1. Introduction
Among molecular conductors, bis(ethylenedithio)tetrathiafulvalene (BEDT-TTF) salts with octahedral anions, so-called
-(BEDT-TTF)
PF
type salts, have a unique arrangement of the donor molecules with a twisted stacking structure (
Figure 1) [
1,
2,
3]. This molecular arrangement is categorized into the
type; the donor column consists of alternate stacking of two interaction modes: the twisted stacking mode (
in
Figure 1c) and the parallel stacking mode (
) slipped along the molecular short axis (ring-over-atom mode) [
3]. The highly conducting direction is not the donor stacking direction but the intercolumnar direction [
1]. After the discovery of the PF
salt, several
-type BEDT-TTF salts with octahedral anions have been developed. The crystal system, however, differs between the PF
(orthorhombic) and SbF
(monoclinic) salts despite the same anion shape [
4]. The AsF
salt has been known as a monoclinic compound, but later the orthorhombic crystal has been found [
4,
5,
6,
7].
The PF
salt has been investigated using X-ray diffraction and Raman spectra; the phase transition at around room temperature is not a
charge-density-wave but a charge order [
8,
9]. The charge ordering transition is due to the nearest neighbor Coulomb repulsion
V [
10,
11,
12]. The orthorhombic AsF
salt also shows a similar charge order to that of the PF
salt [
8]. The charge order has been observed in both the monoclinic AsF
and SbF
salts using Raman spectra [
13]. Although many investigations of the
-type BEDT-TTF salts with octahedral anions have been carried out, the charge ordering transition temperature does not seem to change in the sequence of the anion volume.
In order to clarify the relationship between the transition temperature and the anion volume, the TaF
salt is prepared; TaF
is the largest anion among
MF
anions (
P, As, Sb, and Ta) [
14]. In the present paper, we report two
-type polymorphs of the TaF
salts, monoclinic (
) and orthorhombic (
) phases. Although both salts show a charge ordering transition, the transition temperature of the orthorhombic phase is higher than that of the monoclinic phase. If we distinguish between the monoclinic and orthorhombic phases in the
-type (BEDT-TTF)
F
conductors, the transition temperature increases with increasing the anion volume; this indicates that the chemical pressure affects the charge ordering transition temperature.
2. Results
The crystallographic data of both
- and
-(BEDT-TTF)
TaF
at 298 K are shown in
Table 1. For the monoclinic
phase, we choose the space group
. Although unusual space group
has been used in the SbF
salt [
4], the unit cell transformation
gives the monoclinic angle
and the reported lattice. In the AsF
salt, another unit cell transformation gives the reported
lattice [
5]. The orthorhombic
-TaF
salt and the PF
salt are isostructural, and the space group is
. The unit cell volume of the monoclinic phase is slightly larger than that of the orthorhombic phase.
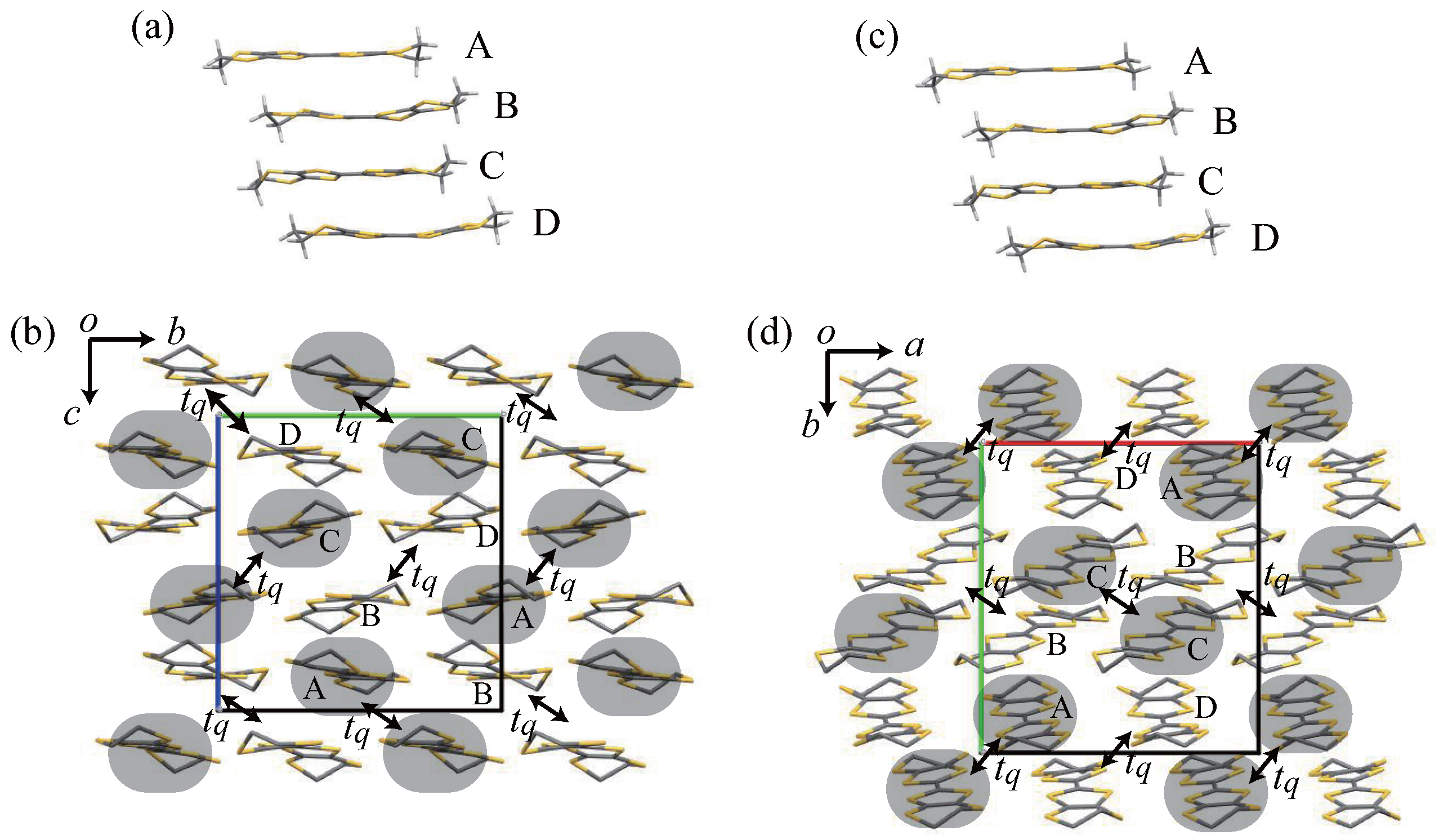
Figure 1a–d show the crystal structure of the TaF
salts. These two phases take similar
-type structures. For the monoclinic phase, the BEDT-TTF molecular planes of the adjacent conducting layers along the
a-axis are almost parallel to each other (
Figure 1a). In the orthorhombic phase, however, donor planes are inclined alternately along the interlayer direction (
Figure 1b). There is one crystallographically independent donor and a half anion, and a unit cell contains eight donors and four anions, affording the donor to anion ratio 2:1. The terminal donor ethylene-groups are disordered. The donors form a stack along the
c-axis for the
phase (
a-axis for the
phase) with the twisted mode
and the ring-over-atom mode
. For the
mode, the interplanar distance is
Å and the twist angle is
in the
phase, and
Å and
in the
phase. For the
mode, the slip distance along the molecular short axis is
Å, and
Å in the
phase. These values are
Å and
Å in the
phase.
An anion is located on an inversion center for the
phase. In the
phase, an anion is on a two-fold rotation axis parallel to the
a-axis, and fluorine atoms are on general positions. The arrangement of anions is an isosceles triangle on an anion layer parallel to the
-plane (
-plane) in the
(
) phase. An interaction between the donor hydrogen and anion fluorine atoms shorter than the sum of the van der Waals radii (
Å) [
15], a so-called hydrogen bond, is found. Although both terminal ethylene groups of the donor have hydrogen bonds in the
phase, one side terminal of the donor has a hydrogen bond in the
phase. This is due to the difference of the unit cell volumes because the unit cell of the
phase is smaller than that of the
phase.
The calculated transfer integrals, the distance between the molecular centers
R, and the twist angles
are shown in
Table 2. All interaction modes (
c,
,
,
p, and
q) are the same as those of
-(BEDT-TTF)
PF
[
2,
3]. The transfer integral of the twisted interaction mode
is larger than that of the ring-over-atom mode
; this twisted dimer structure is characteristic of this structure. The transverse interactions are large, and
is larger than
. The energy band structure and Fermi surface are shown in
Figure 1e,f. The quasi-one-dimensional Fermi surface is shown in an extended zone scheme because the energy bands degenerate at the zone boundary owing to the crystallographic symmetries.
Figure 2 shows the temperature dependence of the electrical resistivity. Both salts exhibit semiconducting behavior in the measurement temperature region, and show anomaly at approximately 300 K. The phase transition temperature
is determined from the peak of
at
K and
K for the
and
phases, respectively. As shown in the inset of
Figure 2, the charge activation energy
is estimated as 27 and 46 meV for the
and
phases, respectively.
The Seebeck coefficient of the
phase shows metallic behavior around 300 K, and has anomaly at approximately 280 K (
Figure 3). For the
phase, the thermopower shows semiconducting behavior even in the high temperature region and has no anomaly at 300 K where the resistivity has an anomaly. The value of the thermopowers around 300 K is approximately 52
V/K. This large value is not in agreement with the energy band structure, indicating a strongly correlated electronic system [
1,
2,
16,
17].
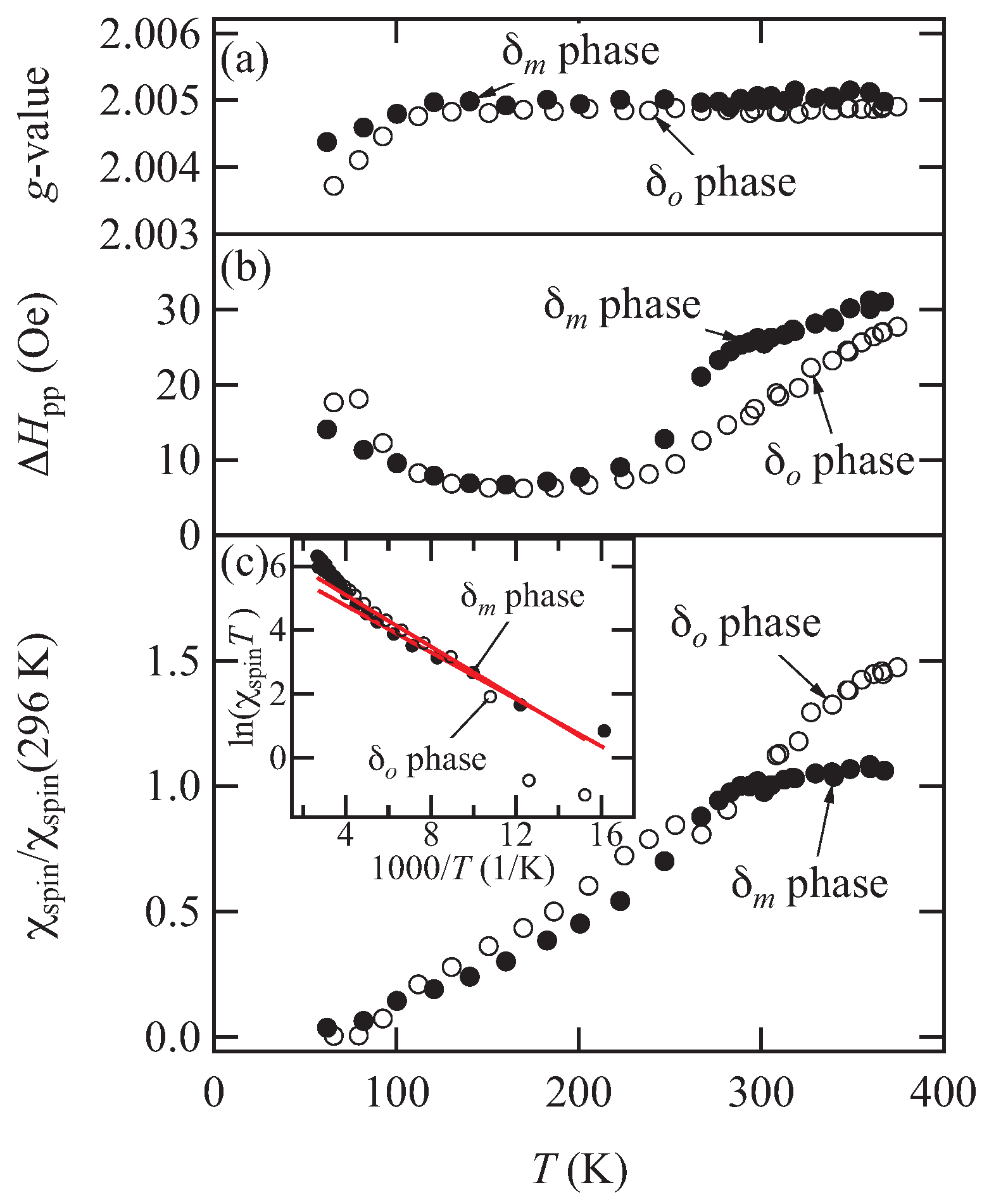
Figure 4 shows the temperature dependence of the electron spin resonance (ESR)
g-values, peak-to-peak linewidths, and the normalized spin susceptibilities measured under the magnetic field perpendicular to the conducting layers. A single Lorentzian lineshape is observed for both phases. The spin susceptibility of the
phase clearly decreases below 280 K without a divergent increase of the linewidth, leading to a spin singlet state. The spin susceptibility of the
phase gradually decreases with decreasing temperature, and the spin singlet state appears at low temperatures. However, the phase transition temperature is not clear. The
g-values are almost independent of the temperature around the phase transition temperature. The spin activation energy
is estimated from the simple singlet–triplet model given by
in the 100–200 K range. The spin activation energy
is about 30 meV.
is in rough agreement with
estimated from the electrical resistivity.
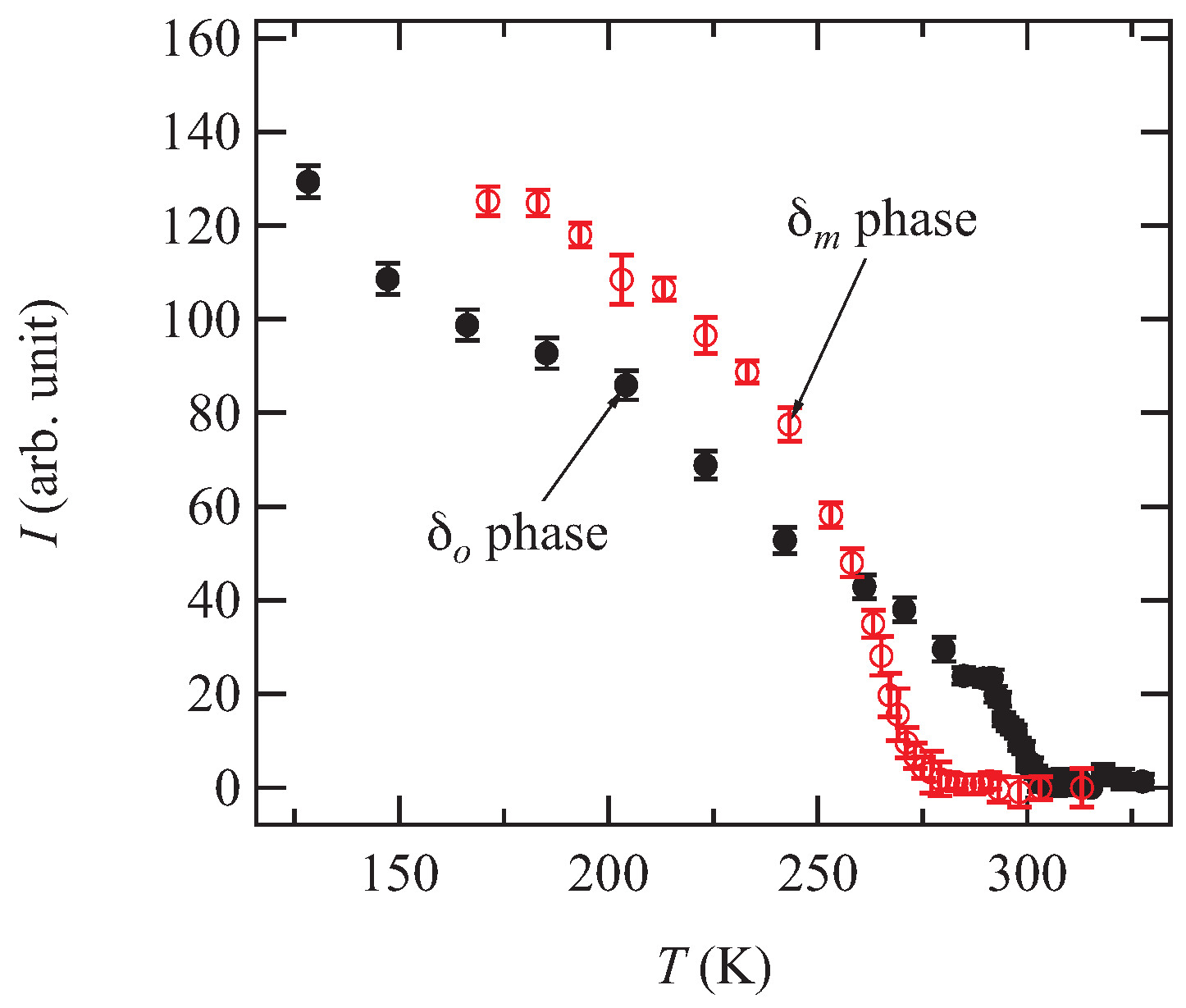
In order to clarify the origin of the phase transition, the low-temperature X-ray diffraction measurements were performed.
Figure 5 shows the X-ray oscillation photographs of both
and
phases. At 171 K, superlattice reflections with the wave vector
are observed for the
phase (
Figure 5a,b). Although the superlattice reflections are observed at 171 K for the
phase, the wave vector is
(
Figure 5c,d).
Figure 6 shows the temperature dependence of the integrated intensity of the superlattice reflections. The superlattice reflection disappears at 277 K and 302 K for the
and
phases, respectively. These results indicate that the temperatures at which the resistivity shows anomaly are the phase transition temperatures.
Figure 7a,b show the crystal structure of the
phase at 171 K. The
C-centered unit cell changes to a primitive cell with the space group
as shown in
Table 1, which is a sub-group of
; the superlattice unit cell is
,
, and
because superlattice reflections with
appear for
, where
n is an integer. Some reflections with
are observed in
reflections. However, in order to analyze the low-temperature average structure, we have chosen
, because a centrosymmetric space group has been suggested by SHELXT; the program SHELXT proposes possible space groups on the basis of phases in the space group
without using systematic absences [
18]. In the present space group, there are four crystallographic independent molecules along the stacking direction (
Figure 7a). The molecules in the nearest neighbor columns in the conducting sheet is connected by a two-fold screw axis; this indicates that the charge ordering pattern is not related by the glide symmetry. Moreover, in the case of
, the maximum shift/error for the parameter refinement does not converge, and the atomic coordinates of four additional independent molecules are connected by the inversion symmetry operation. Although the existence of the inversion center is important for electronic polarity, we do not treat this problem further in the present work.
Figure 7c,d show the crystal structure of the
phase at 39 K. Some reflections with
for
are observed. However, we have chosen
as suggested by SHELXT owing to the same reason as the
phase (
Table 1). This space group is a sub-group of
, where the superlattice is given by
,
, and
on the basis of the superlattice wave vector
. The
n-glide in the original cell corresponds to the
c-glide in the new unit cell. In the
phase, molecules in the nearest neighbor column in the conducting sheet are related by the inversion symmetry. If the space group has no inversion center (
), the nearest neighbor column is composed of four other independent molecules. However, in the case of
, the analysis does not converge similarly to
in the
phase.
A terminal ethylene group at one side is disordered except for the D molecule in the
phase. The A and C molecules are disordered in the
phase. The completely ordered structure has been observed in the PF
and
-AsF
salts. However, this conformational ordering depends on the cooling speed [
8]. The cooling speed approximately 1–3 K/min is too fast to attain a completely ordered structure. Although A and C molecules are flat, B and D molecules are bent for both
and
phases (
Figure 7a,c); the bent TTF skeleton is characteristic of the neutral BEDT-TTF molecule [
19]. The BEDT-TTF molecules have a boat-like structure. The dihedral angles between the central and outer tetrathio-substituted ethylene moieties are approximately
–
in the A and C molecules. These are approximately
–
in the B and D molecules in the
phase, and
–
in the
phase. This clearly shows that the low-temperature phase is a charge ordered state. The charges of the BEDT-TTF molecules are estimated empirically from the bond lengths [
20]:
,
,
, and
in the
phase. In the
phase, the charges are
,
,
, and
. The estimated
Q values are in agreement with the molecular shape; the flat A and C molecules are hole rich, whereas the bent B and C molecules are nearly neutral. The calculated energy levels of the highest occupied molecular orbital (HOMO) of these molecules show that the averaged HOMO level of the bent molecules is lower than that of the flat molecules by ∼0.1 eV. This is in agreement with the charge disproportionation between the flat and bent molecules. The hole-rich donor molecules are connected by the transfer integral
with the largest absolute value in the high temperature structure (
Figure 7b,d); then, a spin-singlet state is realized. Although A and C molecules are connected in the
phase (
Å), A and A or C and C molecules are connected in the
phase (
Å). The absolute value of the transfer integral between A and C molecules
increases (
meV), but
decreases (
meV) despite
in the
phase. This is due to the molecular shape; B and D molecules are bent. The side-by-side molecular center distance is slightly modulated; the averaged difference is approximately
Å. These tendencies are the same as those in the
phase. The anion arrangement is also changed; the isosceles triangle pattern is deformed. There are many hydrogen bonds between the donors and anions in the low-temperature structure. The short hydrogen bonds (≤2.50 Å) are observed in the B and D molecules for both phases.
3. Discussion
The difference between the
and
phases is the inclination of the donor plane in a conducting layer. In the
phase, the plane inclination changes alternately along the normal direction to the conducting layer. The same difference is found in the AsF
salts, which indicates that the monoclinic phase of the AsF
salt differs from the orthorhombic phase discovered by Senadeera et al. [
4,
5,
6,
7]. Nogami et al. have confirmed the lattice constants of the orthorhombic phase of the AsF
salt and solved the structure at 110 K including the superlattice reflections; the superlattice unit cells of both
-PF
and
-AsF
salts are also orthorhombic [
8]. Leung et al. have found two-fold superlattice reflections in the monoclinic AsF
salt above 125 K, where the wave vector
is the same as that of the
-TaF
salt. The Raman spectra of the monoclinic AsF
salt show the charge ordering at approximately 260 K [
13]. Therefore, we have to distinguish between the monoclinic and orthorhombic phases in the AsF
salts; the charge ordering transition temperatures are
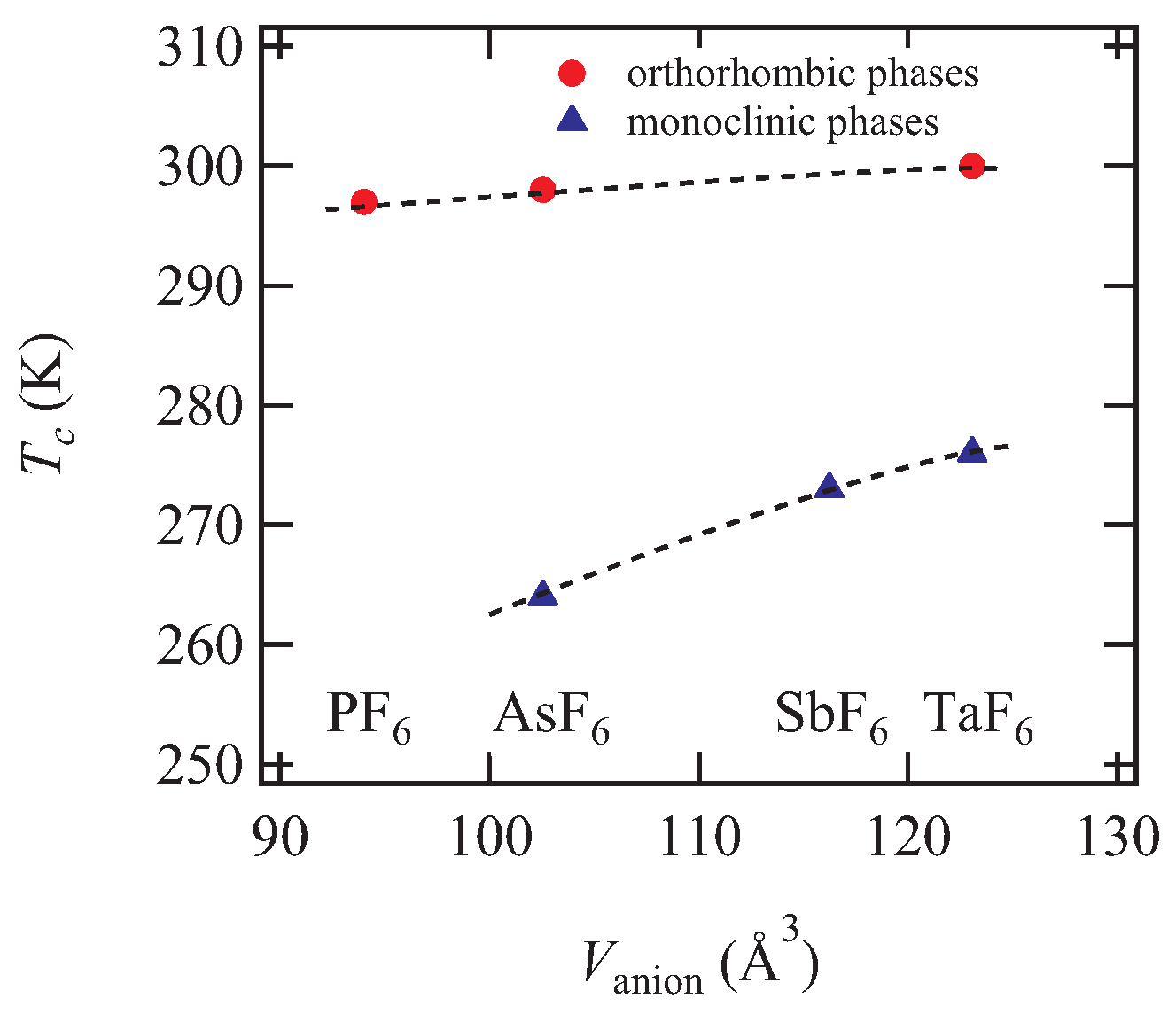
K and 298 K for the monoclinic and orthorhombic phases, respectively. The transition temperature
of the orthorhombic phases is generally higher than that of the monoclinic phases in the other
-type (BEDT-TTF)
F
salts as shown in
Table 3. If we distinguish the crystal system, the transition temperature increases independently with increasing the anion size (
Figure 8). This is in agreement with the concept of chemical pressure; the smaller the anion volume, the higher the pressure region. Actually, the transition temperature decreases with increasing the external pressure in the PF
salt [
6].
The relationship between the charge order and anion has been investigated in
-(BEDT-TTF)
I
[
21]. The hydrogen bonds of the hole-rich donors are shorter than those of the hole-poor donors in
-(BEDT-TTF)
I
; the hydrogen bonds between the donors and anions affect the charge ordering transition. In the present compounds, however, the donors with short hydrogen bonds are hole poor molecules, i.e., bent neutral molecules. This indicates that the anion shift does not affect the present phase transition. Moreover, the shift of the donors is also small; this molecular displacement is known as a bond order wave [
22,
23]. The slight displacement is not dominant in the phase transition that leads to the charge ordered state. The above results show that the origin of the phase transition is due to the electronic correlation between the donor molecules.
The experimentally obtained charge ordered pattern is estimated from the high temperature crystal structure. In the
-type BEDT-TTF compounds, theoretical investigation has revealed several charge ordering patterns depending on
, which have been verified by different kinds of experiments [
10,
11,
12]. However, a simple method to expect the charge ordering pattern has been proposed, since the point charge approximation is appropriate;
V is proportional to
for
Å, where
R is the distance between the molecular centers [
24].
Table 4 shows the transfer integrals and intermolecular distances in the high temperature structure. This shows a trend for the molecular center distances (
), and
∼
. Therefore, we use
as the stacking direction
V and others (
,
, and
) to calculate the potential energy for several charge ordering patterns by the point charge approximation (
Figure 9).
The extended Hubbard model is written as follows [
10,
12]:
where
denotes pairs of the lattice sites
i and
j,
is the creation (annihilation) operator for a hole with spin
at the
i site, and
is the number operator with
.
U is the onsite Coulomb repulsion, and
and
are the transfer integral and the nearest neighbor Coulomb repulsion between sites
i and
j. We neglect
and assume the static limit in order to investigate complicated charge-order patterns [
24,
25,
26]. The static-limit potential energies are listed in
Table 5. The ratios
0.54,
0.65, and
0.76 give the energies on the right side (
energy) in
Table 5. Since
is much larger than other
Vs, the vertical pattern is unlikely. However, because of
, the horizontal pattern is more stable than the checkerboard in disagreement with the experiments. The checkerboard pattern is most stable when
and
make a square lattice [
27]. Although
is obviously large, the relative importance of
and
is less clear. These stripe patterns have very close static energies, and the point charge approximation is insufficient to distinguish the stability of different stripe patterns. This is also in the case of
-(
meso-DMBEDT-TTF)
PF
with the checkerboard charge ordering, where DMBEDT-TTF stands for 2-(5,6-dihydro-1,3-dithiolo[4,5-b][1,4]dithiin-2-ylidene)- 5,6-dihydro-5,6-dimethyl-1,3-dithiolo[4,5-b][1,4]dithiin [
26,
28].
We propose another explanation of the present results. The donor molecules have uniform side-by-side arrangement along the
direction in the
-TaF
salt. This direction is the highest conducting direction, and the Fermi surface is perpendicular to the
direction (
Figure 1c,e). The value of
is smaller than
, and much smaller than the dimerization in the
-type BEDT-TTF salts, where the dimer Mott insulating picture is realized (
meV). The molecular center distance
Å is almost the same as that in the dimer of
-type salts (
Å). Then,
of the
mode is half of the
-type salt and comparatively small. However,
for
q is 90% of that of the
-type, and the dimerization due to
q is important. The energy band in
Figure 1 splits into two by taking the oblique dimerization due to
q into account. The band splitting has been observed as the inter-band transition in the polarized reflectance spectra of the
-PF
salt [
29]. The present compounds show semiconducting behaviors even at 350 K sufficiently higher than the phase transition temperature. This indicates that the high temperature phase is a paramagnetic dimer Mott insulator. In the present compounds, a dimer is surrounded by eight neighboring dimers, i.e., a quasi-square lattice, where all dimers have one hole; the dimers are connected by the horizontal interaction composed of two
c modes and one
mode, the vertical
mode, and the diagonal
p mode. The interdimer Coulomb repulsion leads to the checkerboard type charge order (
) because of the relationship
, and the spin singlet state in the dimer is realized. The spin singlet charge ordering has been observed in the dimer Mott insulator
-(BEDT-TTF)
[Co(CN)
][N(C
H
)
]·2H
O; the charged dimer composed of hole-rich flat donors and the neutral dimer of bent donors exist in the checkerboard charge ordered state [
30].
The wave vector of the superlattice reflection of the
-TaF
salt differs from those of other
MF
salts, but this is only due to the alignment along the interlayer
b-axis. The charge ordering transition temperature increases systematically as the transfer integral
decreases, following a single line both for the monoclinic and orthorhombic phases (
Figure 10).
is almost the same, and the ratio
increases as
increases. This suggests the importance of the dimerization
q [
3].