Slow Magnetic Relaxation of Lanthanide(III) Complexes with a Helical Ligand
Abstract
:1. Introduction
2. Results
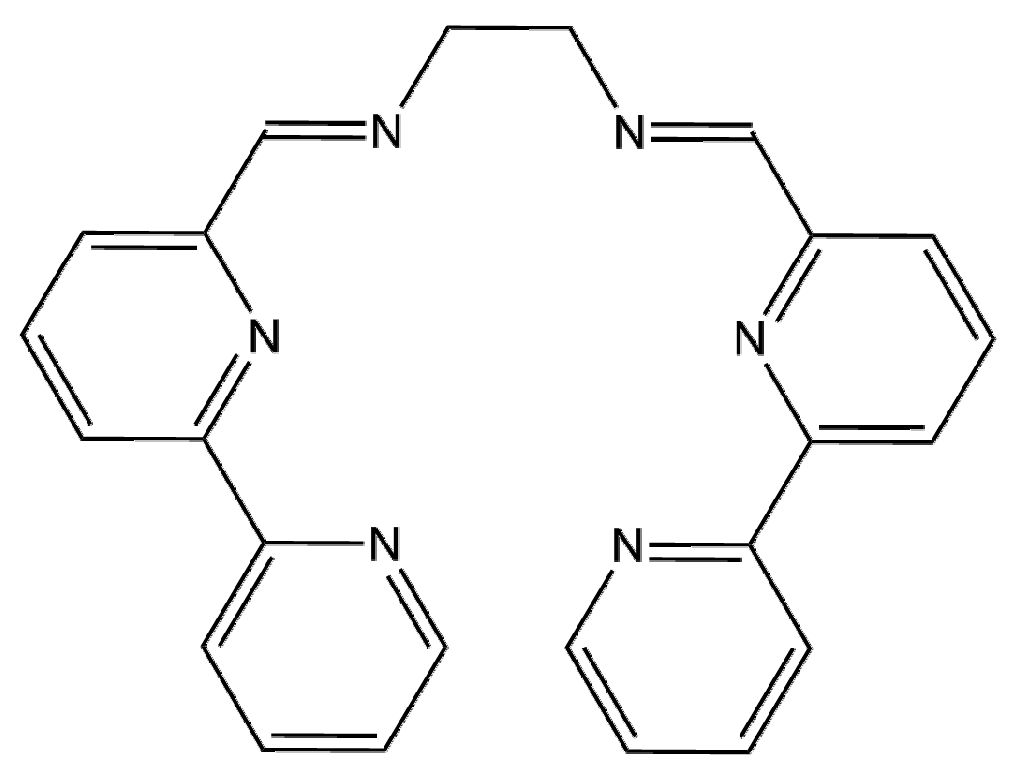
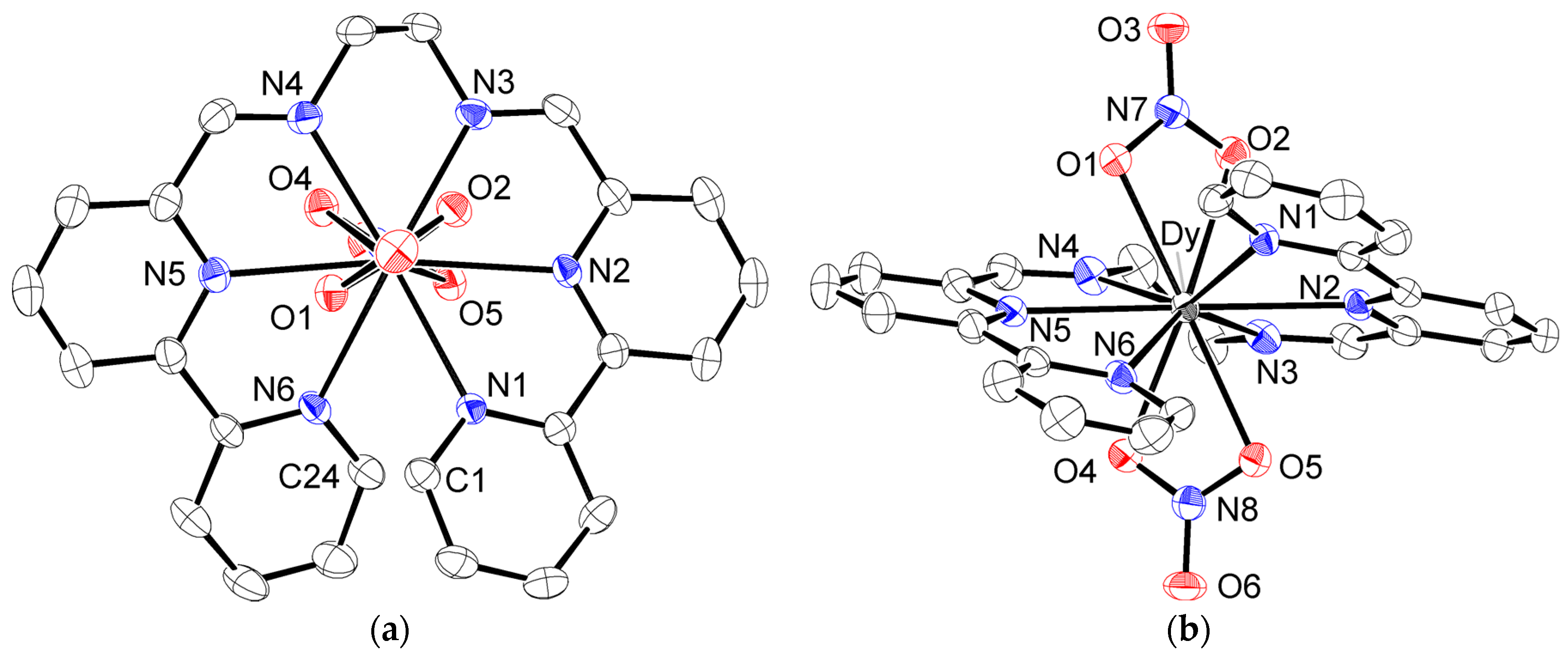
2.1. Synthesis and Characterization
2.2. DC Susceptibility of the Complexes
2.3. Dynamic Susceptibility of the Complexes
2.3.1. Nd(III) Complex 1
2.3.2. Tb(III) Complex 2
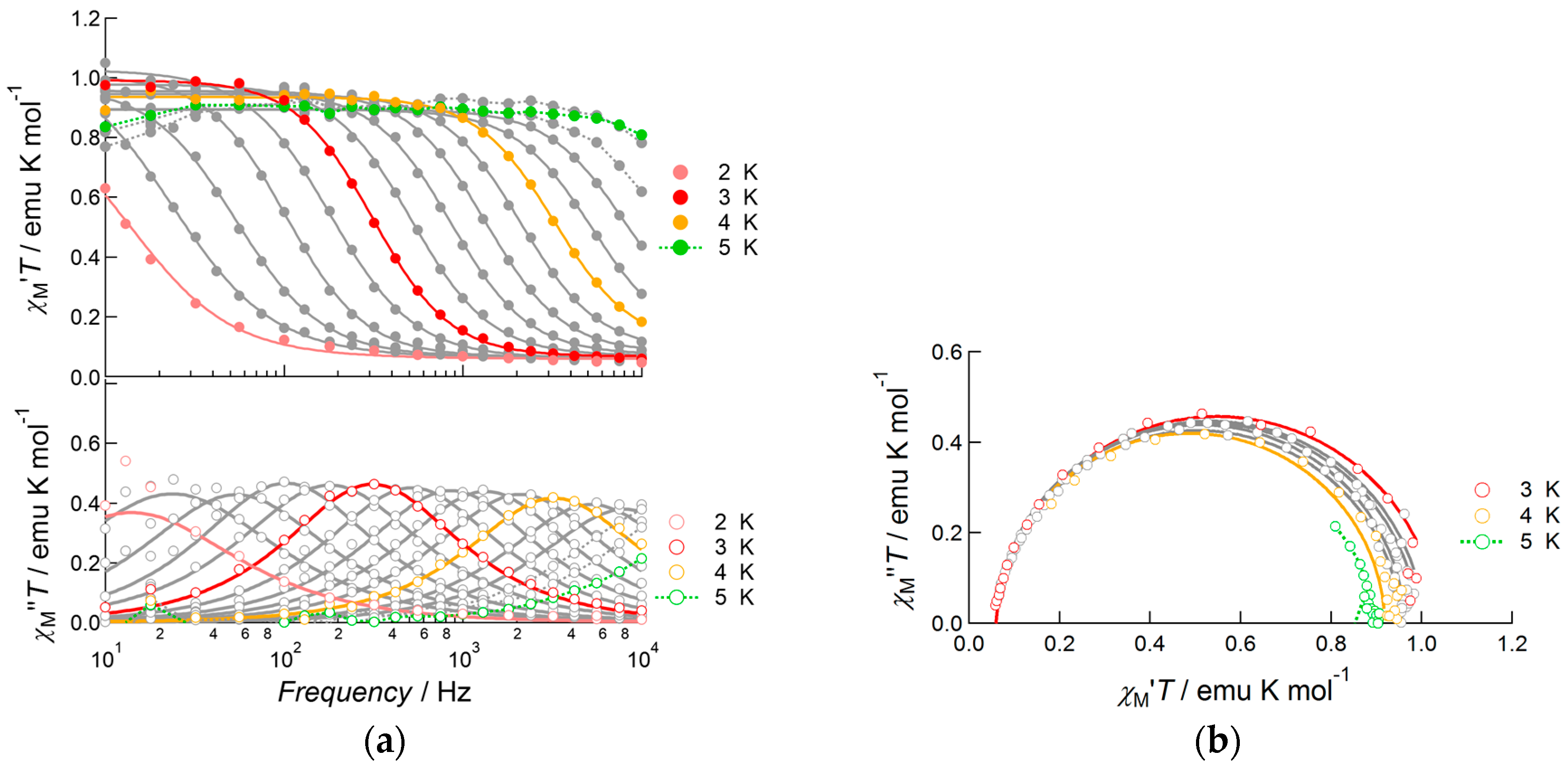
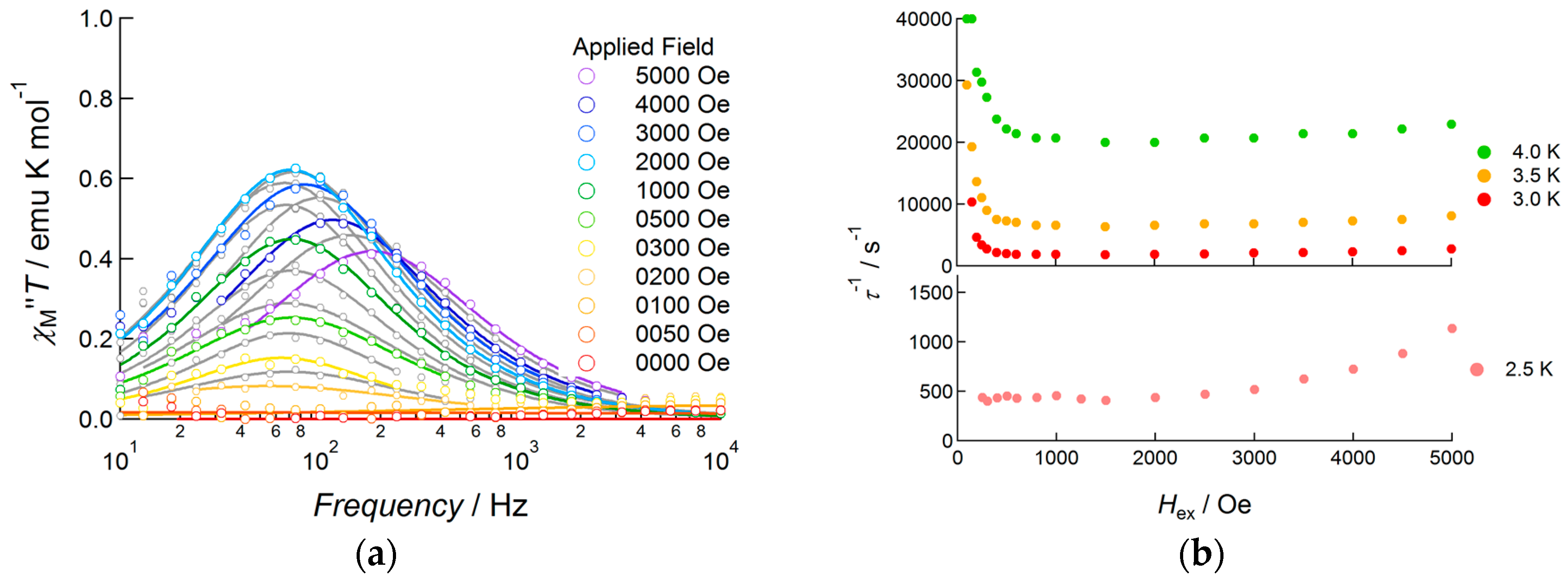
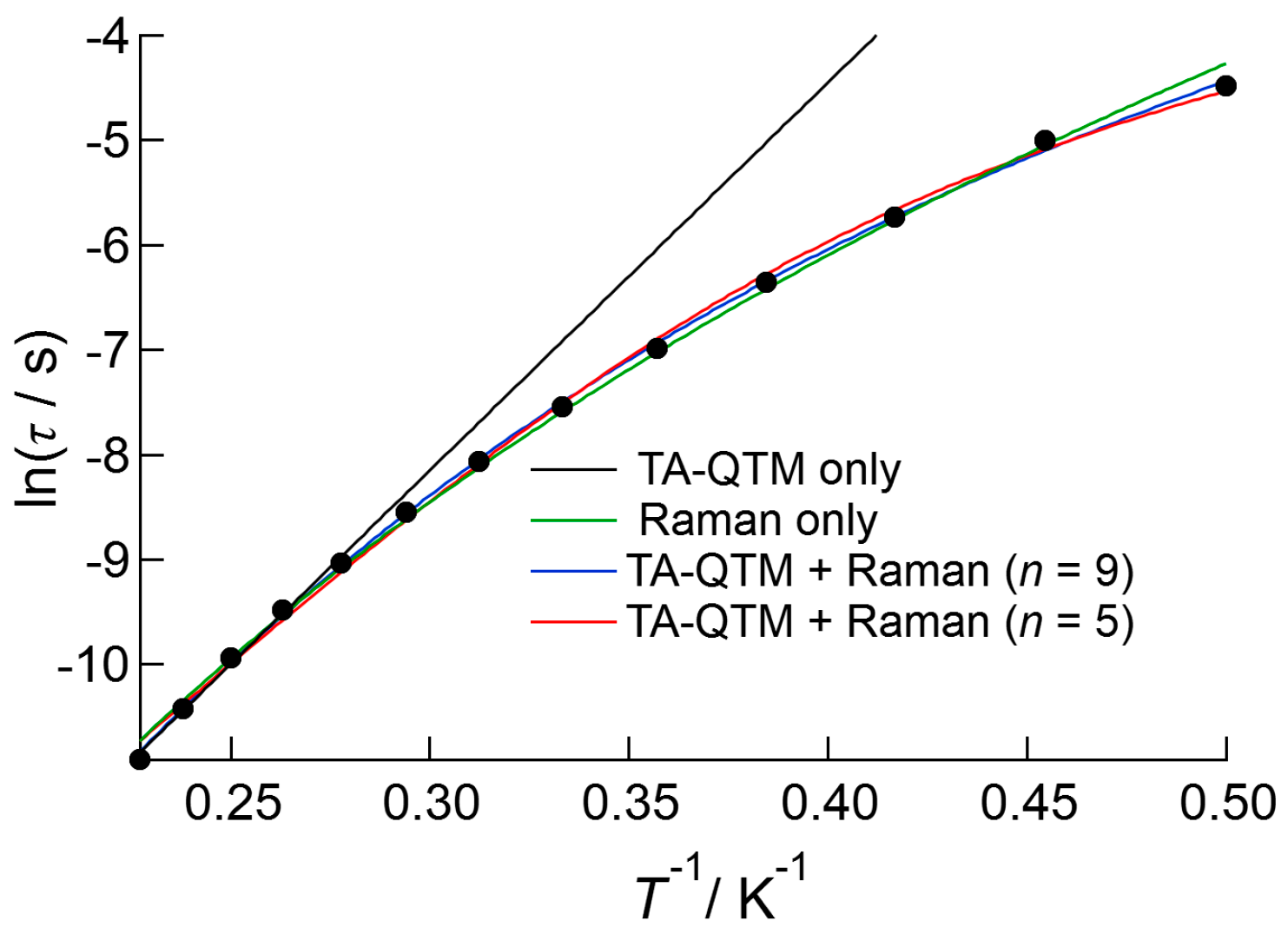
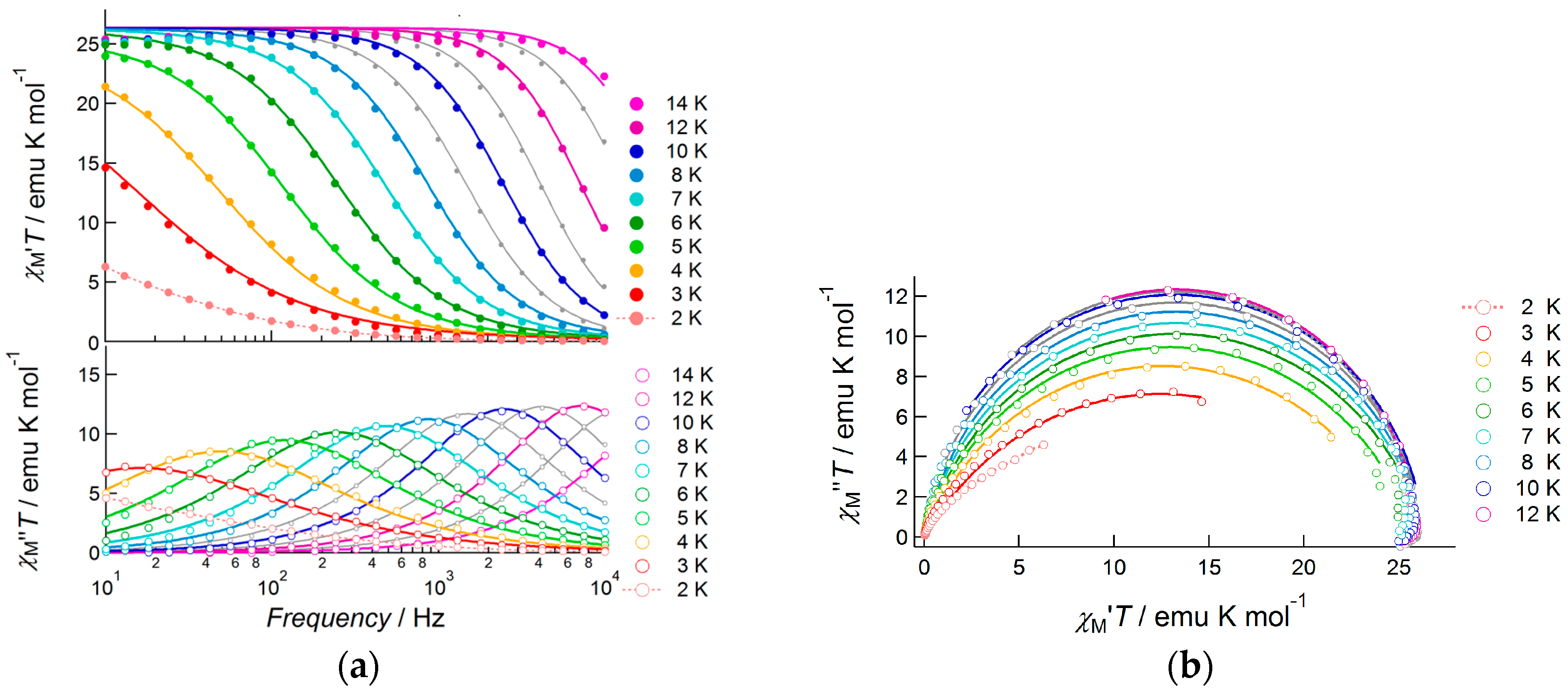
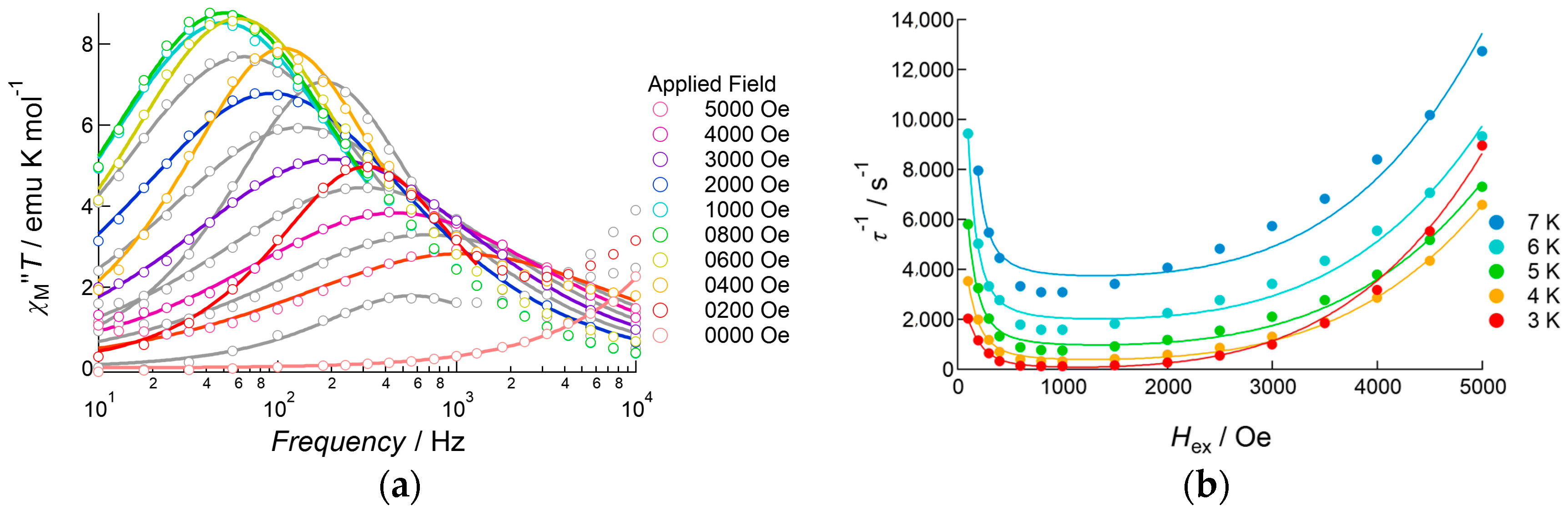
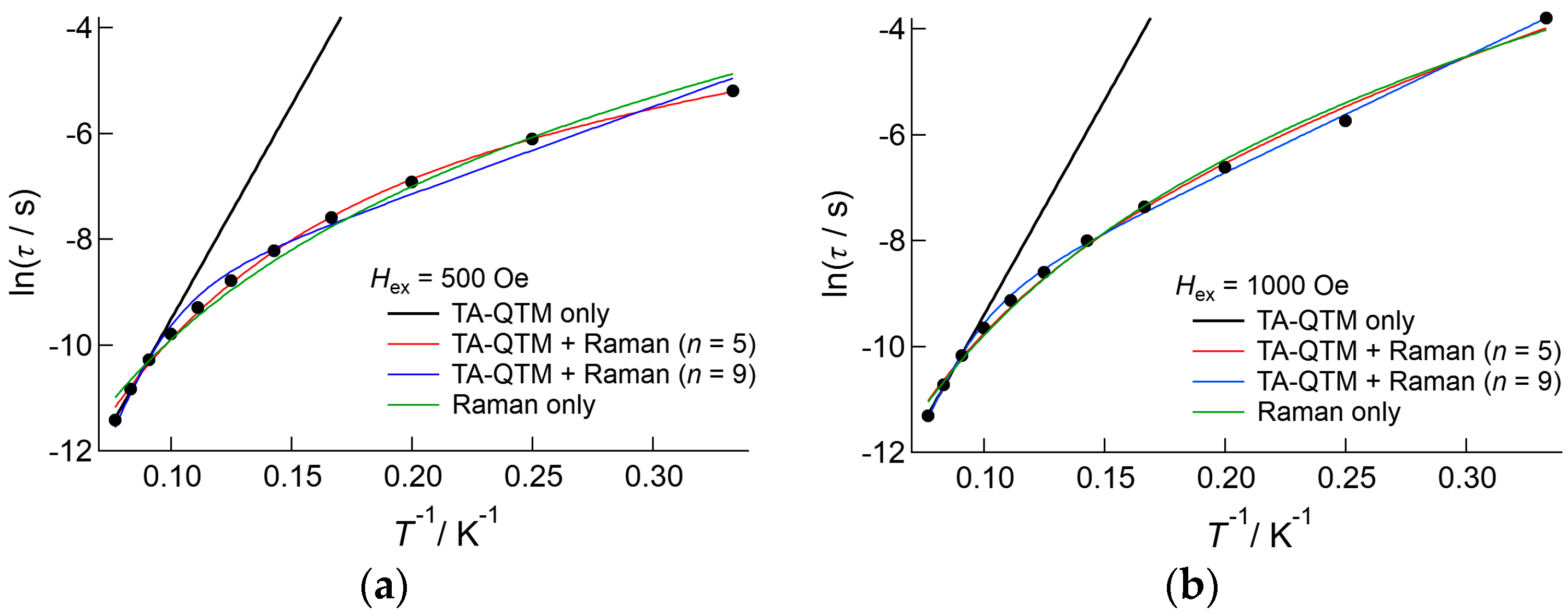
2.3.3. Dy(III) Complex 3
3. Discussion
4. Materials and Methods
4.1. General Procedures and Methods
4.2. Synthesis of Complexes [LnL(NO3)2]PF6·MeCN
4.3. Crystallography
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SMM | Single Molecule Magnet |
| QTM | Quantum Tunneling of Magnetization |
| TM | Thermally Assisted |
| AC and DC | Alternating and Direct Current |
References
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press: New York, NY, USA, 2006; pp. 47–159. [Google Scholar]
- Glaser, T. Rational design of single-molecule magnets: A supramolecular approach. Chem. Comm. 2011, 47, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Layfield, R.A. Organometallic Single-Molecule Magnets. Organometallics 2014, 33, 1084–1099. [Google Scholar] [CrossRef]
- Madhu, N.T.; Tang, J.-K.; Hewitt, I.J.; Clérac, R.; Wernsdorfer, W.; van Slageren, J.; Anson, C.E.; Powell, A.K. What makes a single molecule magnet? Polyhedron 2005, 24, 2864–2869. [Google Scholar] [CrossRef]
- Liddle, S.T.; Van Slageren, J. Improving f-element single molecule magnets. Chem. Soc. Rev. 2015, 24, 6655–6669. [Google Scholar] [CrossRef] [PubMed]
- Feltham, H.L.C.; Brooker, S. Review of purely 4f and mixed-metal nd-4f single-molecule magnets containing only one lanthanide ion. Coord. Chem. Rev. 2014, 276, 1–33. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Y.-N.; Tang, J. Recent advances in dysprosium-based single molecule magnets: Structural overview and synthetic strategies. Coord. Chem. Rev. 2013, 257, 1728–1763. [Google Scholar] [CrossRef]
- Woodru, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, D. Angular distribution of 4f electrons in the presence of a crystal field. J. Phys. 1986, 47, 677–681. [Google Scholar] [CrossRef]
- Walter, U. Charge Distributions of Crystal Field States. Z. Phys. B: Condens. Matter 1986, 62, 299–309. [Google Scholar] [CrossRef]
- Rinehart, J.R.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ohtsu, H.; Kodama, D.; Kasai, T.; Sakurai, S.; Ishii, A.; Suzuki, K. Luminescence behaviour in acetonitrile and in the solid state of a series of lanthanide complexes with a single helical ligand. New J. Chem. 2014, 38, 1225–1234. [Google Scholar] [CrossRef]
- Takahara, C.; Then, P.L.; Kataoka, Y.; Nakano, M.; Yamamura, T.; Kajiwara, T. Slow magnetic relaxation of light lanthanidebased linear LnZn2 trinuclear complexes. Dalton Trans. 2015, 44, 18276–18283. [Google Scholar] [CrossRef] [PubMed]
- Hino, S.; Maeda, M.; Kataoka, Y.; Nakano, M.; Yamamura, T.; Kajiwara, T. SMM Behavior Observed in Ce(III)Zn(II)2 Linear Trinuclear Complex. Chem. Lett. 2013, 42, 1276–1278. [Google Scholar] [CrossRef]
- Hino, S.; Maeda, M.; Yamashita, K.; Kataoka, Y.; Nakano, M.; Yamamura, T.; Nojiri, H.; Kofu, M.; Yamamuro, O.; Kajiwara, T. Linear Trinuclear Zn(II)–Ce(III)–Zn(II) Complex which Behaves as Single-molecule Magnet. Dalton Trans. 2013, 42, 2683–2686. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, J.J.; Gorelsky, S.I.; Korobkov, I.; Murugesu, M. Slow Magnetic Relaxation in Uranium(III) and Neodymium(III) Cyclooctatetraenyl Complexes. Organometallics 2015, 34, 1415–1418. [Google Scholar] [CrossRef]
- Singh, S.K.; Gupta, T.; Ungur, L.; Rajaraman, G. Magnetic Relaxation in Single-Electron Single-Ion Cerium(III) Magnets: Insights from Ab Initio Calculations. Chem. Eur. J. 2015, 21, 13812–13819. [Google Scholar] [CrossRef] [PubMed]
- Habib, F.; Long, J.; Lin, P.-H.; Korobkov, I.; Ungur, L.; Wernsdorfer, W.; Chibotaru, L.F.; Murugesu, M. Supramolecular architectures for controlling slow magnetic relaxation in field-induced single-molecule magnets. Chem. Sci. 2012, 3, 2158–2164. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Abragam, A.; Bleaney, B. Electron Paramagnetic Resonance of Transition Ions; Oxford University Press: Oxford, UK, 1970; pp. 60–74 and pp. 555–560. [Google Scholar]
- Carlin, R.L. Magnetochemistry; Springer: Berlin/Heidelberg, Germany, 1986; pp. 36–51. [Google Scholar]
- Vrábel, P.; Orendáč, M.; Orendáčová, A.; Čižmár, E.; Tarasenko, R.; Zvyagin, S.; Wosnitza, J.; Prokleška, J.; Sechovský, V.; Pavlík, V.; et al. Slow spin relaxation induced by magnetic field in [NdCo(bpdo)(H2O)4(CN)6]·3H2O. J. Phys. Condens. Matter. 2013, 25, 186003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; Duan, G.-P.; Sato, O.; Gao, S. Structures and magnetism of cyano-bridged grid-like two-dimensional 4f–3d arrays. J. Mater. Chem. 2006, 16, 2625–2634. [Google Scholar] [CrossRef]
- Ma, B.-Q.; Gao, S.; Su, G.; Xu, G.-X. Cyano-Briged 4f-3d Coordination Polymers with a Unique Two-Dimensional Topological Architecture and Unusual Magnetic Behavior. Angew. Chem. Int. Ed. 2001, 40, 434–437. [Google Scholar] [CrossRef]
- Crystal Clear, Version 1.3.5; Operating Software for the CCD Detector System; Rigaku and Molecular Structure Corp.: Tokyo, Japan; The Woodlands, TX, USA, 2003.
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97: Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, H.; Ooka, S.; Iwasawa, D.; Hasegawa, M.; Kajiwara, T. Slow Magnetic Relaxation of Lanthanide(III) Complexes with a Helical Ligand. Magnetochemistry 2016, 2, 43. https://doi.org/10.3390/magnetochemistry2040043
Wada H, Ooka S, Iwasawa D, Hasegawa M, Kajiwara T. Slow Magnetic Relaxation of Lanthanide(III) Complexes with a Helical Ligand. Magnetochemistry. 2016; 2(4):43. https://doi.org/10.3390/magnetochemistry2040043
Chicago/Turabian StyleWada, Hisami, Sayaka Ooka, Daichi Iwasawa, Miki Hasegawa, and Takashi Kajiwara. 2016. "Slow Magnetic Relaxation of Lanthanide(III) Complexes with a Helical Ligand" Magnetochemistry 2, no. 4: 43. https://doi.org/10.3390/magnetochemistry2040043






