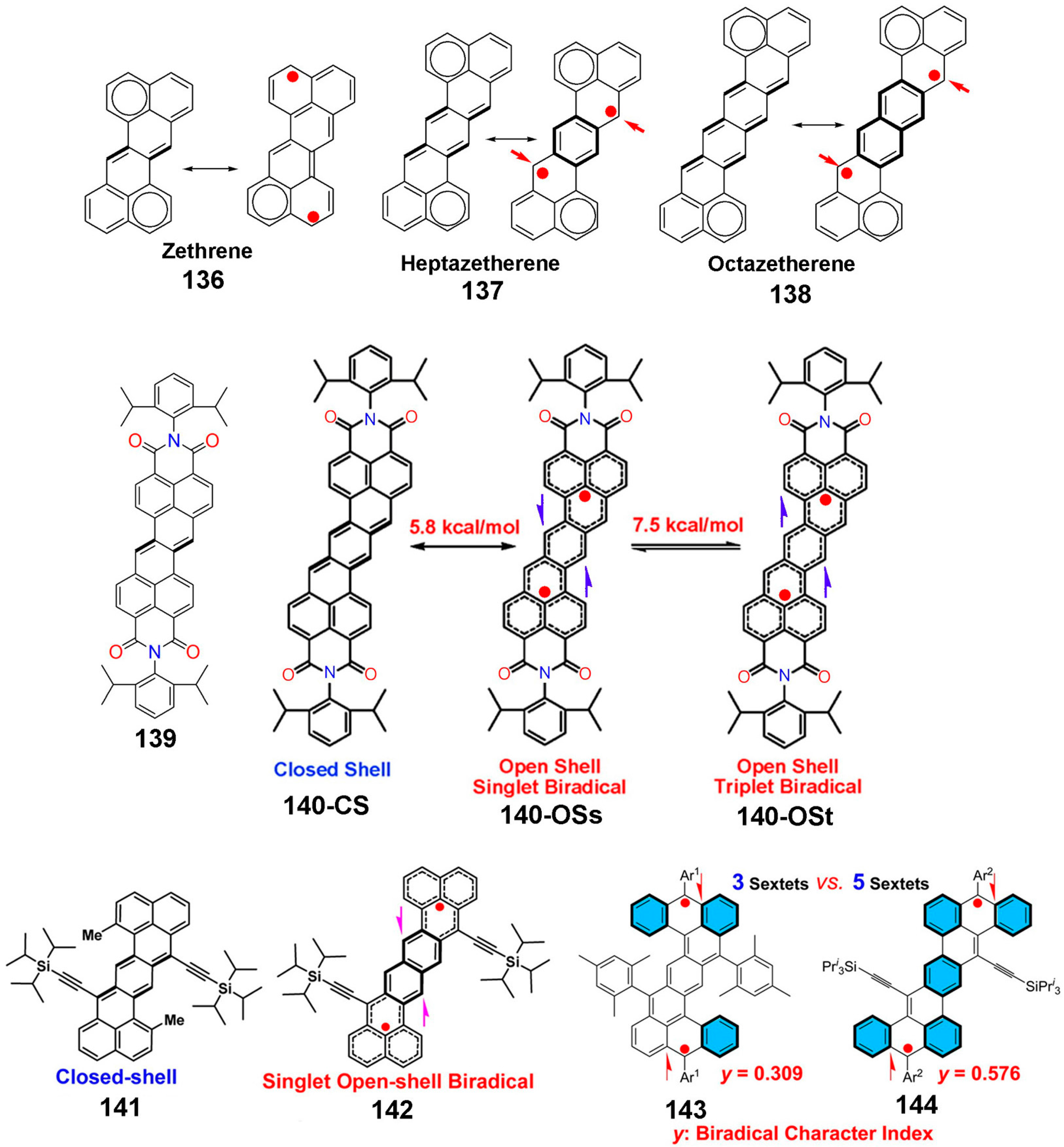
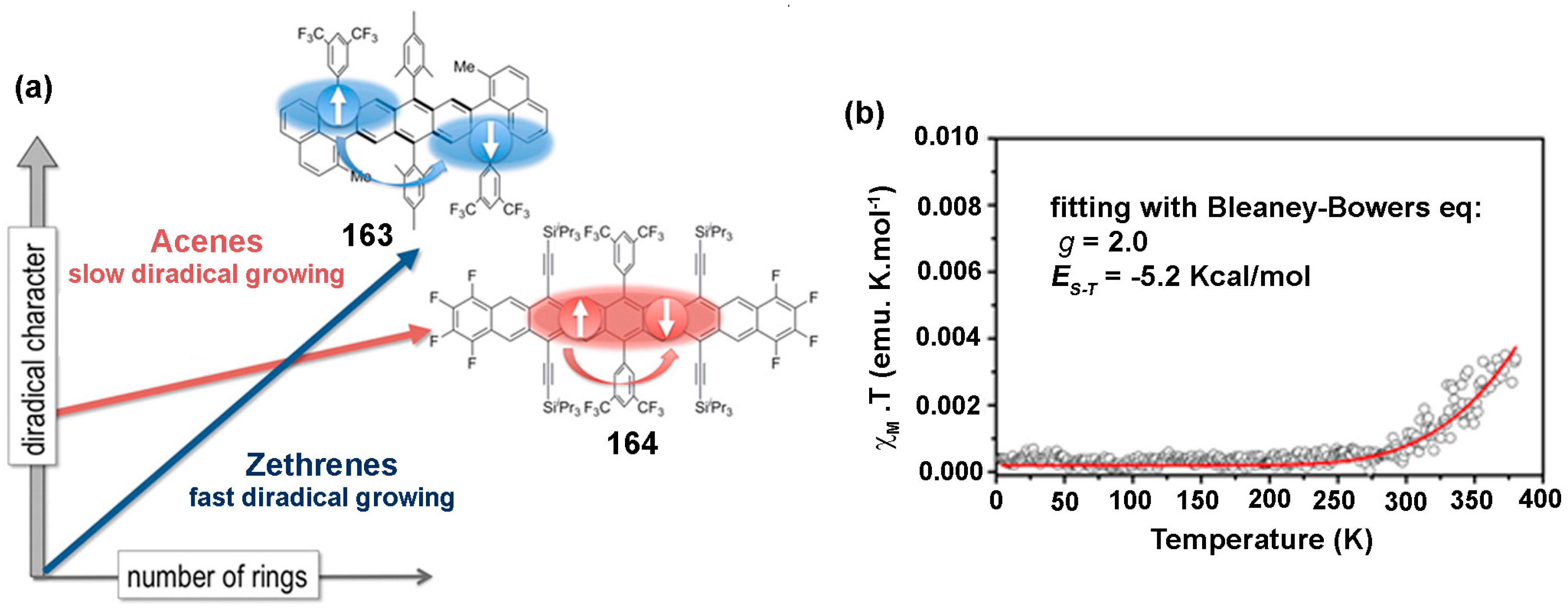
8.1. Zethrenes
8.2. P-Quinodimethane (p-QDM)
8.3. Tetraradicaloids
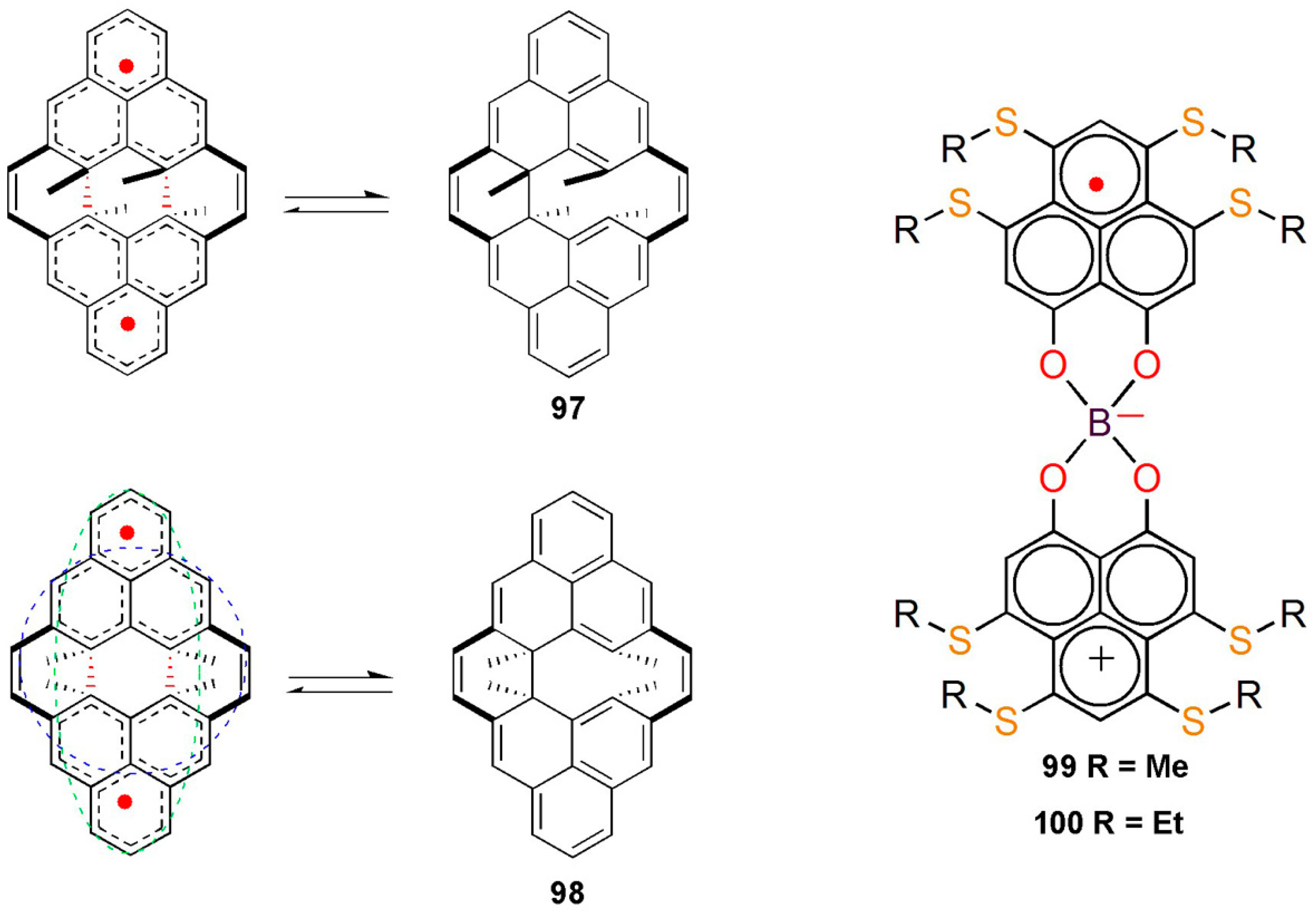
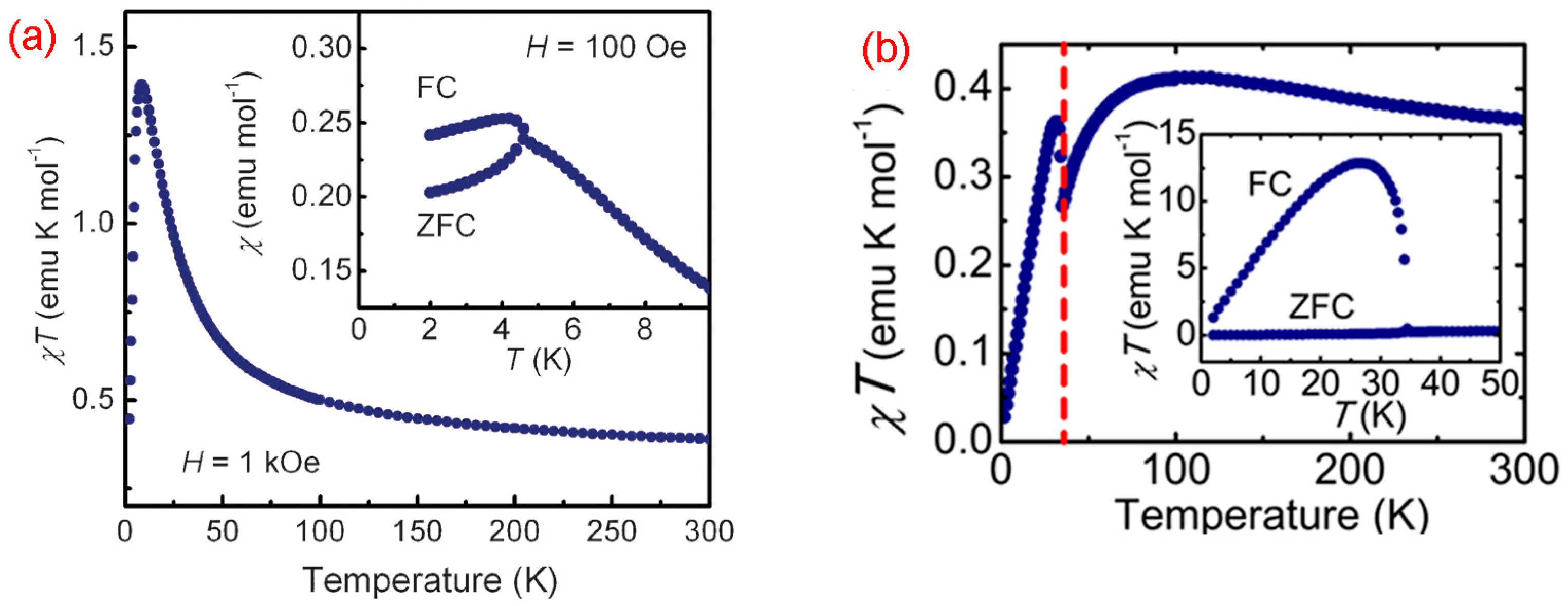
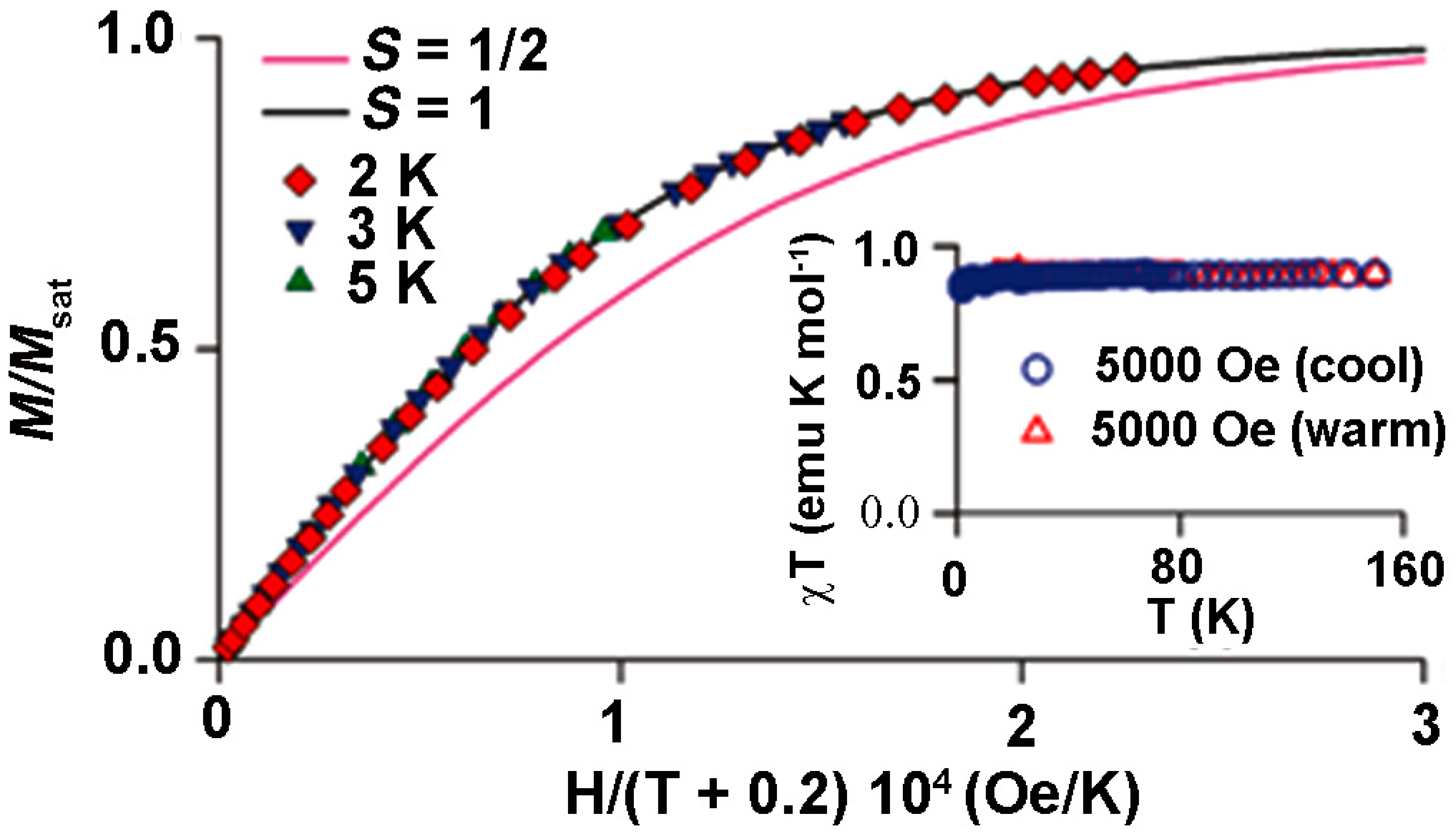
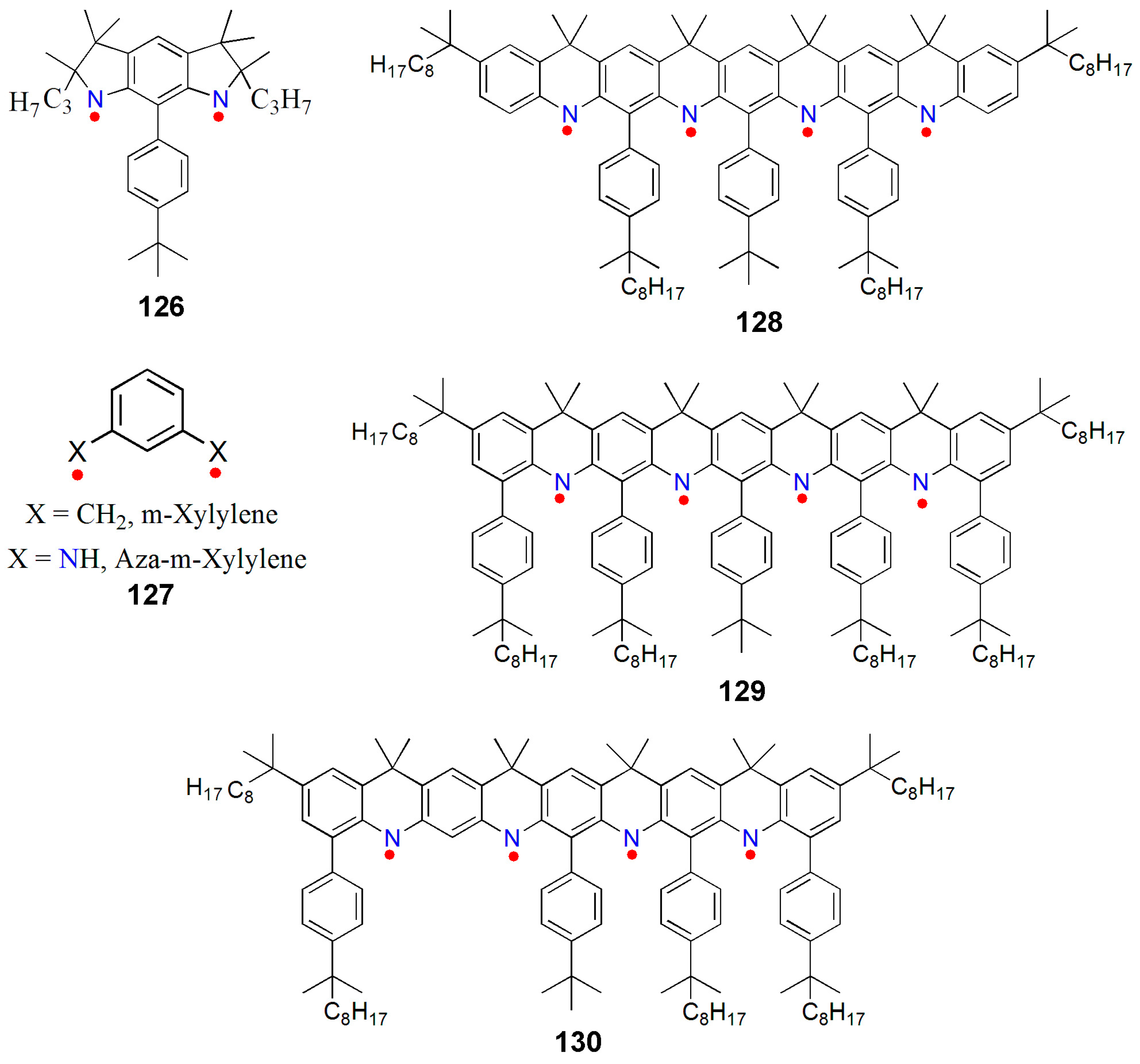
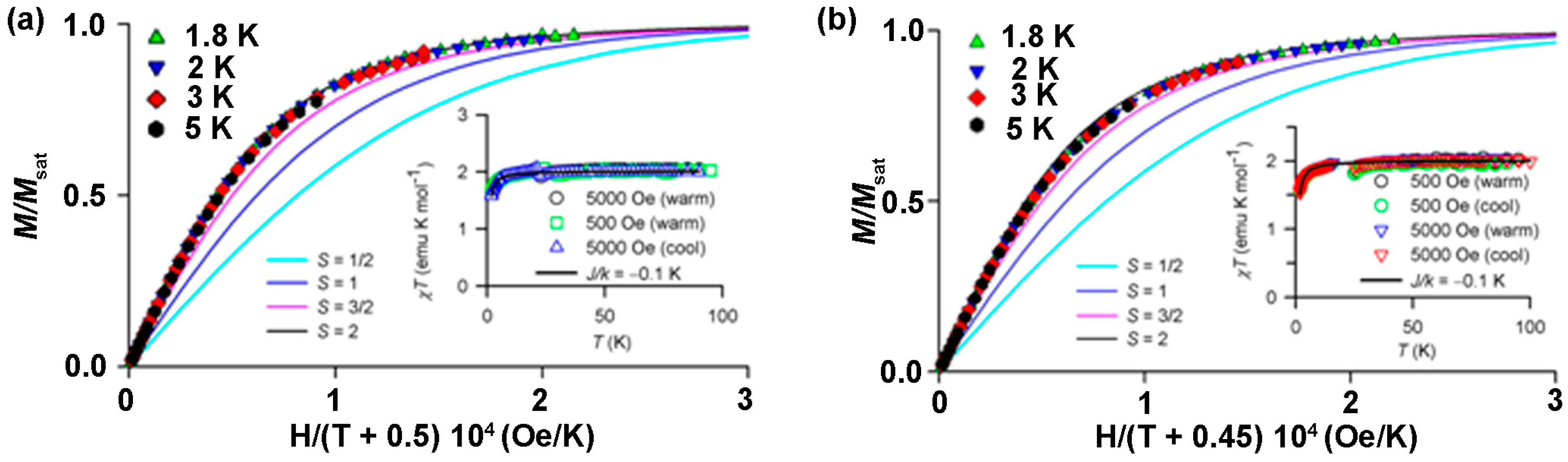
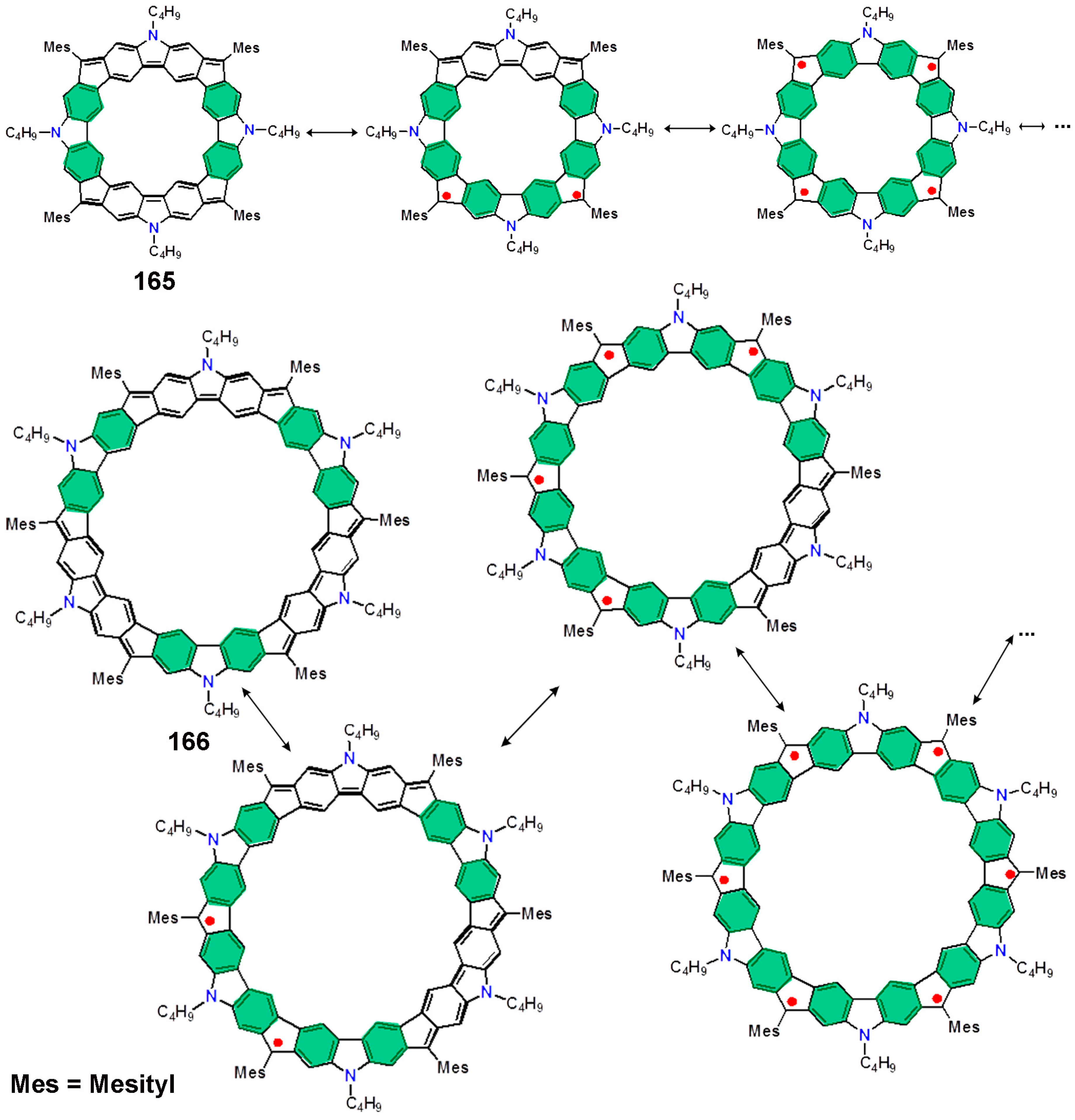
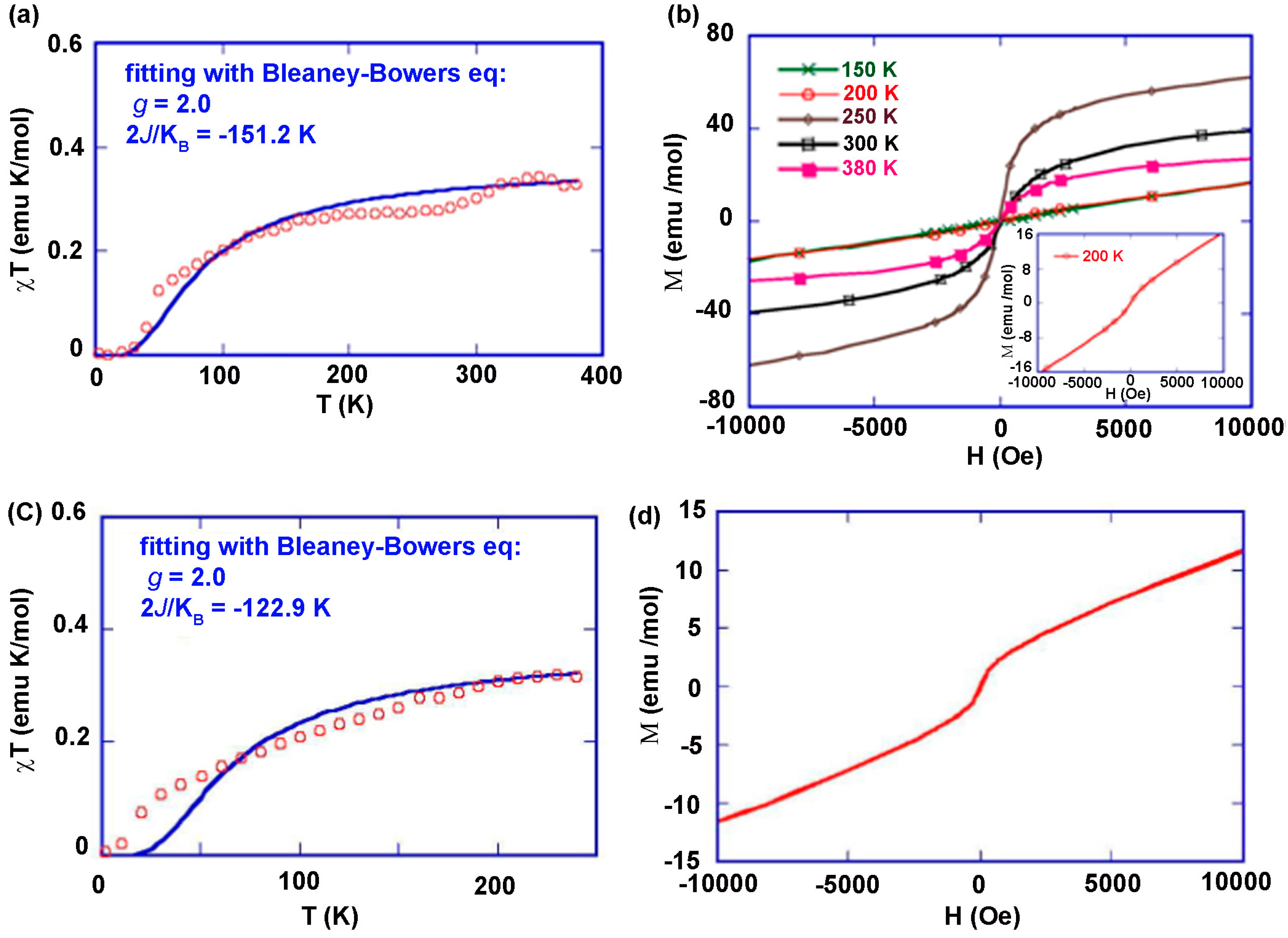
8.4. Macrocyclic Polyradicals
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Iwamura, H. What role has organic chemistry played in the development of molecule-based magnets? Polyhedron 2013, 66, 3–14. [Google Scholar] [CrossRef]
- Gomberg, M. An instance of trivalent carbon: Triphenylmethyl. J. Am. Chem. Soc. 1900, 22, 757–771. [Google Scholar] [CrossRef]
- Hicks, R.G. (Ed.) Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds; John Wiley & Sons Ltd.: Chichester, UK, 2010.
- Abe, M. Diradicals. Chem. Rev. 2013, 113, 7011–7088. [Google Scholar] [CrossRef] [PubMed]
- International Union of Pure and Applied Chemistry. IUPAC Compendium of Chemical Terminology, Release 2.3.2; International Union of Pure and Applied Chemistry (IUPAC): Research Triangle Park, NC, USA, 2012; p. 168. [Google Scholar]
- International Union of Pure and Applied Chemistry. IUPAC Compendium of Chemical Terminology, Release 2.3.2; International Union of Pure and Applied Chemistry (IUPAC): Research Triangle Park, NC, USA, 2012; p. 427. [Google Scholar]
- Amorati, R.; Lucarini, M.; Mugnaini, V.; Pedulli, G.F.; Minisci, F.; Recupero, F.; Fontana, F.; Astolfi, P.; Greci, L. Hydroxylamines as Oxidation Catalysts: Thermochemical and Kinetic Studies. J. Org. Chem. 2003, 68, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Samanta, S.R.; Rosen, B.M.; Percec, V. Single Electron Transfer in Radical Ion and Radical-Mediated Organic, Materials and Polymer Synthesis. Chem. Rev. 2014, 114, 5848–5958. [Google Scholar] [CrossRef] [PubMed]
- Asandei, A.D.; Moran, I.W.; Saha, G.; Chen, Y. Ti-Catalyzed Living radical polymerization of Styrene Initiated by Epoxide radical ring Opening. ACS Symp. Ser. 2006, 944, 125–139. [Google Scholar]
- Asandei, A.D.; Chen, Y. Cp2TiCl-Catalyzed SET Reduction of Aldehydes: A New Initiating Protocol for Living Radical Polymerization. Macromolecules 2006, 39, 7549–7554. [Google Scholar] [CrossRef]
- Suga, T.; Sakata, M.; Aoki, K.; Nishide, H. Synthesis of Pendant Radical- and Ion-Containing Block Copolymers via Ring-Opening Metathesis Polymerization for Organic Resistive Memory. ACS Macro Lett. 2014, 3, 703–707. [Google Scholar] [CrossRef]
- Gatteschi, D. Molecular Magnetism: A basis for new materials. Adv. Mater. 1994, 6, 635–645. [Google Scholar] [CrossRef]
- Fujita, W.; Awaga, K. Room-Temperature Magnetic Bistability in Organic Radical Crystals. Science 1999, 286, 261–262. [Google Scholar] [CrossRef] [PubMed]
- Janoschka, T.; Hager, M.D.; Schubert, U.S. Powering up the Future: Radical Polymers for Battery Applications. Adv. Mater. 2012, 24, 6397–6409. [Google Scholar] [CrossRef] [PubMed]
- Suga, T.; Ohshiro, H.; Sugita, S.; Oyaizu, K.; Nishide, H. Emerging N-Type Redox-Active Radical Polymer for a Totally Organic Polymer-Based Rechargeable Battery. Adv. Mater. 2009, 21, 1627–1630. [Google Scholar] [CrossRef]
- Gaudenzi, R.; Burzurí, E.; Reta, D.; Moreira, I.P.R.; Bromley, S.T.; Rovira, C.; Veciana, J.; van der Zant, H.S.J. Exchange Coupling Inversion in a High-Spin Organic Triradical Molecule. Nano Lett. 2016, 16, 2066–2071. [Google Scholar] [CrossRef] [PubMed]
- Frisenda, R.; Gaudenzi, R.; Franco, C.; Mas-Torrent, M.; Rovira, C.; Veciana, J.; Alcon, I.; Bromley, S.T.; Burzurí, E.; van der Zant, H.S.J. Kondo Effect in a Neutral and Stable All Organic Radical Single Molecule Break Junction. Nano Lett. 2015, 15, 3109–3114. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, W.L.; Gross, A.; Langen, R.; Lietzow, M.A. Recent advances in site-directed spin labeling of proteins. Curr. Opin. Struct. Biol. 1998, 8, 649–656. [Google Scholar] [CrossRef]
- Fanucci, G.E.; Cafiso, D.S. Recent advances and applications of site-directed spin labeling. Curr. Opin. Struct. Biol. 2006, 16, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Cambi, L.; Szego, L. Über die magnetische Susceptibilität der komplexen Verbindungen. Ber. Dtsch. Chem. Ges. B 1931, 64, 2591–2598. [Google Scholar] [CrossRef]
- Ratera, I.; Veciana, J. Playing with organic radicals as building blocks for functional molecular materials. Chem. Soc. Rev. 2012, 41, 303–349. [Google Scholar] [CrossRef] [PubMed]
- Fourmigué, M.; Lieffrig, J. Organizing Radical Species in the Solid State with Halogen Bonding. Top. Curr. Chem. 2015, 359, 91–114. [Google Scholar] [PubMed]
- Tamura, M.; Nakazawa, Y.; Shiomi, D.; Nozawa, K.; Hosokoshi, Y.; Ishikawa, M.; Takahashi, M.; Kinoshita, M. Bulk ferromagnetism in the β-phase crystal of the p-nitrophenyl nitronyl nitroxide radical. Chem. Phys. Lett. 1991, 186, 401–404. [Google Scholar] [CrossRef]
- Banister, A.J.; Bricklebank, N.; Lavender, I.; Rawson, J.M.; Gregory, C.I.; Tanner, B.K.; Clegg, W.; Elsegood, M.R.J.; Palacio, F. Spontaneous magnetization in a sulfur-nitrogen radical at 36 K. Angew. Chemie Int. Ed. 1996, 35, 2533–2535. [Google Scholar] [CrossRef]
- Piloty, O.; Schwerin, B.G. Ueber die Existenz von Derivaten des vierwertigen Stickstoffs (I. Mittheilung). Chem. Ber. 1901, 34, 1870–1887. [Google Scholar] [CrossRef]
- Thiele, J.; Balhorn, H. Ueber einen chinoïden Kohlenwasserstoff. Chem. Ber. 1904, 37, 1463–1470. [Google Scholar] [CrossRef]
- Montgomery, L.K.; Huffman, J.C.; Jurczak, E.A.; Grendze, M.P. The molecular structures of Thiele’s and Chichibabin’s hydrocarbons. J. Am. Chem. Soc. 1986, 108, 6004–6011. [Google Scholar] [CrossRef] [PubMed]
- Tschitschibabin, A.E. Über einige phenylierte Derivatedes p, p-Ditolyls. Chem. Ber. 1907, 40, 1810–1819. [Google Scholar] [CrossRef]
- Goldschmidt, S.; Renn, K. Zweiwertiger sticlstoff: Über das α,α-diphenyl-β-trinitrophenyl hydrazyl. Ber. Dtsch. Chem. Ges. B 1922, B55, 628–643. [Google Scholar] [CrossRef]
- Foti, M.C. Use and Abuse of the DPPH• Radical. J. Agric. Food Chem. 2015, 63, 8765–8876. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.H. Syntheses and properties of the perinaphthyl radical. Chem. Ind. 1956, 1504–1505. [Google Scholar]
- Paskovichlb, D.H.; Reddoch, A.H. Phenalenyl dimer cation and its electron paramagnetic resonance spectrum. J. Am. Chem. Soc. 1972, 94, 6938–6939. [Google Scholar] [CrossRef]
- Coppinger, G.M. A Stable Phenoxy Radical Inert to Oxygen. J. Am. Chem. Soc. 1957, 79, 501–502. [Google Scholar] [CrossRef]
- Lebedev, O.L.; Kazarnovskii, S.N. Zhur. Obshch. Khim. 1960, 30, 1631–1635.
- Neiman, M.; Rozantzev, É.G.; Mamedova, Y.G. Free radical reactions involving no unpaired electrons. Nature 1962, 196, 472–473. [Google Scholar] [CrossRef]
- Neiman, M.; Rozantzev, É.G.; Mamedova, Y.G. A new organic radical reaction involving no free valence. Nature 1963, 200, 256. [Google Scholar] [CrossRef]
- Forrester, A.R.; Thomson, R.H. Stable nitroxide radical. Nature 1964, 203, 74–75. [Google Scholar] [CrossRef]
- Keana, J.F.W. Newer aspects of the synthesis and chemistry of nitroxide spin lebels. Chem. Rev. 1978, 78, 37–64. [Google Scholar] [CrossRef]
- Kuhn, R.; Trischmann, H. Surprisingly Stable Nitrogenous Free Radicals. Angew. Chem. Int. Ed. 1963, 2, 155. [Google Scholar] [CrossRef]
- Neugebauer, F.A. Hydrazidinyl Radicals: 1,2,4,5Tetraazapentenyls, Verdazyls, and Tetrazolinyls. Angew. Chem. Int. Ed. 1973, 12, 455–464. [Google Scholar] [CrossRef]
- Barr, C.L.; Chase, P.A.; Hicks, R.G.; Lemaire, M.T.; Stevens, C.L. Synthesis and characterization of verdazyl radicals bearing pyridine or pyrimidine substituents: A new family of chelating spin-bearing ligands. J. Org. Chem. 1999, 64, 8893–8897. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.; Neugebauer, F.A.; Trischmann, H. Tetraazapentenyl radicals. Angew. Chem. Int. Ed. 1964, 3, 232. [Google Scholar] [CrossRef]
- Kosower, E.M.; Poziomek, E.J. Stable Free Radicals. I. Isolation and Distillation of 1-Ethyl-4-carbomethoxypyridinyl. J. Am. Chem. Soc. 1964, 86, 5515–5523. [Google Scholar] [CrossRef]
- Kuhn, R.; Neugebauer, F.A.; Trischmann, H. Stabile N-haltige Biradikale und Triradikale. Monatsh. Chem. 1966, 97, 525–553. [Google Scholar] [CrossRef]
- Keana, J.F.W.; Keana, S.B.; Beetham, D. A New Versatile Ketone Spin Label. J. Am. Chem. Soc. 1967, 89, 3055–3056. [Google Scholar] [CrossRef]
- Osiecki, J.H.; Ullman, E.F. Studies of Free Radicals. I. a-Nitronyl Nitroxides a New Class of Stable Radicals. J. Am. Chem. Soc. 1968, 90, 1078–1079. [Google Scholar] [CrossRef]
- Blatter, H.M.; Lukaszewski, H. A new stable free radical. Tetrahedron Lett. 1968, 9, 2701–2705. [Google Scholar] [CrossRef]
- Berezin, A.A.; Constantinides, C.P.; Drouza, C.; Manoli, M.; Koutentis, P.A. From blatter radical to 7-substituted 1,3-diphenyl-1,4-dihydrothiazolo[5′,4′:4,5]benzo[1,2-e][1,2,4]triazin-4-yls: Toward multifunctional materials. Org. Lett. 2012, 14, 5586–5589. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, F.A. Crystalline tetrazolinyl radical. Angew. Chem. Int. Ed. 1969, 8, 520–521. [Google Scholar] [CrossRef] [PubMed]
- Ullman, E.F.; Boocock, D.G.B.J. “Conjugated” nitronyl-nitroxide and imino-nitroxide biradicals. Chem. Soc. Chem. Commun. 1969, 1161–1162. [Google Scholar] [CrossRef]
- Alies, F.; Luneau, D.; Laugier, J.; Rey, P. Ullman’s Nitroxide Biradicals Revisited. Structural and Magnetic Properties. J. Phys. Chem. 1993, 97, 2922–2925. [Google Scholar] [CrossRef]
- Ballester, M.; Castaǹer, J.; Olivella, S. Syntheses and isolation of the perchlodiphenylaminyl, an exceptionally stable radical. Tetrahedron Lett. 1974, 15, 615–616. [Google Scholar] [CrossRef]
- Yozo, M.; Masayoshi, K. ESR studies of nitrogen-centered free radicals, N-Aryl-N-phenylthioaminyls. Bull. Chem. Soc. Jpn. 1977, 50, 1142–1146. [Google Scholar]
- Markovski, L.N.; Polumbrik, O.M.; Talanov, V.; Shermolovich, Y.G. 1,2,3,5-Dithiadiazolyles, a new type of persistent sulfurnitrogencontaining radicals. Tetrahedron Lett. 1982, 23, 761–762. [Google Scholar] [CrossRef]
- Barclay, T.M.; Cordes, A.W.; Laat, R.H.; Goddard, J.D.; Haddon, R.C.; Jeter, D.Y.; Mawhinney, R.C.; Oakley, R.T.; Palstra, T.T.M.; Patenaude, G.W.; et al. The heterocyclic diradical benzo-1,2:4,5-bis(1,3,2-dithiazolyl). Electronic, molecular and solid state structure. J. Am. Chem. Soc. 1997, 119, 2633–2641. [Google Scholar] [CrossRef]
- Mayer, R. S,N-Compounds via Amines and Sulphur Halides. Phosphorus, Sulfur Silicon Relat. Elem. 1985, 23, 277. [Google Scholar] [CrossRef]
- Preuss, K.E. Metal complexes of thiazyl radicals. Dalt. Trans. 2007, 23, 2357–2369. [Google Scholar] [CrossRef]
- Haider, K.; Soundararajan, N.; Shaffer, M.; Platz, M.S. EPR spectroscopy of a diaza derivative of meta-xylylene. Tetrahedron Lett. 1989, 30, 1225–1228. [Google Scholar] [CrossRef]
- Akita, T.; Mazaki, Y.; Kobayashi, K. Crystal Structures and Magnetic Properties of Nitronyl Nitroxide and Imino Nitroxide Radicals Attached to Thieno[3,2-b]-Thieno[2,3-b] thiophene Rings. J. Org. Chem. 1995, 60, 2092–2098. [Google Scholar] [CrossRef]
- Miura, Y.; Kurokawa, S.; Nakatsuji, M.K.; Teki, Y. Pyridyl-substituted thioaminyl stable free radicals: Isolation, ESR spectra, and magnetic characterization. J. Org. Chem. 1998, 63, 8295–8303. [Google Scholar] [CrossRef]
- Chi, X.; Itkis, M.E.; Patrik, B.O.; Barklay, T.M.; Reed, R.W.; Oakley, R.T.; Cordes, A.W.; Haddon, R.C. The first phenalenyl-based neutral radical molecular conductor. J. Am. Chem. Soc. 1999, 121, 10395–10402. [Google Scholar] [CrossRef]
- Mandal, S.K.; Samanta, S.; Itkis, M.E.; Jensen, D.W.; Reed, R.W.; Oakley, R.T.; Tham, F.S.; Donnadieu, B.; Haddon, R.C. Resonating valence bond ground state in oxygen-functionalized phenalenyl-based neutral radical molecular conductors. J. Am. Chem. Soc. 2006, 128, 1982–1994. [Google Scholar] [CrossRef] [PubMed]
- Beer, L.; Brusso, J.L.; Cordes, A.W.; Haddon, R.C.; Itkis, M.E.; Kirschbaum, K.; MacGregor, D.S.; Barklay, T.M.; Pinkerton, A.A.; Reed, R.W. Resonance-stabilized 1,2,3-dithiazolo-1,2,3-dithiazolyis as neutral π-radical conductors. J. Am. Chem. Soc. 2002, 124, 9498–9509. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.M.; Leitch, A.A.; Cvrkalj, K.; Myles, D.J.T.; Reed, R.W.; Dube, P.A.; Oakley, R.T. Ferromagnetic ordering in bisthiaselenazolyl radicals: Variations on a tetragonal theme. J. Am. Chem. Soc. 2008, 130, 14791–14801. [Google Scholar] [CrossRef] [PubMed]
- Sugano, T.; Blundell, S.J.; Hayes, W.; Day, P. Magnetism in organic diradical ion salts based on nitronyl nitroxide. Polyhedron 2005, 24, 2108–2111. [Google Scholar] [CrossRef]
- Rajca, A.; Shiraishi, K.; Pink, M.; Rajca, S. Triplet (S = 1) ground state aminyl diradical. J. Am. Chem. Soc. 2007, 129, 7232–7233. [Google Scholar] [CrossRef] [PubMed]
- Rajca, A.; Olankitwanit, A.; Rajca, S. Triplet ground state derivative of aza-m-xylylene diradical with large singlet-triplet energy gap. J. Am. Chem. Soc. 2011, 133, 4750–4753. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Furui, T.; Kuratsu, M.; Kozaki, M.; Shiomi, D.; Sato, K.; Takui, T.; Okada, K. Nitroxide-substituted nitronyl nitroxide and iminonitroxide. J. Am. Chem. Soc. 2010, 132, 15908–15910. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, J.K.; Martin, D.; Moore, C.E.; Rheingold, A.L.; Bertrand, G. Bottleable (Amino) (carboxy) Radicals Derived from Cyclic (Alkyl)(Amino) Carbenes. J. Am. Chem. Soc. 2013, 135, 18766–18769. [Google Scholar] [CrossRef] [PubMed]
- Bardelang, D.; Casano, G.; Poulhès, F.; Karoui, H.; Filippini, J.; Rockenbauer, A.; Rosas, R.; monnier, V.; Siri, D.; Gaudel-Siri, A.; et al. Spin exchange monitoring of the strong positive homotropic allosteric binding of a tetraradical by a synthetic receptor in water. J. Am. Chem. Soc. 2014, 136, 17570–17577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, N.M.; Bauer, J.J.; Pink, M.; Rajca, S.; Rajca, A. High Spin Organic Diradical with Robust Stability. J. Am. Chem. Soc. 2016, 138, 9377–9380. [Google Scholar] [CrossRef] [PubMed]
- Bechman, F.; Paul, T. Verhalten von Ketonen und Aldehyden gegen Natrium bei Gegenwart indifferenter Lösungsmittel. Justus Liebigs Ann. Chem. 1891, 266, 1–28. [Google Scholar] [CrossRef]
- Schlenk, W.; Appenrodt, J.; Michael, A.; Thal, A. Metal additions to multiple bonds. Ber. Dtsch. Chem. Ges. 1914, 47, 473–490. [Google Scholar] [CrossRef]
- Holy, N.L. Reactions of the radical anions and dianions of aromatic hydrocarbons. Chem. Rev. 1974, 74, 243–277. [Google Scholar] [CrossRef]
- Scott, N.D.; Walker, J.F.; Hansley, V.L. Sodium Naphthalene. I. A New Method for the Preparation of Addition Com-pounds of Alkali Metals and Polycyclic Aromatic Hydrocarbons. J. Am. Chem. Soc. 1936, 58, 2442–2444. [Google Scholar] [CrossRef]
- Acker, S.D.; Hertler, W.R. Substituted Quinodimethans. I. Preparation and Chemistry of 7,7,8,8-Tetracyanoquinodimethan. J. Am. Chem. Soc. 1962, 84, 3370–3374. [Google Scholar] [CrossRef]
- Eastman, J.W.; Engelsma, G.; Celvin, M. Free Radicals, Ionsand Donor-acceptor Complexes in the Reaction: Chloranil + N,N-Dimethylaniline → Crystal Violet. J. Am. Chem. Soc. 1962, 84, 1339–1345. [Google Scholar] [CrossRef]
- Brandon, R.L.; Osiecki, J.H.; Ottenberg, A. The Reactions of Metallocenes with Electron Acceptors. J. Org. Chem. 1966, 31, 1214–1217. [Google Scholar] [CrossRef]
- Miller, J.S.; Krusic, P.J.; Dixon, D.A.; Reiff, M.W.; Zhang, J.H.; Anderson, E.C.; Epstein, A.J. Radical Ion Salts of 2,3-Dichloro-5,6-dicyanobenzoquinone and Metallocenes. J. Am. Chem. Soc. 1986, 108, 4459–4466. [Google Scholar] [CrossRef]
- Walker, D.; Hiebert, J.D. 2,3-Dichloro-5,6-Dicyanobenzoquinone and Its Reactions. Chem. Rev. 1967, 67, 153–195. [Google Scholar] [CrossRef] [PubMed]
- Nelsen, S.F. Heterocyclic Radical Anions. 11. Naphthalic and 1,4,5,8-Naphthalenetetracarboxylic Acid Derivatives. J. Am. Chem. Soc. 1967, 190, 5925–5931. [Google Scholar] [CrossRef]
- Leffler, J.E.; Watts, G.B.; Tanigaki, T.; Dolan, E.; Miller, D.S. Triarylboron anion radicals and the reductive cleavage of boron compounds. J. Am. Chem. Soc. 1970, 92, 6825–6830. [Google Scholar] [CrossRef]
- Wheland, R.C.; Gillson, J.L. Synthesis of Electrically Conductive Organic. J. Am. Chem. Soc. 1976, 98, 3916–3925. [Google Scholar] [CrossRef]
- Kaim, W.; Bock, H. Radical Anion and Radical Trianion of 1,4-Bis (dirnethylphosphino) benzene. J. Am. Chem. Soc. 1978, 100, 6504–6506. [Google Scholar] [CrossRef]
- Rohrbach, W.D.; Gerson, F.; Moeckel, R.; Boekelheide, V. Synthesis of anti-4,5,14,15-tetramethyl[22](2,7)naphthalenophane-1,11-diene and its dianion. ESR and ENDOR studies of its radical anion and other related radical ions. J. Org. Chem. 1984, 49, 4128–4132. [Google Scholar] [CrossRef]
- Heywang, G.; Born, L.; Fitzky, H.-G.; Hassel, T.; Hocker, J.; Muller, H.-K.; Pittel, B.; Roth, S. Radical Anion Salts of Naphthalenetetracarboxylic Acid Derivatives-a Novel Class of Electrically Conducting Compounds. Angew. Chem. Int. Ed. 1989, 28, 483–485. [Google Scholar] [CrossRef]
- Zhao, J.; Dowd, P.; Grabowski, J.J. Anion-molecule approaches to non-kekule molecules: The radical anion of cyclopentadienylidenetrimethylenemethane and derivatives. J. Am. Chem. Soc. 1996, 118, 8871–8874. [Google Scholar] [CrossRef]
- Schmitt, S.; Baumgarten, M.; Simon, J.; Hafner, K. 2,4,6,8-Tetracyanoazulene: A New Building Block for ‘Organic Metals’. Angew. Chem. Int. Ed. 1998, 37, 1077–1081. [Google Scholar] [CrossRef]
- Gosztola, D.; Niemczyk, M.P.; Svec, W.; Lukas, A.S.; Wasielewski, M.R. Excited Doublet States of Electrochemically Generated Aromatic Imide and Diimide Radical Anions. J. Phys. Chem. A 2000, 104, 6545–6655. [Google Scholar] [CrossRef]
- Ishida, S.; Iwamoto, T.; Kira, M. Radical anion of isolable dialkylsilylene. J. Am. Chem. Soc. 2003, 125, 3212–3213. [Google Scholar] [CrossRef] [PubMed]
- Nelsen, S.F.; Weaver, M.N.; Konradsson, A.E.; Telo, J.P.; Clark, T. Electron transfer within 2,7-dinitronaphthalene radical anion. J. Am. Chem. Soc. 2004, 126, 15431–15438. [Google Scholar] [CrossRef] [PubMed]
- Makarov, A.Y.; Irtegova, I.G.; Vasilieva, N.V.; Bagryanskaya, I.Y.; Borrmann, T.; Gatilov, Y.V.; Lork, E.; Mews, R.; Stohrer, W.-D.; Zibarev, A.V. [1,2,5]Thiadiazolo[3,4-c][1,2,5]thiadiazolidyl: A Long-Lived Radical Anion and Its Stable Salts. Inorg. Chem. 2005, 44, 7194–7199. [Google Scholar] [CrossRef] [PubMed]
- Denning, M.S.; Irwin, M.; Goicoechea, J.M. Synthesis and Characterization of the 4,4′-Bipyridyl Dianion and Radical Monoanion. A Structural Study. Inorg. Chem. 2008, 47, 6118–6120. [Google Scholar] [CrossRef] [PubMed]
- Nelsen, S.F.; Weaver, M.N.; Telo, J.P. Solvent Control of Charge Localization in 11-Bond Bridged Dinitroaromatic Radical Anions. J. Am. Chem. Soc. 2007, 129, 7036–7043. [Google Scholar] [CrossRef] [PubMed]
- Kozaki, M.; Sugimura, K.; Ohnishi, H.; Okada, K. Preparation, Properties, and Reduction of a Novel TCNQ-Type Thienoquinoid. Org. Lett. 2006, 8, 5235–5238. [Google Scholar] [CrossRef] [PubMed]
- Rosokha, S.V.; Lu, J.; Han, B.; Kochi, J.K. Unusual structural effects of intermolecular π-bonding in the tetracyanopyrazine (ion-radical) dimer. New J. Chem. 2009, 33, 545–553. [Google Scholar] [CrossRef]
- Ueda, A.; Ogasawara, K.; Nishida, S.; Ise, T.; Yoshino, T.; Nakazawa, S.; Sato, K.; Takui, T.; Nakasuji, K.; Morita, Y. A bowl-shaped ortho-semiquinone radical anion: Quantitative evaluation of the dynamic behavior of structural and electronic features. Angew. Chem. Int. Ed. 2010, 49, 6333–6337. [Google Scholar] [CrossRef] [PubMed]
- Ajayakumar, M.R.; Asthana, D.; Mukhopadhyay, P. Core-modified naphthalenediimides generate persistent radical anion and cation: New panchromatic NIR probes. Org. Lett. 2012, 14, 4822–4825. [Google Scholar] [CrossRef] [PubMed]
- Kushida, T.; Yamaguchi, S. A radical anion of structurally constrained triphenylborane. Organometallics 2013, 32, 6654–6657. [Google Scholar] [CrossRef]
- Roznyatovskiy, V.V.; Gardner, D.M.; Eaton, S.W.; Wasielewski, M.R. Radical Anions of trifluoromethylated perylene and naphthalene imide and diimide electron acceptors. Org. Lett. 2014, 16, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Weng, X.; Zhao, Y.; Sui, Y.; Wang, X. A crystalline phosphaalkene radical anion. J. Am. Chem. Soc. 2014, 136, 9834–9837. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, A.; Shuku, Y.; Suizu, R.; Matsushita, M.M.; Tsuchiizu, M.; Mañeru, D.R.; Illas, F.; Robert, V.; Awaga, K. Discovery of the K4 Structure Formed by a Triangular π Radical Anion. J. Am. Chem. Soc. 2015, 137, 7612–7615. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.J.; Klen, J.R. Tris-[8]annulenyl isocyanurate trianion triradical and hexa-anion from the alkali metal reduction of [8]annulenyl isocyanate. J. Org. Chem. 2015, 80, 5851–5858. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Haehnel, M.; Krummenacher, I.; Biegger, P.; Geyer, F.L.; Tverskoy, O.; Schaffroth, M.; Han, J.; Dreuw, A.; Marder, T.B.; et al. The Radical Anion and Dianion of Tetraazapentacene. Angew. Chem. Int. Ed. 2016, 55, 10498–10501. [Google Scholar] [CrossRef] [PubMed]
- Wurster, C.; Sendtner, R. Zur Kenntniss des Dimethylparaphenylendiamins. Ber. Dtsch. Chem. Ges. 1879, 12, 1803–1807. [Google Scholar] [CrossRef]
- Wurster, C.; Scholig, E. Ueber die Einwirkung oxydirender Agentien auf Tetramethylparaphenylendiamin. Ber. Dtsch. Chem. Ges. 1879, 12, 1807–1813. [Google Scholar] [CrossRef]
- Wieland, H. Zur Kenntnis der tertiären aromatischen Hydrazine und Amine. (III). Chem. Ber. 1907, 40, 4260–4281. [Google Scholar] [CrossRef]
- Weitz, E.; Schwechtin, H.W. Über den Ammonium-Charakter der Triarylamine. (VII. Mitteilung über freie Ammonium-Radikale.). Chem. Ber. 1926, 59, 2307–2314. [Google Scholar] [CrossRef]
- Michaelis, L. “Ein Reduktions-Indikator im Potentialbereich der Wasserstoffüberspannung”. Biochem. Zeitschrift 1932, 250, 564–567. [Google Scholar]
- Michaelis, L.; Hill, E.S.J. The Viologen Indicator. Gen. Physiol. 1933, 16, 859–873. [Google Scholar] [CrossRef]
- Bockman, T.M.; Kochi, J.K. Isolation and Oxidation-Reduction of methylviologen cation radicals. Novel disproportionation in charge-transfersalts by X-ray crystallography. J. Org. Chem. 1990, 55, 4127–4135. [Google Scholar] [CrossRef]
- Lewis, I.C.; Singer, L.S. Electron spin resonance of radical cations produced by the oxidation of aromatic hydrocarbons with SbCl5. J. Chem. Phys. 1965, 43, 2712–2727. [Google Scholar] [CrossRef]
- McKinney, T.M.; Geske, D.H. The triethylenediamine cation radical. J. Am. Chem. Soc. 1965, 87, 3013–3014. [Google Scholar] [CrossRef]
- Nelson, R.F.; Adams, R.N. The anion and cation radicals of N,N-dimethyl-p-nitroaniline. J. Phys. Chem. 1968, 72, 740–742. [Google Scholar] [CrossRef]
- Ishizu, K.; Dearman, H.H.; Huang, M.T.; White, J.R. Interaction of the 5-Methylphenazinium cation radical with deoxyribonucleic acid. Biochemistry 1969, 8, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Wudl, F.; Wobschall, D.; Hufnagel, E.J. Electrical conductivity by the bis-1,3-dithiole-bis-1,3-dithiolium system. J. Am. Chem. Soc. 1972, 94, 670–672. [Google Scholar] [CrossRef]
- Drews, M.J.; Wong, P.S.; Jones, P.R. Bonding studies in group IV substituted anilines. III. Electron spin resonance spectra of the radical cations and the ground-state bonding description. J. Am. Chem. Soc. 1972, 94, 9122–9128. [Google Scholar] [CrossRef]
- Effenberger, F.; Mack, K.E.; Niess, R.; Reisinger, F.; Steinbach, A.; Stohrer, W.D.; Stezowski, J.J.; Rommel, I.; Maier, A. Aminobenzenes. 19’ dimeric a-complexes: Intermediates in the oxidative dimerization of aromatics. J. Org. Chem. 1988, 53, 4379–4386. [Google Scholar] [CrossRef]
- Effenberger, F.; Stohrer, W.D.; Mack, K.E.; Reisinger, F.; Seufert, W.; Kramer, H.E.A.; FOll, R.; Vogelmann, E. Experimental and theoretical aspects of the formation of radical cations from tripyrrolidinobenzenes and their follow-up reactions. J. Am. Chem. Soc. 1990, 112, 4849–4857. [Google Scholar] [CrossRef]
- Schmittel, M.; Gescheidt, G.; Rock, M. The first spectroscopic identification of an enol radical cation in solution: The anisyldimesitylethenol radical cation. Angew. Chem. Int. Ed. 1994, 33, 1961–1963. [Google Scholar] [CrossRef]
- Adam, W.; Kammel, T. Generation of 4,5-diazacyclopentane-1,3-diyl radical cations by chemical electron transfer (CET) oxidation of urazole-bridged bicyclic housanes (Bicyclo[2.1.0]pentanes) and their chemical transformations. J. Org. Chem. 1996, 61, 3172–3176. [Google Scholar] [CrossRef] [PubMed]
- Bryce, M.R.; Moore, A.J. Vinylogous tetrathiafulvalene (TTF) л-electron donors and derived radical cations: ESR spectroscopic, magnetic, and X-ray structural studies. Chem. Mater. 1996, 8, 1182–1188. [Google Scholar]
- Lu, J.; Chen, Y.; Wen, X.; Wu, L.M.; Jia, X.; Liu, Y.C.; Liu, Z.L. 14N/15N and 12C/13C Equilibrium isotope effects on the Eelectron-transfer reaction between N-methylphenothiazine and its radical cation. J. Phys. Chem. A 1999, 103, 6998–7007. [Google Scholar] [CrossRef]
- Rathore, R.; Burns, C.L.; Deselnicu, M.I. Multiple-electron transfer in a single step. Design and synthesis of highly charged cation-radical salts. Org. Lett. 2001, 18, 2887–2890. [Google Scholar] [CrossRef]
- Hiraoka, S.; Okamoto, T.; Kozaki, M.; Shiomi, D.; Sato, K.; Takui, T.; Okada, K. A stable radical-substituted radical cation with strongly ferromagnetic interaction: Nitronyl nitroxide-substituted 5,10-diphenyl-5,10-dihydrophenazine radical cation. J. Am. Chem. Soc. 2004, 126, 58–59. [Google Scholar] [CrossRef] [PubMed]
- Shorafa, H.; Mollenhauer, D.; Paulus, B.; Seppelt, K. The Two Structures of the Hexafluorobenzene Radical Cation C6F6•+. Angew. Chem. Int. Ed. 2009, 48, 5845–5847. [Google Scholar] [CrossRef] [PubMed]
- Back, O.; Donnadieu, B.; Parameswaran, P.; Frenking, G.; Bertrand, G. Isolation of crystalline carbene-stabilized P2-radical cations and P2-dications. Nat. Chem. 2010, 2, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, X.; Zhou, Z.; Li, Y.; Sui, Y.; Ma, J.; Wang, X.; Power, P.P. Reversible σ-dimerizations of persistent organic radical cations. Angew. Chem. Int. Ed. 2013, 52, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ajayakumar, M.R.; Hundal, G.; Mukhopadhyay, P. Extraordinary stability of naphthalenediimider Ion and its ultra-electron-deficient precursor: Strategic role of the phosphonium group. J. Am. Chem. Soc. 2014, 136, 12004–12010. [Google Scholar] [CrossRef] [PubMed]
- Welker, E.A.; Tiley, B.L.; Sasaran, C.M.; Zuchero, M.A.; Tong, W.S.; Vettleson, M.J.; Richards, R.A.; Geruntho, J.J.; Stoll, S.; Wolbach, J.P.; et al. Conformational change with steric interactions affects the inner sphere component of concerted proton–electron transfer in a pyridyl-appended radical cation system. J. Org. Chem. 2015, 80, 8705–8712. [Google Scholar] [CrossRef] [PubMed]
- Kayahara, E.; Kouyama, T.; Kato, T.; Yamago, S. Synthesis and characterization of [n]CPP (n = 5, 6, 8, 10, and 12) radical cation and dications: Size-dependent absorption, spin, and charge delocalization. J. Am. Chem. Soc. 2016, 138, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.J.; Turk, M.R.; Kiesewetter, M.K.; Reiter, R.C.; Stevenson, C.D. The cyclooctatriene-η2-ynyl potassium zwitterionic radical: Evidence for a potassium organometallic. J. Am. Chem. Soc. 2003, 125, 11212–11213. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Blum, C.; Liu, S.-X.; Keene, T.D.; Frei, G.; Neels, A.; Decurtins, S. Isolable Zwitterionic Pyridinio-semiquinone π-Radicals. Mild and Efficient Single-Step Access to Stable Radicals. Org. Lett. 2009, 11, 2261–2262. [Google Scholar] [CrossRef] [PubMed]
- Yong, G.P.; Li, C.F.; Li, Y.Z.; Luo, S.W. New zwitterionic radical salts: Dimers in solution and unusual magnetic and luminescent properties in the solid state. Chem. Commun. 2010, 46, 3194–3196. [Google Scholar] [CrossRef] [PubMed]
- Sanna, E.; Martinez, L.; Rotger, C.; Blasco, S.; Gonzalez, J.; Espana, G.E.; Costa, A. Squaramide-based reagent for selective chromogenic sensing of Cu(II) through a zwitterion radical. Org. Lett. 2010, 12, 3840–3843. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Bialas, D.; Wurthner, F. Ambient stable zwitterionic perylene bisimide-centered radical. Angew. Chem. Int. Ed. 2015, 54, 3611–3614. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Sumpter, B.G.; Meunier, V.; Tian, Y.H.; Kertesz, M. Cyclo-biphenalenyl biradicaloid molecular materials: Conformation, tautomerization, magnetism, and thermochromism. Chem. Mater. 2011, 23, 874–885. [Google Scholar] [CrossRef]
- Bag, P.; Pal, S.K.; Itkis, M.E.; Sarkar, A.; Tham, F.S.; Donnadieu, B.; Haddon, R.C. Synthesis of tetrachalcogenide-substituted phenalenyl derivatives: Preparation and solid-state characterization of bis(3,4,6,7-tetrathioalkyl-phenalenyl) boron radicals. J. Am. Chem. Soc. 2013, 135, 12936–12939. [Google Scholar] [CrossRef] [PubMed]
- Aboaku, S.; Filho, A.P.; Bindilatti, V.; Oliveira, N.F.; Schlueter, J.A., Jr.; Lahti, P.M. Aminophenylnitronylnitroxides: Highly networked hydrogen-bond assembly in organic radical materials. Chem. Mater. 2011, 23, 4844–4856. [Google Scholar] [CrossRef]
- Seber, G.; Freitas, R.S.; Oliveira, N.F.; Schlueter, J.A., Jr.; Lahti, P.M. Crystal packing and magnetism in phenolic nitronylnitroxides: 2-(3′,5′-dimethoxy-4′-hydroxyphenyl)-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazole-1-oxyl. Cryst. Growth Des. 2013, 13, 893–900. [Google Scholar] [CrossRef]
- Mostovich, E.A.; Borozdina, Y.; Enkelmann, V.; Langer, K.R.; Wolf, B.; Lang, M.; Baumgarten, M. Planar biphenyl-bridged biradicals as building blocks for the design of quantum magnets. Cryst. Growth Des. 2012, 12, 54–59. [Google Scholar] [CrossRef]
- Constantinides, C.P.; Berezin, A.A.; Manoli, M.; Leitus, G.M.; Zissimou, G.A.; Bendikov, M.; Rawson, J.M.; Koutentis, P.A. Structural, Magnetic, and Computational Correlations of Some Imidazolo-Fused 1,2,4-Benzotriazinyl Radicals. Chem. Eur. J. 2014, 20, 5388–5396. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, C.P.; Koutentis, P.A.; Rawson, J.M. Antiferromagnetic interactions in 1D heisenberg linear chains of 7-(4-fluorophenyl) and 7-phenyl-substituted 1,3-diphenyl-1,4-dihydro-1,2,4-benzotriazin-4-yl radicals. Chem. Eur. J. 2012, 18, 15433–15438. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, C.P.; Koutentis, P.A.; Rawson, J.M. Ferromagnetic interactions in a 1D alternating linear chain of p-stacked 1,3-diphenyl-7-(thien-2-yl)-1,4-dihydro-1,2,4-benzotriazin-4-yl radicals. Chem. Eur. J. 2012, 18, 7109–7116. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, C.P.; Carter, E.; Murphy, D.M.; Manoli, M.; Leitus, G.M.; Bendikov, M.; Rawsond, J.M.; Koutentis, P.A. Spin-triplet excitons in 1,3-diphenyl-7-(fur-2-yl)-1,4-dihydro-1,2,4-benzotriazin-4-yl. Chem. Commun. 2013, 49, 8662–8664. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Cramen, J.; McDonald, R.; Frank, N.L. Ferromagnetic spin-delocalized electron donors for multifunctional materials: P-conjugated benzotriazinyl radicals. Chem. Commun. 2011, 47, 3201–3203. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, C.P.; Berezin, A.A.; Zissimou, G.A.; Manoli, M.; Leitus, G.M.; Bendikov, M.; Probert, M.R.; Rawson, J.M.; Koutentis, P.A. A Magnetostructural investigation of an abrupt spin transition for 1-Phenyl-3-trifluoromethyl-1,4-dihydrobenzo[e][1,2,4]triazin-4-yl. J. Am. Chem. Soc. 2014, 136, 11906–11909. [Google Scholar] [CrossRef] [PubMed]
- Ciccullo, F.; Gallagher, N.M.; Geladari, O.; Chassé, T.; Rajca, A.; Casu, M.B. A Derivative of the Blatter Radical as a Potential Metal-Free Magnet for Stable Thin Films and Interfaces. ACS Appl. Mater. Interfaces 2016, 8, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
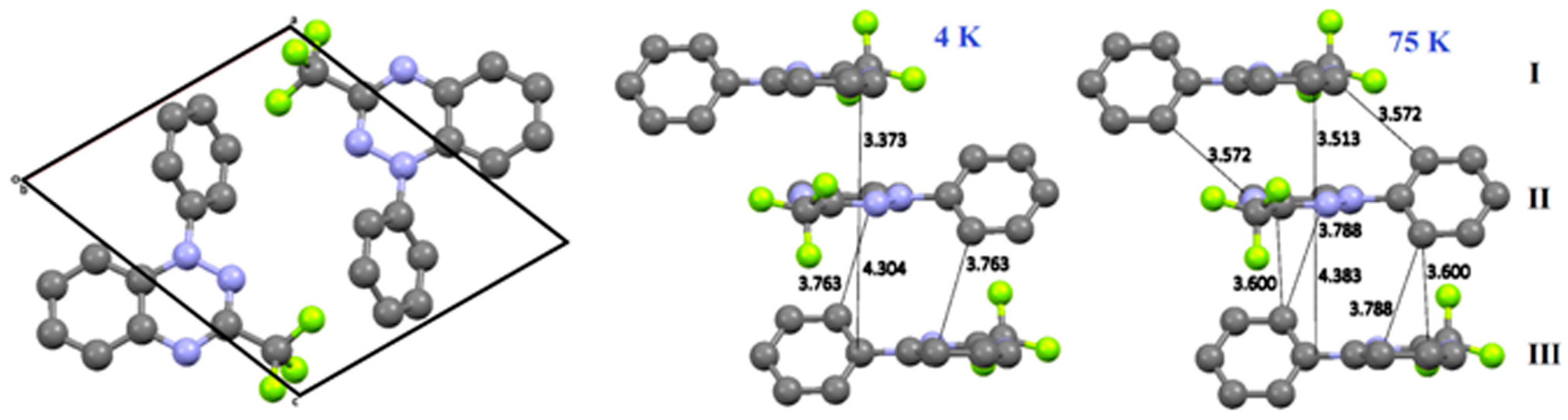
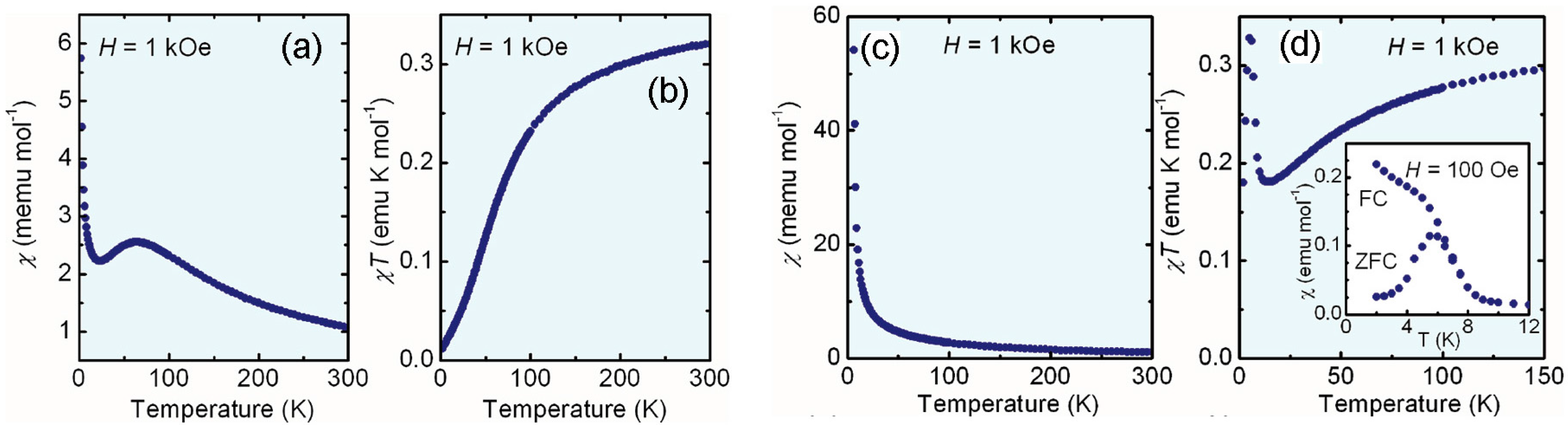
- Winter, S.M.; Datta, S.; Hill, S.; Oakley, R.T. Magnetic anisotropy in a heavy atom radical ferromagnet. J. Am. Chem. Soc. 2011, 133, 8126–8129. [Google Scholar] [CrossRef] [PubMed]
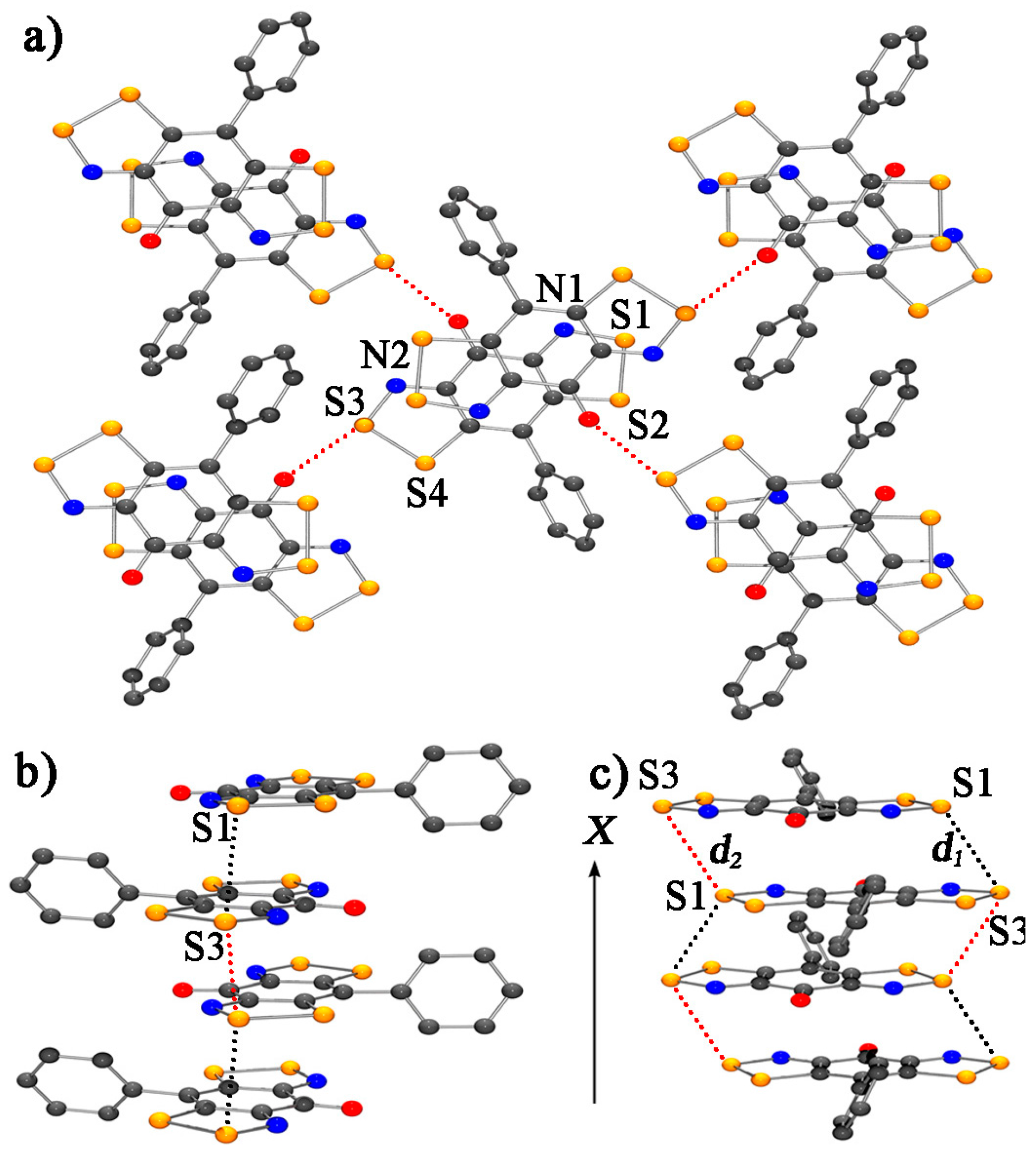
- Yu, X.; Mailman, A.; Lekin, K.; Assoud, A.; Robertson, C.M.; Noll, B.C.; Campana, C.F.; Howard, J.A.K.; Dube, P.A.; Oakley, R.T. Semiquinone-bridged bisdithiazolyl radicals as neutral radical conductors. J. Am. Chem. Soc. 2012, 134, 2264–2275. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Mailman, A.; Dube, P.A.; Assouda, A.; Oakley, R.T. The first semiquinone-bridged bisdithiazolyl radical conductor: A canted antiferromagnet displaying a spin-flop transition. Chem. Commun. 2011, 47, 4655–4657. [Google Scholar] [CrossRef] [PubMed]
- Mailman, A.; Winter, S.M.; Wong, J.W.L.; Robertson, C.M.; Assoud, A.; Dube, P.A.; Oakley, R.T. Multiple orbital effects and magnetic ordering in a neutral radical. J. Am. Chem. Soc. 2015, 137, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Rajca, A.; Olankitwanit, A.; Wang, Y.; Boratynski, P.J.; Pink, M.; Rajca, S. High-spin S = 2 ground state aminyl tetraradicals. J. Am. Chem. Soc. 2013, 135, 18205–18215. [Google Scholar] [CrossRef] [PubMed]
- Ueda, A.; Yamada, S.; Isono, T.; Kamo, H.; Nakao, A.; Kumai, R.; Nakao, H.; Murakami, Y.; Yamamoto, K.; Nishio, Y.; et al. Hydrogen-bond-dynamics-based switching of conductivity and magnetism: A phase transition caused by deuterium and electron transfer in a hydrogen-bonded purely organic conductor crystal. J. Am. Chem. Soc. 2014, 136, 12184–12192. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Sung, Y.M.; Bao, N.; Tan, D.; Lee, R.; Zafra, J.L.; Lee, B.S.; Ishida, M.; Ding, J.; Navarrete, J.T.L.; et al. Stable Tetrabenzo-Chichibabin’s hydrocarbons: Tunable ground state and unusual transition between their closed-shell and open-shell resonance forms. J. Am. Chem. Soc. 2012, 134, 14513–14525. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Huang, K.W.; Wu, J. Soluble and stable heptazethrenebis(dicarboximide) with a singlet open-shell ground state. J. Am. Chem. Soc. 2011, 133, 11896–11899. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Heng, W.K.; Lee, B.S.; Aratani, N.; Zafra, J.L.; Bao, N.; Lee, R.; Sung, Y.M.; Sun, Z.; Huang, K.W.; et al. Kinetically blocked stable heptazethrene and octazethrene: Closed-shell or open-shell in the ground state? J. Am. Chem. Soc. 2012, 134, 14913–14922. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lee, S.; Park, K.H.; Zhu, X.; Zhang, W.; Zheng, B.; Hu, P.; Zeng, Z.; Das, S.; Li, Y.; et al. Dibenzoheptazethrene isomers with different biradical characters: An exercise of Clar’s aromatic sextet rule in singlet biradicaloids. J. Am. Chem. Soc. 2013, 135, 18229–18236. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Ishida, M.; Zafra, J.L.; Zhu, X.; Sung, Y.M.; Bao, N.; Webster, R.D.; Lee, B.S.; Li, R.W.; Zeng, W.; et al. Pushing extended p-quinodimethanes to the limit: Stable tetracyano-oligo(N-annulated perylene) quinodimethanes with tunable ground states. J. Am. Chem. Soc. 2013, 135, 6363–6371. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Lee, S.; Herng, T.S.; Aratani, N.; Goncalves, T.P.; Qi, Q.; Shi, X.; Yamada, H.; Huang, K.W.; Ding, J.; et al. Toward tetraradicaloid: The effect of fusion mode on radical character and chemical reactivity. J. Am. Chem. Soc. 2016, 138, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Phan, H.; Herng, T.S.; Hu, P.; Zeng, W.; Dong, S.Q.; Das, S.; Shen, Y.; Ding, J.; Casanova, D.; et al. Higher order π-conjugated polycyclic hydrocarbons with open-shell singlet ground state: Nonazethrene versus nonacene. J. Am. Chem. Soc. 2016, 138, 10323–10330. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Herng, T.S.; Zafra, J.L.; Burrezo, P.M.; Kitano, M.; Ishida, M.; Gopalakrishna, T.Y.; Hu, P.; Osuka, A.; Casado, J.; et al. Fully fused quinoidal/aromatic carbazole macrocycles with polyradical characters. J. Am. Chem. Soc. 2016, 138, 7782–7790. [Google Scholar] [CrossRef] [PubMed]
- Nishide, H.; Oyaizu, K. Toward Flexible Batteries. Science 2008, 319, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Suzuki, S.; Sato, K.; Takui, T. Synthetic organic spin chemistry for structurally well-defined open-shell graphene fragments. Nat. Chem. 2011, 3, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Nishida, S.; Sato, K.; Nakazawa, S.; Rahimi, R.D.; Toyota, K.; Shiomi, D.; Morita, Y.; Kitagawa, M.; Takui, T. ESR and 1H-,19F-ENDOR/TRIPLE Study of Fluorinated Diphenylnitroxides as Synthetic Bus Spin-Qubit Radicals with Client Qubits in Solution. J. Phys. Chem. Lett. 2011, 2, 449–453. [Google Scholar] [CrossRef]
- Ajayakumar, M.R.; Mukhopadhyay, P. Naphthalene-bis-hydrazimide: Radical Anions and ICT as New Bimodal Probes for Differential Sensing of a Library of Amines. Chem. Commun. 2009, 3702–3704. [Google Scholar] [CrossRef] [PubMed]
- Ajayakumar, M.R.; Yadav, S.; Ghosh, S.; Mukhopadhyay, P. Single-Electron Transfer Driven Cyanide Sensing: A New Multimodal Approach. Org. Lett. 2010, 46, 2646–2649. [Google Scholar] [CrossRef] [PubMed]
- Ajayakumar, M.R.; Mandal, K.; Rawat, K.; Asthana, D.; Pandey, R.; Sharma, A.; Yadav, S.; Ghosh, S.; Mukhopadhyay, P. Single Electron Transfer-Driven Multi-Dimensional Signal Read-out Function of TCNQ as an “Off-the-Shelf” Detector for Cyanide. ACS Appl. Mater. Interfaces 2013, 5, 6996–7000. [Google Scholar] [CrossRef] [PubMed]

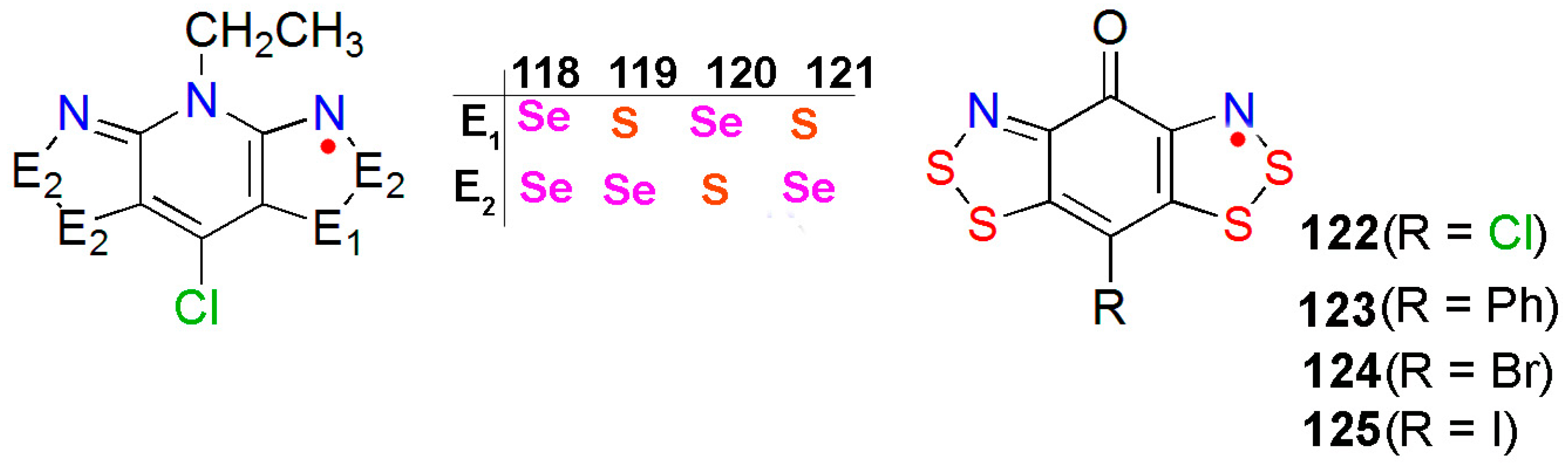


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Kumar, Y.; Keshri, S.K.; Mukhopadhyay, P. Recent Advances in Organic Radicals and Their Magnetism. Magnetochemistry 2016, 2, 42. https://doi.org/10.3390/magnetochemistry2040042
Kumar S, Kumar Y, Keshri SK, Mukhopadhyay P. Recent Advances in Organic Radicals and Their Magnetism. Magnetochemistry. 2016; 2(4):42. https://doi.org/10.3390/magnetochemistry2040042
Chicago/Turabian StyleKumar, Sharvan, Yogendra Kumar, Sudhir Kumar Keshri, and Pritam Mukhopadhyay. 2016. "Recent Advances in Organic Radicals and Their Magnetism" Magnetochemistry 2, no. 4: 42. https://doi.org/10.3390/magnetochemistry2040042




