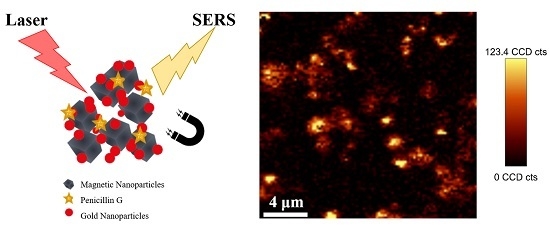
SERS Detection of Penicillin G Using Magnetite Decorated with Gold Nanoparticles
Abstract
:1. Introduction
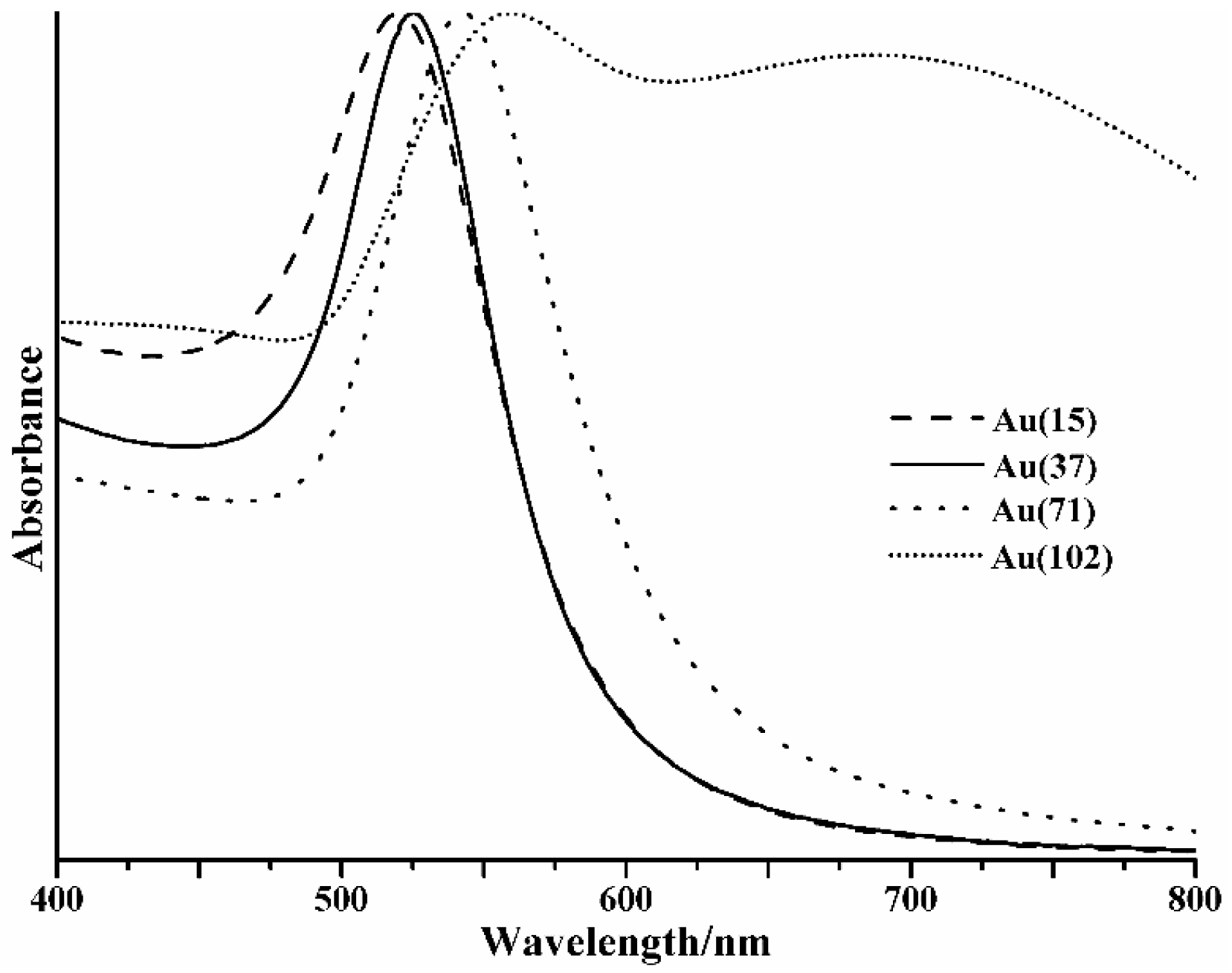
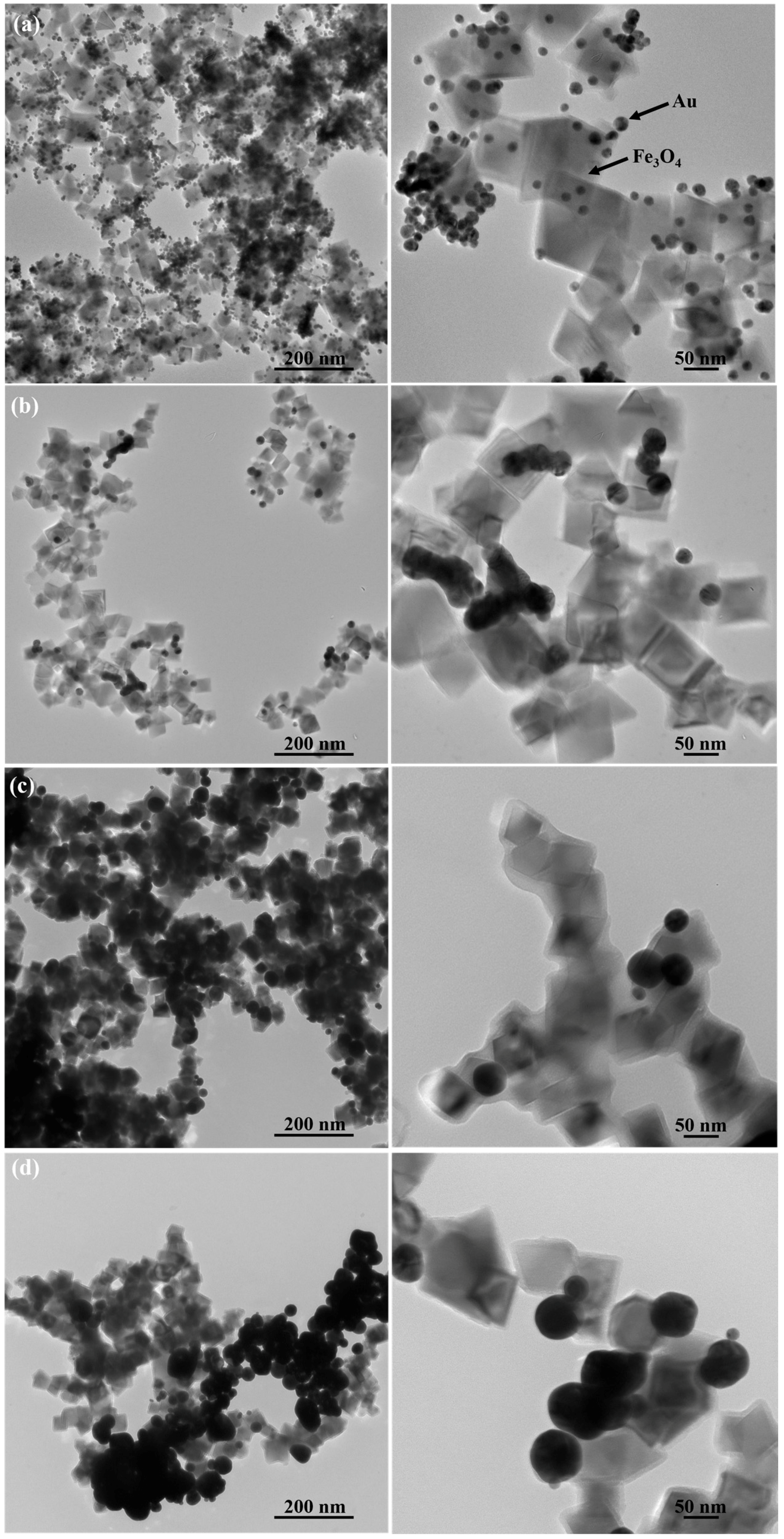
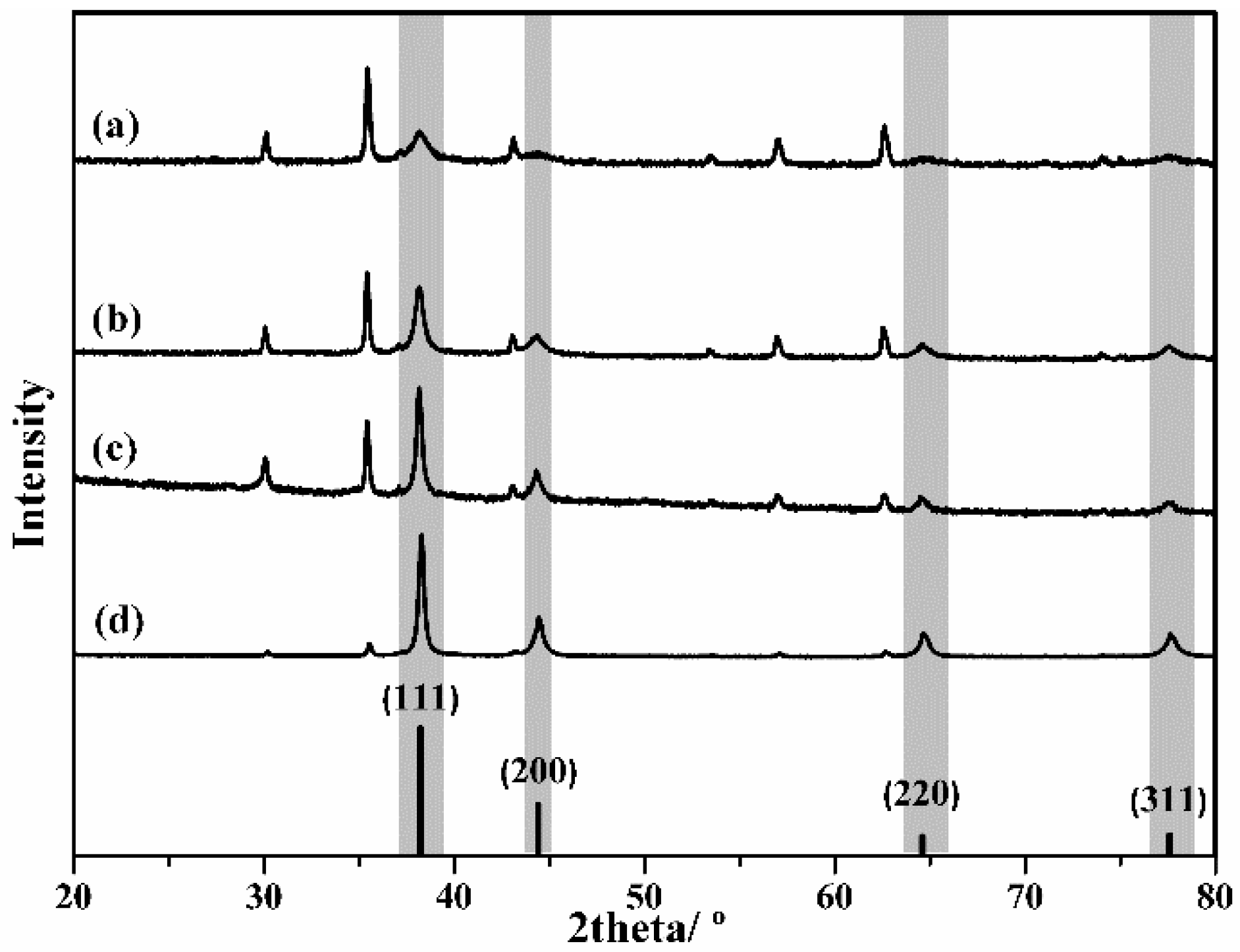
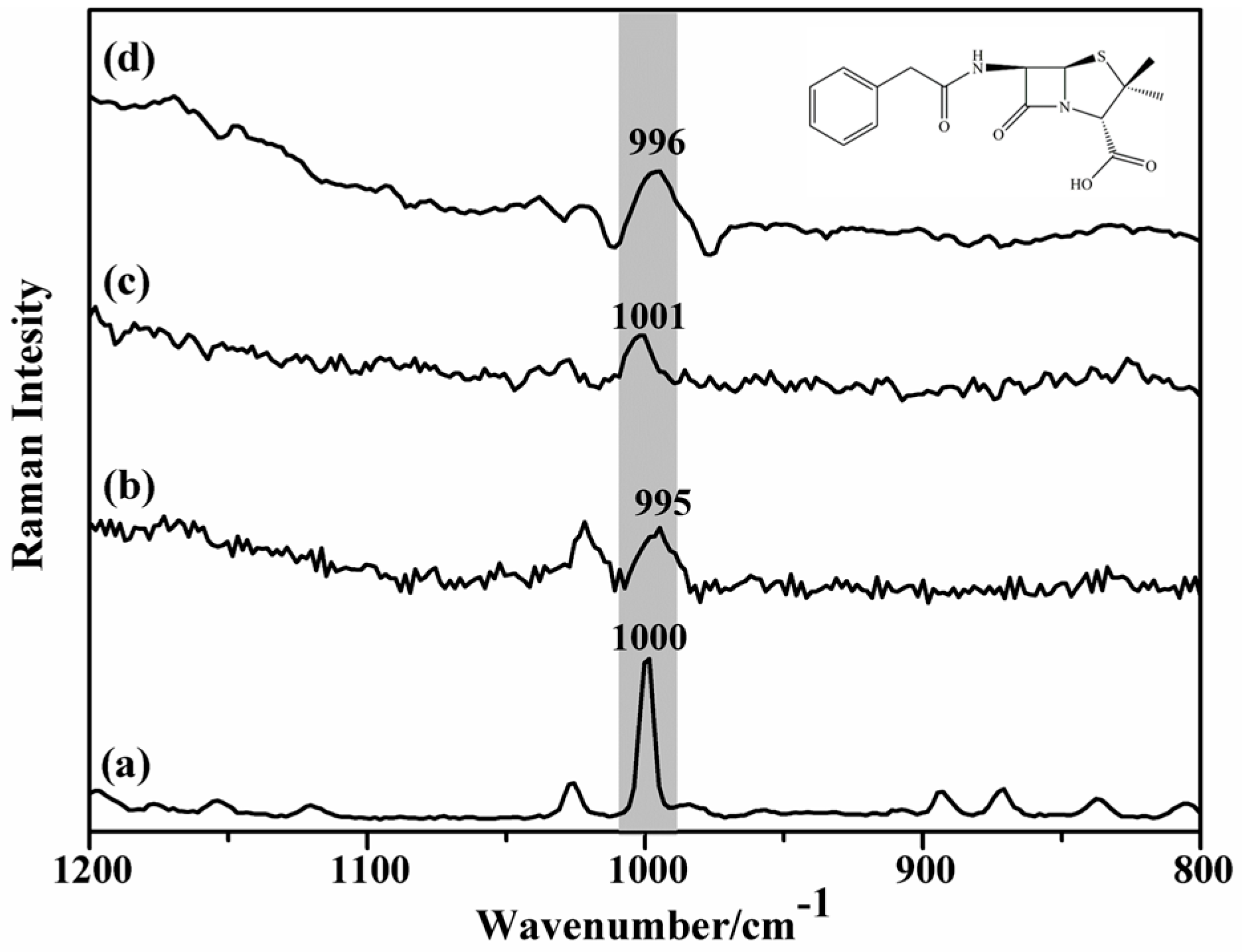
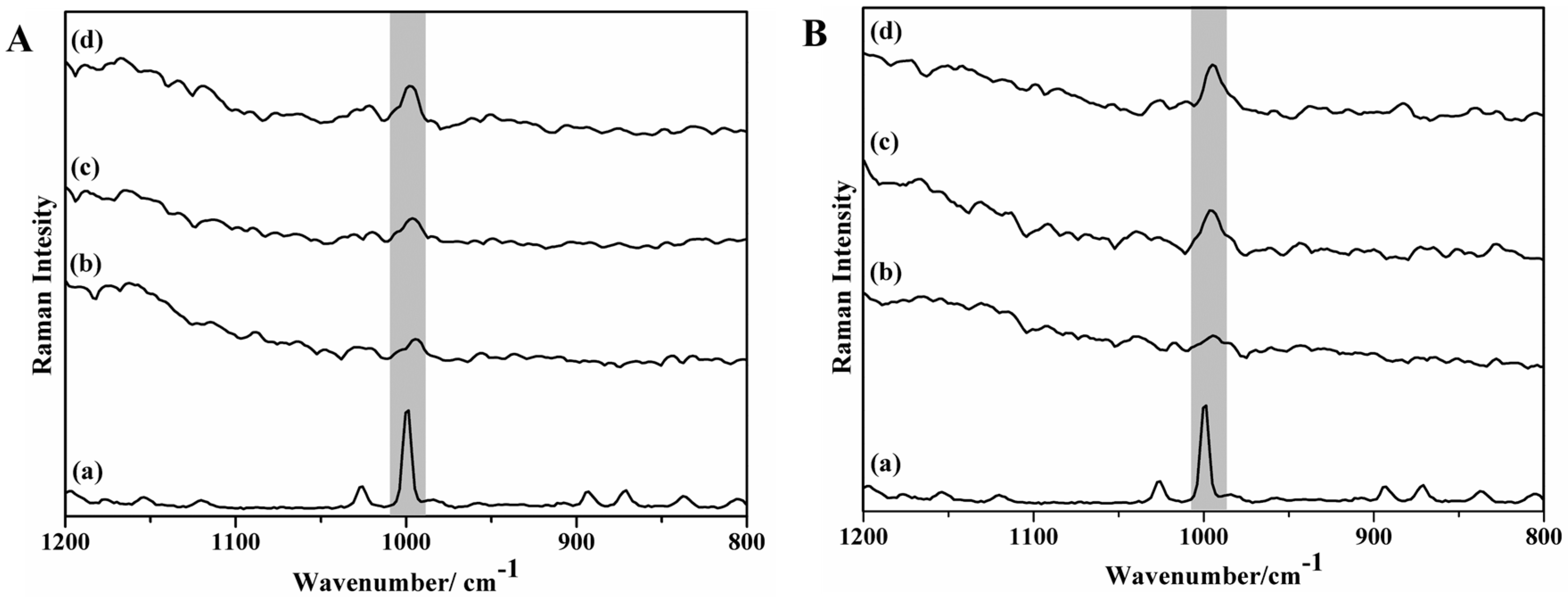
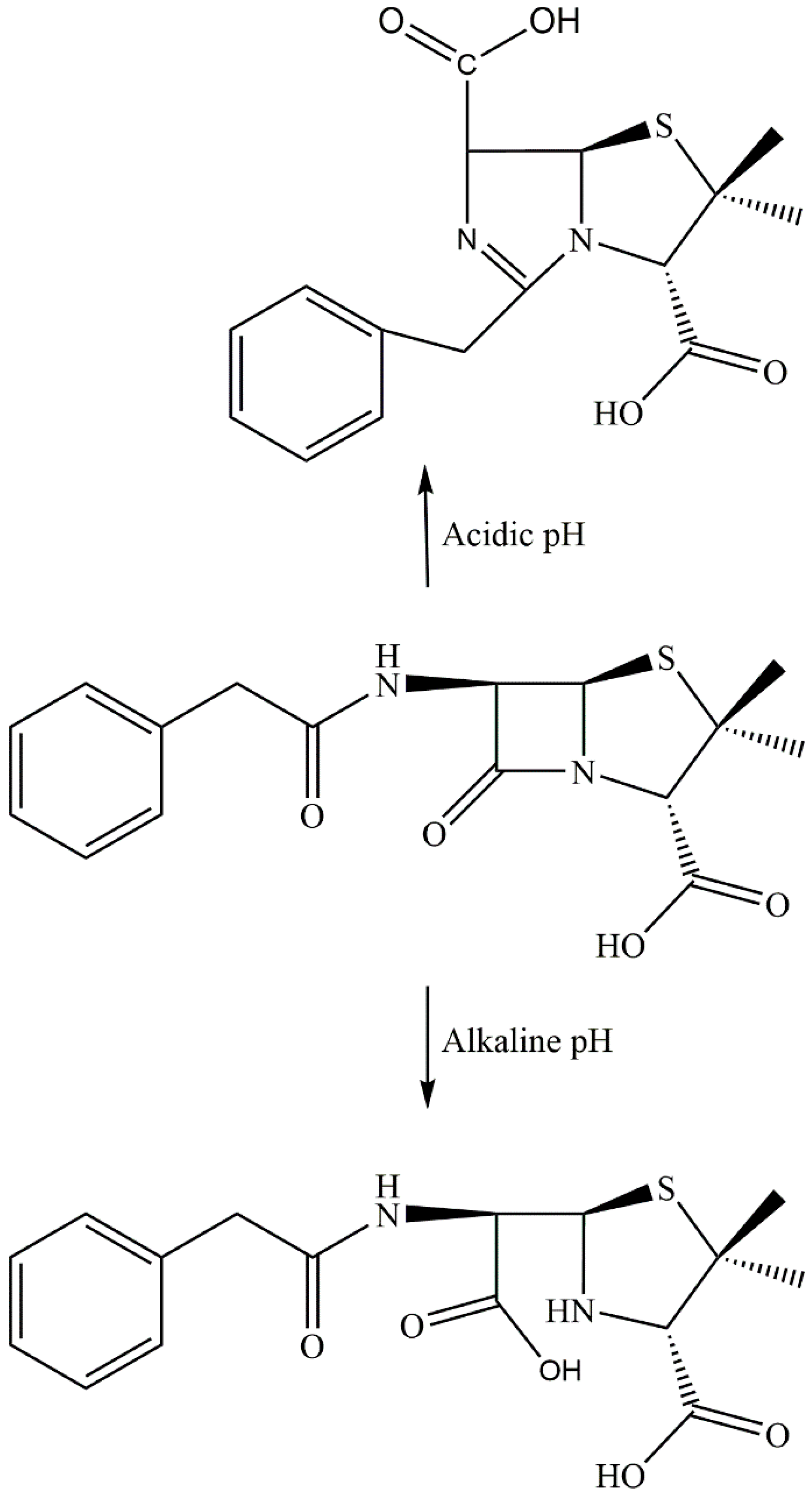
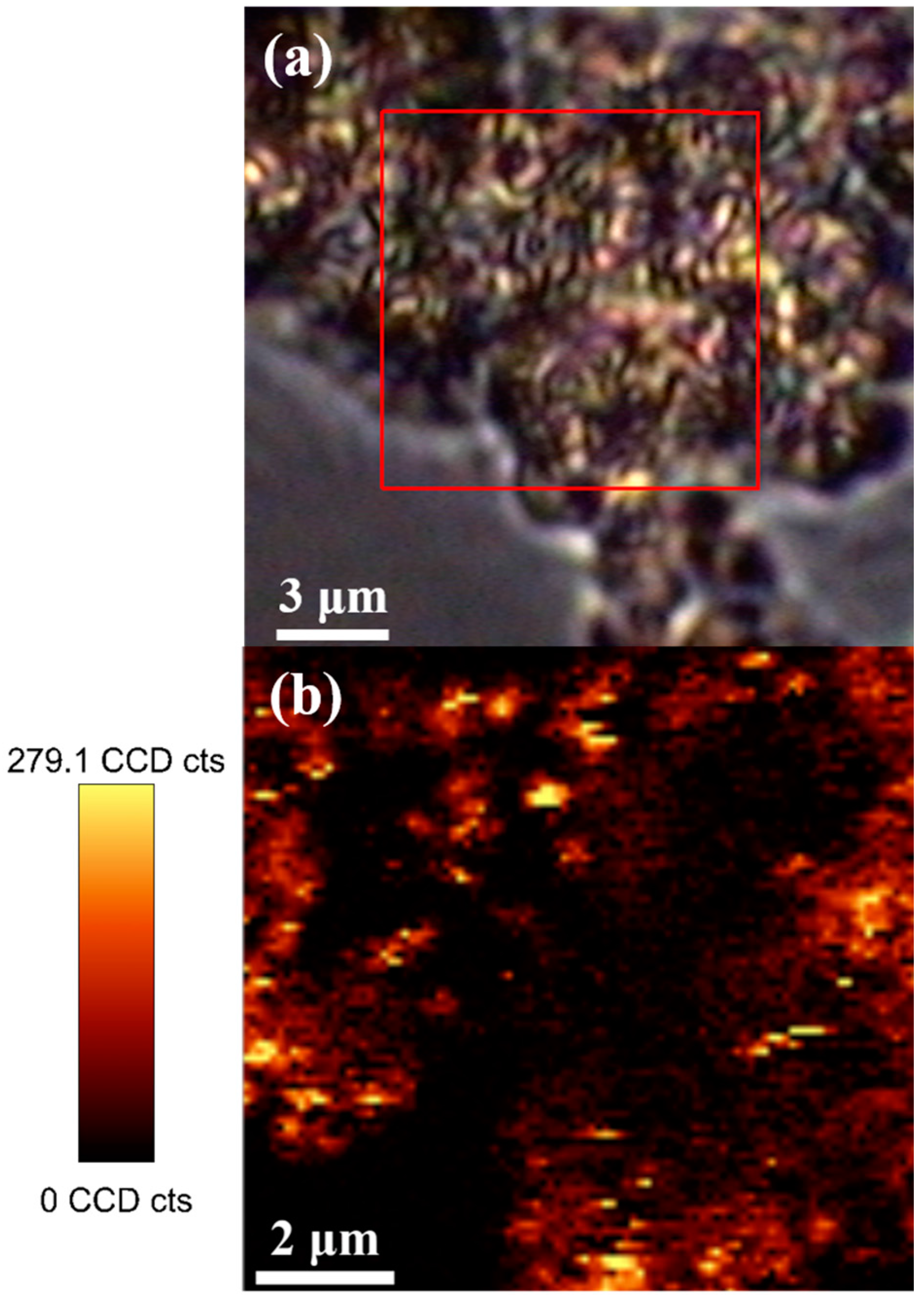
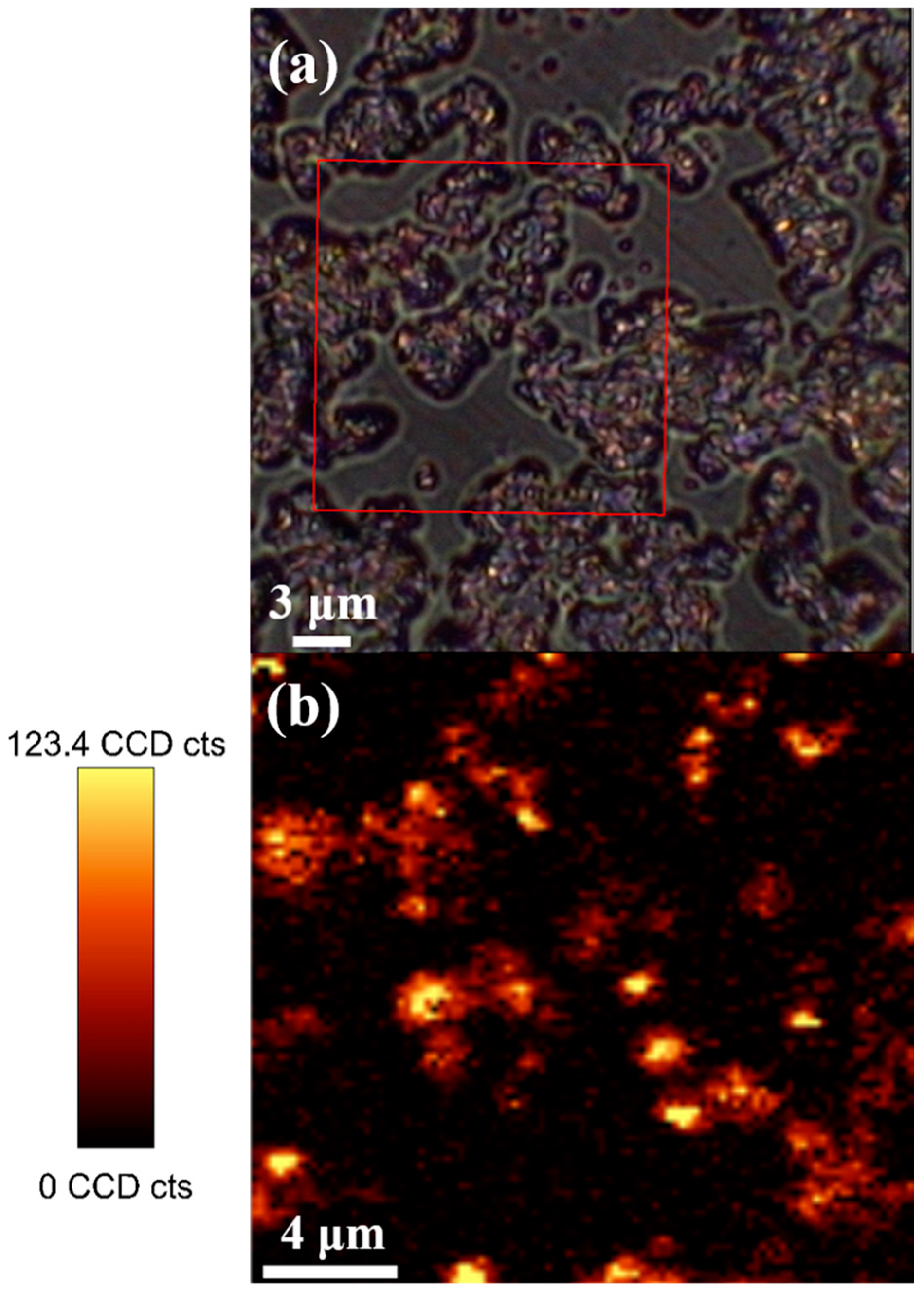
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of MNP-Au Nanocomposites
3.2.1. Surface Functionalization of Magnetite Particles
3.2.2. Seeded-Growth Synthesis of Gold Nanoparticles
3.2.3. Adsorption of Gold Nanoparticles at the Surface of Magnetite Nanoparticles
3.3. SERS Experiments
Uptake of PG from Water and SERS Detection
3.4. Instrumentation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part II. Chemosphere 2009, 75, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Aroca, R. Surface-Enhanced Vibrational Spectroscopy; John Wiley & Sons, Ltd.: Chichester, UK, 2006; ISBN 9780470035641. [Google Scholar]
- Fateixa, S.; Nogueira, H.I.S.; Trindade, T. Hybrid nanostructures for SERS: Materials development and chemical detection. Phys. Chem. Chem. Phys. 2015, 17, 21046–21071. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Brimer, A.; Slocik, J.M.; Tian, L.; Naik, R.R.; Singamaneni, S. Multifunctional analytical platform on a paper strip: Separation, preconcentration, and subattomolar detection. Anal. Chem. 2013, 85, 3977–3983. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhao, J.; Wu, C.J.; Zhang, S.W.; Deng, Z.W.; Hu, X.X.; Chen, M.L.; Peng, B. Fabrication of silver-decorated sulfonated polystyrene microspheres for surface-enhanced Raman scattering and antibacterial applications. RSC Adv. 2015, 5, 69543–69554. [Google Scholar] [CrossRef]
- Li, Y.T.; Qu, L.L.; Li, D.W.; Song, Q.X.; Fathi, F.; Long, Y.T. Rapid and sensitive in-situ detection of polar antibiotics in water using a disposable Ag-graphene sensor based on electrophoretic preconcentration and surface-enhanced Raman spectroscopy. Biosens. Bioelectron. 2013, 43, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.J.; Littleford, R.E.; Smith, W.E.; Goodacre, R. Rapid monitoring of antibiotics using Raman and surface enhanced Raman spectroscopy. Analyst 2005, 130, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Calborean, A.; Maniu, D.; Chis, V.; Iliescu, T.; Rastogi, V.K. Raman and SERS investigations of trihydrate amoxicillin. J. Optoelectron. Adv. Mater. 2007, 9, 680–685. [Google Scholar]
- Iliescu, T.; Baia, M.; Pavel, I. Raman and SERS investigations of potassium benzylpenicillin. J. Raman Spectrosc. 2006, 37, 318–325. [Google Scholar] [CrossRef]
- Filgueiras, A.L.; Paschoal, D.; Santos, F.D.; Sant, A.C. Adsorption study of antibiotics on silver nanoparticle surfaces by surface-enhanced Raman scattering spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhu, X.; Sun, Y.; Wang, H.; Qian, H.; Yao, W. Rapid detection method for nitrofuran antibiotic residues by surface-enhanced Raman spectroscopy. Eur. Food Res. Technol. 2012, 235, 555–561. [Google Scholar] [CrossRef]
- Alivisatos, A.P. Perspectives on the physical chemistry of semiconductor nanocrystals. J. Phys. Chem. 1996, 100, 13226–13239. [Google Scholar] [CrossRef]
- Esteves, A.C.C.; Trindade, T. Synthetic studies on II/VI semiconductor quantum dots. Curr. Opin. Solid State Mater. Sci. 2002, 6, 347–353. [Google Scholar] [CrossRef]
- Khajeh, M.; Laurent, S.; Dastafkan, K. Nanoadsorbents: Classification, preparation, and applications (with emphasis on aqueous media). Chem. Rev. 2013, 113, 7728–7768. [Google Scholar] [CrossRef] [PubMed]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Riley, M.; Vermerris, W. Recent advances in nanomaterials for gene delivery—A review. Nanomaterials 2017, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Dave, P.N.; Chopda, L.V. Application of iron oxide nanomaterials for the removal of heavy metals. J. Nanotechnol. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tavares, D.S.; Daniel-da-Silva, A.L.; Lopes, C.B.; Silva, N.J.O.; Amaral, V.S.; Rocha, J.; Pereira, E.; Trindade, T. Efficient sorbents based on magnetite coated with siliceous hybrid shells for removal of mercury ions. J. Mater. Chem. A 2013, 1, 8134–8143. [Google Scholar] [CrossRef]
- Pinheiro, P.C.; Sousa, C.T.; Araújo, J.P.; Guiomar, A.J.; Trindade, T. Functionalization of nickel nanowires with a fluorophore aiming at new probes for multimodal bioanalysis. J. Colloid Interface Sci. 2013, 410, 21–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras-Cáceres, R.; Abalde-Cela, S.; Guardia-Girós, P.; Fernández-Barbero, A.; Pérez-Juste, J.; Alvarez-Puebla, R.A.; Liz-Marzán, L.M. Multifunctional microgel magnetic/optical traps for SERS ultradetection. Langmuir 2011, 27, 4520–4525. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhai, J.; Wang, Y.; Guo, S.; Ren, W.; Dong, S. Fabrication of iron oxide core/gold shell submicrometer spheres with nanoscale surface roughness for efficient surface-enhanced Raman scattering. J. Phys. Chem. C 2009, 113, 7009–7014. [Google Scholar] [CrossRef]
- Yu, W.; Huang, Y.; Pei, L.; Fan, Y.; Wang, X.; Lai, K. Magnetic Fe3O4/Ag hybrid nanoparticles as surface-enhanced raman scattering substrate for trace analysis of furazolidone in fish feeds. J. Nanomater. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, Y. Stable magnetic hot spots for simultaneous concentration and ultrasensitive surface-enhanced Raman scattering detection of solution analytes. J. Phys. Chem. C 2012, 116, 13329–13335. [Google Scholar] [CrossRef]
- Baniukevic, J.; Hakki Boyaci, I.; Goktug Bozkurt, A.; Tamer, U.; Ramanavicius, A.; Ramanaviciene, A. Magnetic gold nanoparticles in SERS-based sandwich immunoassay for antigen detection by well oriented antibodies. Biosens. Bioelectron. 2013, 43, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Harpster, M.H.; Park, H.J.; Johnson, P.A.; Wilson, W.C. Surface-enhanced raman scattering detection of DNA derived from the west nile virus genome using magnetic capture of raman-active gold nanoparticles. Anal. Chem. 2011, 83, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Kadasala, N.R.; Wei, A. Trace detection of tetrabromobisphenol A by SERS with DMAP-modified magnetic gold nanoclusters. Nanoscale 2015, 7, 10931–10935. [Google Scholar] [CrossRef] [PubMed]
- Han, X.X.; Schmidt, A.M.; Marten, G.; Fischer, A.; Weidinger, I.M.; Hildebrandt, P. Magnetic silver hybrid nanoparticles for surface-enhanced resonance raman spectroscopic detection and decontamination of small toxic molecules. ACS Nano 2013, 7, 3212–3220. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-L.; Xu, M.-M.; Guo, Q.-H.; Yuan, Y.-X.; Shen, L.-M.; Gu, R.-A.; Yao, J.-L. Surface enhanced Raman spectroscopic studies on magnetic Fe3O4@AuAg alloy core—Shell nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 114, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, J.; Lee, H.B.; Shin, K.S. Silanization of Ag-deposited magnetite particles: An efficient route to fabricate magnetic nanoparticle-based raman barcode materials. ACS Appl. Mater. Interfaces 2010, 2, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Jing, C. Preparation of thiol modified Fe3O4@Ag magnetic SERS probe for PAHs detection and identification. J. Phys. Chem. C 2011, 115, 17829–17835. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Wang, C.; Rong, Z.; Ding, H.; Li, H.; Li, S.; Shao, N.; Dong, P.; Xiao, R.; et al. Facile synthesis of Au-coated magnetic nanoparticles and their application in bacteria detection via a SERS method. ACS Appl. Mater. Interfaces 2016, 8, 19958–19967. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.A.; Adams, S.A.; López-luke, T.; Torres-Castro, A.; Zhang, J.Z. Magnetic Fe3O4-Au core-shell nanostructures for surface enhanced Raman scattering. Ann. Phys. 2012, 679, 670–679. [Google Scholar] [CrossRef]
- Quaresma, P.; Osório, I.; Carvalho, P.A.; Pereira, A.; Langer, J.; Araújo, J.P.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Franco, R.; Baptista, P.V.; et al. Star-shaped magnetite@gold nanoparticles for protein magnetic separation and SERS detection. RSC Adv. 2014, 4, 3659–3667. [Google Scholar] [CrossRef]
- Hu, F.; Lin, H.; Zhang, Z.; Liao, F.; Shao, M.; Lifshitz, Y.; Lee, S.-T. Smart liquid SERS substrates based on Fe3O4/Au nanoparticles with reversibly tunable enhancement factor for practical quantitative detection. Sci. Rep. 2015, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Qin, X.; Yin, D.; Gong, M.; Yang, L.; Zhao, B.; Ruan, W. Rapid monitoring of benzylpenicillin sodium using Raman and surface enhanced Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 140, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Reipa, V.; Horvath, J.J. Surface-Enhanced Raman Study of Benzylpenicillin. Appl. Spectrosc. 1992, 46, 1009–1013. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, M.; Zhao, J.; Yuan, H.; Li, Y.; Tao, J.; Guo, H. Determination of benzylpenicillin potassium residues in duck meat using surface enhanced raman spectroscopy with Au nanoparticles. J. Spectrosc. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Yang, M.; Yang, L.; Han, X.; Jiang, X.; Zhao, B. High sensitive detection of penicillin G residues in milk by surface-enhanced Raman scattering. Talanta 2017, 167, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Thokchom, A.K.; Kim, D.J.; Kim, T. Inkjet-printed Ag micro-/nanostructure clusters on Cu substrates for in-situ pre-concentration and surface-enhanced Raman scattering. Sensors Actuators B Chem. 2017, 243, 176–183. [Google Scholar] [CrossRef]
- Zhao, K.; Wu, C.; Deng, Z.; Guo, Y.; Peng, B. Preparation of silver decorated silica nanocomposite rods for catalytic and surface-enhanced Raman scattering applications. RSC Adv. 2015, 5, 52726–52736. [Google Scholar] [CrossRef]
- El-Zahry, M.R.; Refaat, I.H.; Mohamed, H.A.; Rosenberg, E.; Lendl, B. Utility of surface enhanced Raman spectroscopy (SERS) for elucidation and simultaneous determination of some penicillins and penicilloic acid using hydroxylamine silver nanoparticles. Talanta 2015, 144, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Girginova, P.I.; Daniel-da-Silva, A.L.; Lopes, C.B.; Figueira, P.; Otero, M.; Amaral, V.S.; Pereira, E.; Trindade, T. Silica coated magnetite particles for magnetic removal of Hg2+ from water. J. Colloid Interface Sci. 2010, 345, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Li, X.; Li, H.; Zhang, W. Quantifying thiol-gold interactions towards the efficient strength control. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, C.; Eychmüller, A. Seeded growth synthesis of uniform gold nanoparticles with diameters of 15–300 nm. J. Phys. Chem. C 2011, 115, 4502–4506. [Google Scholar] [CrossRef]
- Niu, J.; Zhu, T.; Liu, Z. One-step seed-mediated growth of 30–150 nm quasispherical gold nanoparticles with 2-mercaptosuccinic acid as a new reducing agent. Nanotechnology 2007, 18, 325607–325614. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, J.; Pérez-Juste, J.; García De Abajo, F.J.; Liz-Marzán, L.M. Seeded growth of submicron Au colloids with quadrupole plasmon resonance modes. Langmuir 2006, 22, 7007–7010. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, O.C.; Esteves, A.C.C.; Trindade, T. The synthesis of SiO2@CdS nanocomposites using single-molecule precursors. Chem. Mater. 2002, 14, 2900–2904. [Google Scholar] [CrossRef] [Green Version]
- Polavarapu, L.; Pérez-Juste, J.; Xu, Q.-H.; Liz-Marzán, L.M. Optical sensing of biological, chemical and ionic species through aggregation of plasmonic nanoparticles. J. Mater. Chem. C 2014, 2, 7460–7476. [Google Scholar] [CrossRef]
- Gómez-Graña, S.; Fernández-López, C.; Polavarapu, L.; Salmon, J.B.; Leng, J.; Pastoriza-Santos, I.; Pérez-Juste, J. Gold nanooctahedra with tunable size and microfluidic-induced 3D assembly for highly uniform SERS-active supercrystals. Chem. Mater. 2015, 27, 8310–8317. [Google Scholar] [CrossRef]
- Fernández-López, C.; Polavarapu, L.; Solís, D.M.; Taboada, J.M.; Obelleiro, F.; Contreras-Cáceres, R.; Pastoriza-Santos, I.; Pérez-Juste, J. Gold nanorod-pNIPAM hybrids with reversible plasmon coupling: Synthesis, modeling, and SERS properties. ACS Appl. Mater. Interfaces 2015, 7, 12530–12538. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, H.; Kneipp, K. Surface-enhanced hyper Raman scattering in silver colloidal solutions. J. Raman Spectrosc. 2005, 36, 551–554. [Google Scholar] [CrossRef]
- Radziuk, D.; Moehwald, H. Prospects for plasmonic hot spots in single molecule SERS towards the chemical imaging of live cells. Phys. Chem. Chem. Phys. 2015, 17, 21072–21093. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.D.; Baheti, K.G.; Chatterjee, N.R. Degradation of β-lactam antibiotics. Curr. Sci. 2004, 87, 1684–1695. [Google Scholar]
- Lopes, J.L.; Marques, K.L.; Girão, A.V.; Pereira, E.; Trindade, T. Functionalized magnetite particles for adsorption of colloidal noble metal nanoparticles. J. Colloid Interface Sci. 2016, 475, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Goubert-Renaudin, S.; Schneider, R.; Walcarius, A. Synthesis of new dithiocarbamate-based organosilanes for grafting on silica. Tetrahedron Lett. 2007, 48, 2113–2116. [Google Scholar] [CrossRef]
- Fernandes, T.; Soares, S.; Trindade, T.; Daniel-da-Silva, A. Magnetic hybrid nanosorbents for the uptake of paraquat from water. Nanomaterials 2017, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Sreeju, N.; Rufus, A.; Philip, D. Studies on catalytic degradation of organic pollutants and anti-bacterial property using biosynthesized CuO nanostructures. J. Mol. Liq. 2017, 242, 690–700. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, P.C.; Fateixa, S.; Trindade, T. SERS Detection of Penicillin G Using Magnetite Decorated with Gold Nanoparticles. Magnetochemistry 2017, 3, 32. https://doi.org/10.3390/magnetochemistry3040032
Pinheiro PC, Fateixa S, Trindade T. SERS Detection of Penicillin G Using Magnetite Decorated with Gold Nanoparticles. Magnetochemistry. 2017; 3(4):32. https://doi.org/10.3390/magnetochemistry3040032
Chicago/Turabian StylePinheiro, Paula C., Sara Fateixa, and Tito Trindade. 2017. "SERS Detection of Penicillin G Using Magnetite Decorated with Gold Nanoparticles" Magnetochemistry 3, no. 4: 32. https://doi.org/10.3390/magnetochemistry3040032