2. Orientational and Quadrupolar Glasses: Basics
While spin glasses arise from the combination of frustration (of dipolar magnetic interactions) and disorder, the molecular orientational and quadrupolar glasses arise from the frustration of short-range interactions that are dominated by intermolecular electric quadrupole-quadrupole interactions resulting from the non-spherical electrostatic charge distribution of the molecules. The classic examples are ortho-para hydrogen mixtures [
21,
25,
26,
27] and solid N
-Ar alloys [
28,
29]. The order parameters are given by two sets of parameters: (i) the local axes (x,y,z) for the mean orientation of the molecular axes and (ii) the degree of alignment or quadrupolarization about those axes. The latter in the classical case is given by the expectation values of the spherical harmonics,
and
For solid hydrogen, the orbital angular momentum
J is a good quantum number. There are two molecular species: ortho-H
(with orbital angular momentum
J = 1 and total nuclear spin
I = 1) and para-H
(with
J = 0 and
I = 0). Only the ortho-H
molecules have an electrostatic quadrupole moment and are subject to orientational or quadrupole ordering. The para-H
molecules provide the disorder. Although the para-H
molecules are the true ground state, the ortho-para conversion is a very slow process in pure solid hydrogen, and one can conduct experiments for a wide range of ortho-para mixtures (for a review of the properties of sold hydrogen, see Silvera [
30]). For the ortho-H
molecules, the order parameters are are given by
and
, which are the expectation values of the tensor operator equivalents of the spherical harmonics to within a simple numerical factor. Above a critical concentration of 55% ortho-H
, the ortho molecules order in a four-sublattice anti-ferro-orientational Pa3 structure [
30]. Below 55%, the ortho molecules form a quadrupolar glass with no sudden phase transition [
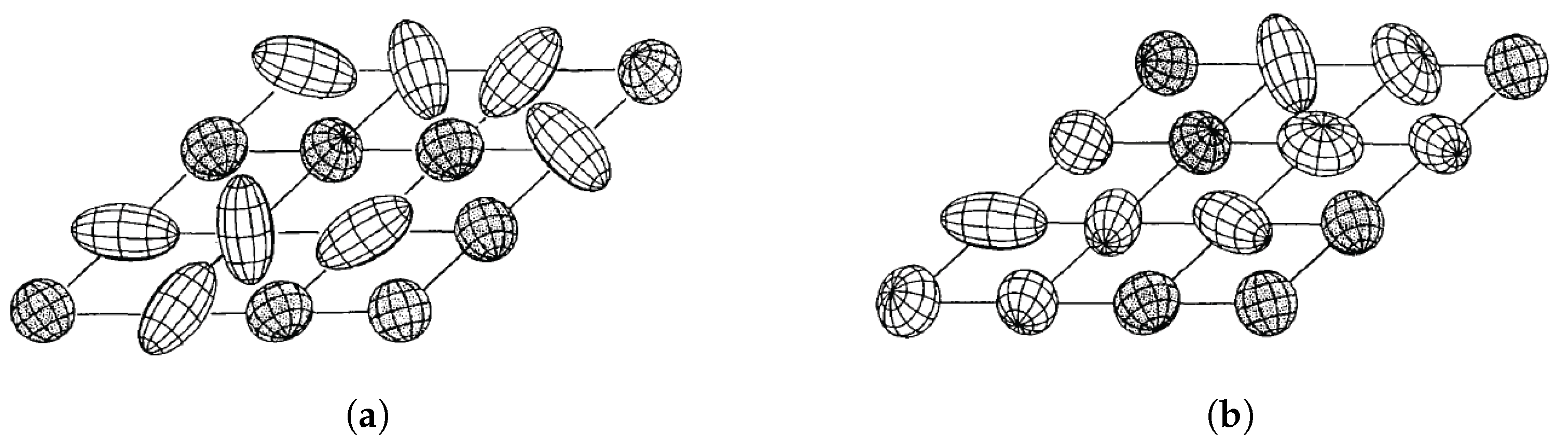
31]. The onset of glass formation was signaled by the observation of a broad distribution of local order parameters at low temperatures. For a simple orientational glass, only the axes (x,y,z) are randomly distributed, while for the quadrupolar glass, both the axes and the quadrupolarizations vary randomly. The distinction between the two types of molecular glasses is illustrated in
Figure 1 [
31].
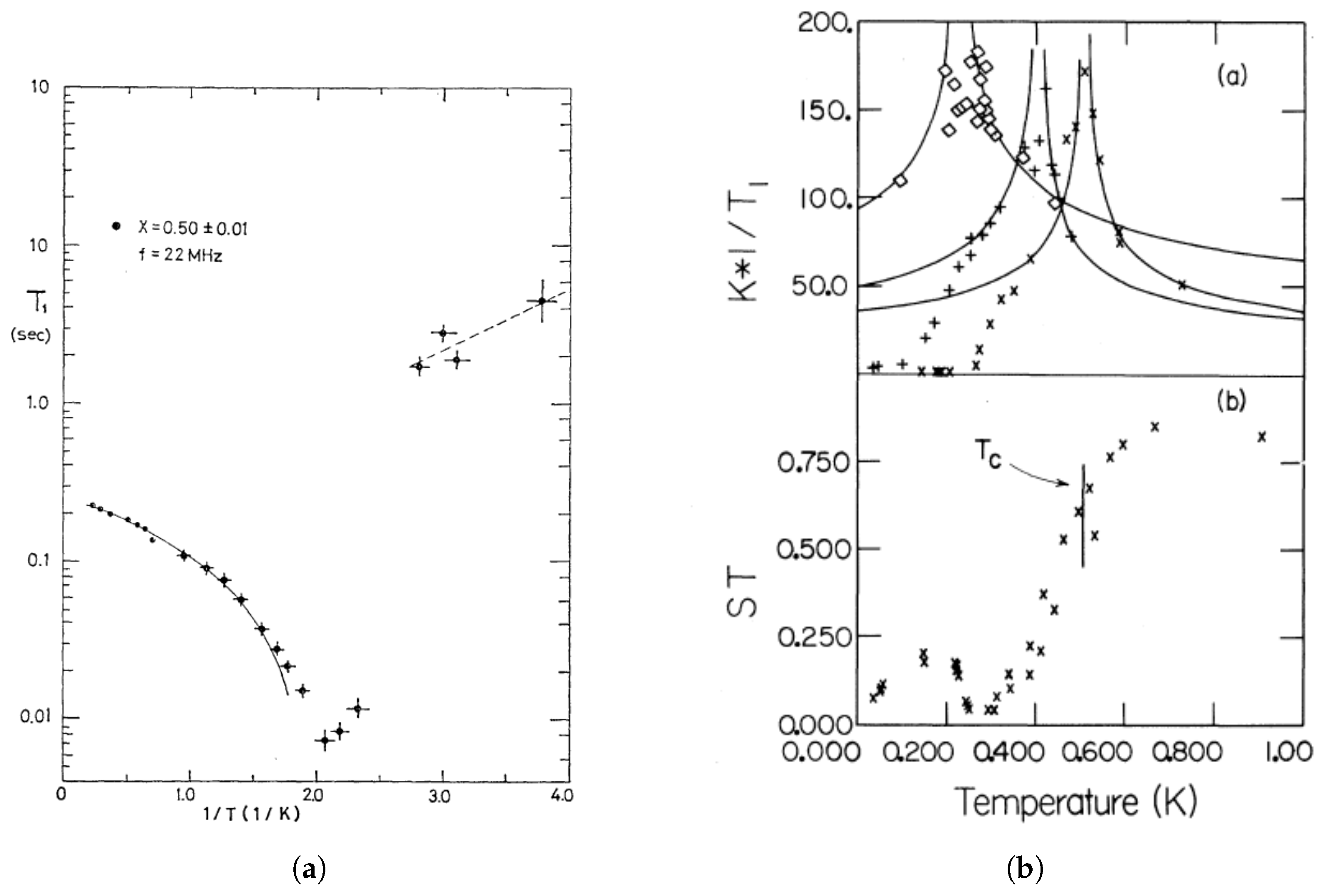
In addition to the static observations of the order parameters, experiments also showed strong temperature dependencies for the dynamics of the molecules (principally their thermal fluctuations about the mean molecular alignments) as inferred from various studies of the nuclear spin relaxation times. Two independent measurements of the relaxation times in solid ortho-para H
mixtures are shown in
Figure 2. The change in the static order parameter,
(as defined above and represented by the variable
in
Figure 2b), is considerably less dramatic than the changes observed in the dynamical parameters that determine the nuclear spin-lattice relaxation times. The main difference in the two measurements shown in
Figure 2 is the frequency of the measurements; 22 MHz for
Figure 2a (Ishimoto et al. [
25]) and 33 MHz for
Figure 2b (Cochran et al. [
26]). This difference was the first evidence of the characteristic frequency dependence of the response functions observed below the glass formation and will be discussed further in the next section.
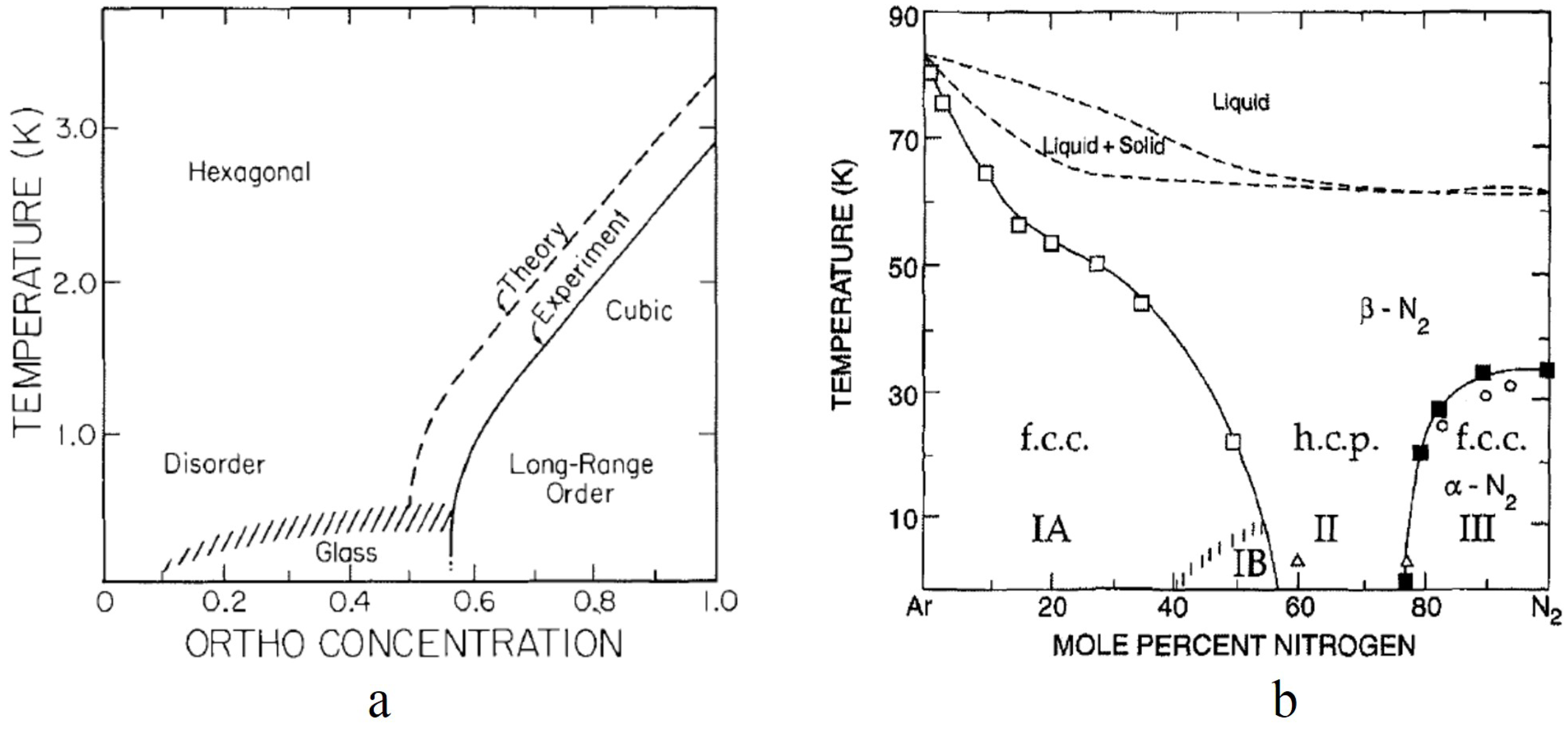
Studies of solid nitrogen-argon mixtures [
33,
34,
35] showed similar features to those referenced above for solid ortho-para H
mixtures. The randomness for the N
-Ar solid mixtures is introduced by the replacement of nitrogen molecules with argon atoms, which are spherically symmetrical and carry no electrostatic quadrupole moment. At high nitrogen concentrations (more than 77%), long-range Pa3 ordering is observed similar to pure ortho-H
. Below that critical concentration, glass behavior is observed at low temperatures. As indicated in
Figure 3a,b, a lattice change is observed when the long-rage ordering occurs with the hcp lattice transitioning to an fcc lattice as the orientational ordering occurs. The critical concentration depends on the concentration dependence of the anisotropic interactions. A detailed restricted trace calculation [
36] showed that the orientational fluctuations for ortho-para hydrogen mixtures varied as
where
x is the ortho concentration leading to a predicted critical concentration of 50%. No detailed calculation has been made for solid N
-Ar mixtures. For the latter, it is noteworthy that for N
concentrations less than 56%, a cubic lattice is maintained at low temperatures. This is important for any analysis of the glass formation because in the hcp structure, the symmetry of the electric quadrupole-quadrupole interaction is incompatible with the non-cubic lattice symmetry. As a consequence, replacing a quadrupole by a spherical diluent is equivalent to adding a local conjugate quadrupolar field so that for the hcp lattice, the glass formation is analogous to that of a spin glass in a static magnetic field, and any phase transition would be rounded out. The solid N
-Ar mixtures at low concentrations (Phase II of
Figure 3b) therefore provide a better testbed for understanding quadrupolar or orientational glass formation.
3. NMR Methods
NMR methods are sensitive to long-range ordering or orientational or quadrupolar glass ordering because the nuclear spin-spin interactions depend on the local order parameters. Both the nuclear dipole-dipole interactions and the anisotropic chemical shift interaction vary with the values of the local order parameter and thus alter the NMR spectra.
The intra-molecular dipole-dipole interaction between the two nuclei of one molecule can be written as [
27]:
where we have introduced the irreducible orthonormal tensor operators
and
to describe the orientational and nuclear spin degrees of freedom, respectively. Under rotations, these operators transform equivalently to the spherical harmonics
. In the manifold
, the orientational operators are [
27,
39]:
with:
and
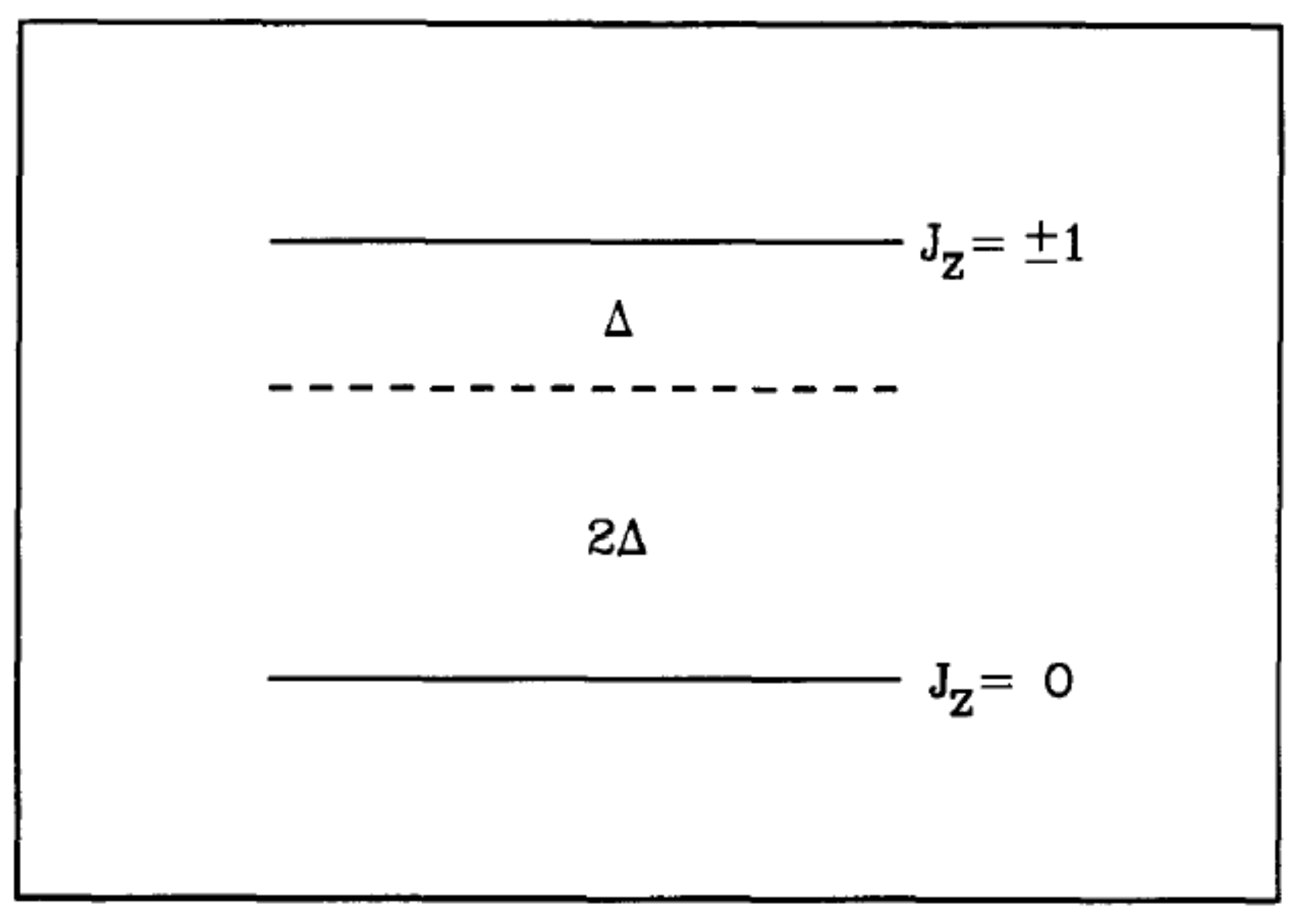
The strength of the intra-molecular dipole-dipole interaction for H
is given by
= 173.06 kHz. For a high magnetic field, the dipole interaction is only a weak perturbation of the nuclear Zeeman interaction,
, where
is the nuclear gyromagnetic ratio and
is the magnetic field aligned along the
z-axis. For the Zeeman term alone, the energy levels for one ortho-H
molecule with total nuclear spin I= 1 consist of three equally-spaced levels corresponding to
with energy separations
where
is the nuclear Larmor frequency. In the absence of any orientational ordering, the dipolar interaction averages to zero, and this energy separation is unperturbed. However, for a non-trivial orientational ordering, the expectation value of the secular component of
(the part that commutes with the Zeeman interaction),
, is non-zero, and the energy levels are perturbed as shown in
Figure 4.
If the orientation of the magnetic field with respect to local axes of molecule
i is defined by the polar angles
), we have for axial symmetry
(which is usually assumed in the literature) [
21,
27],
where the local order parameter
From Equation (
4) and
Figure 4, we see that each molecule with a non-zero order parameter
contributes a doublet to the NMR absorption line shape at:
The experiments are almost always carried out for powdered samples, and we need to sum the contributions to the spectrum over a powder distribution
of the crystalline orientations
If
is the line intensity at frequency
for a fixed
, we have
. The line shapes therefore consist of a doublet given by:
These are the familiar Pake doublets and are shown in
Figure 5 for
.
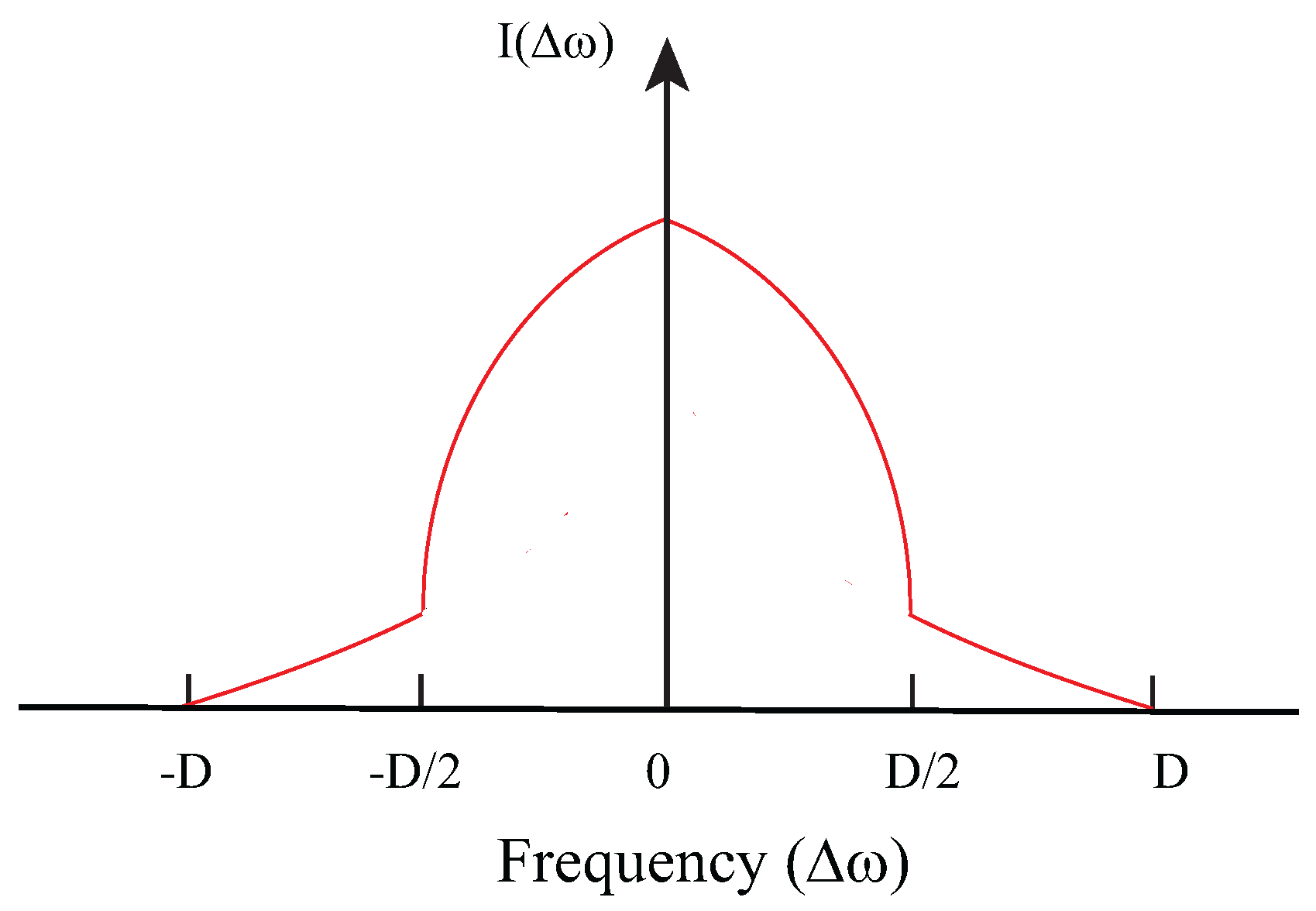
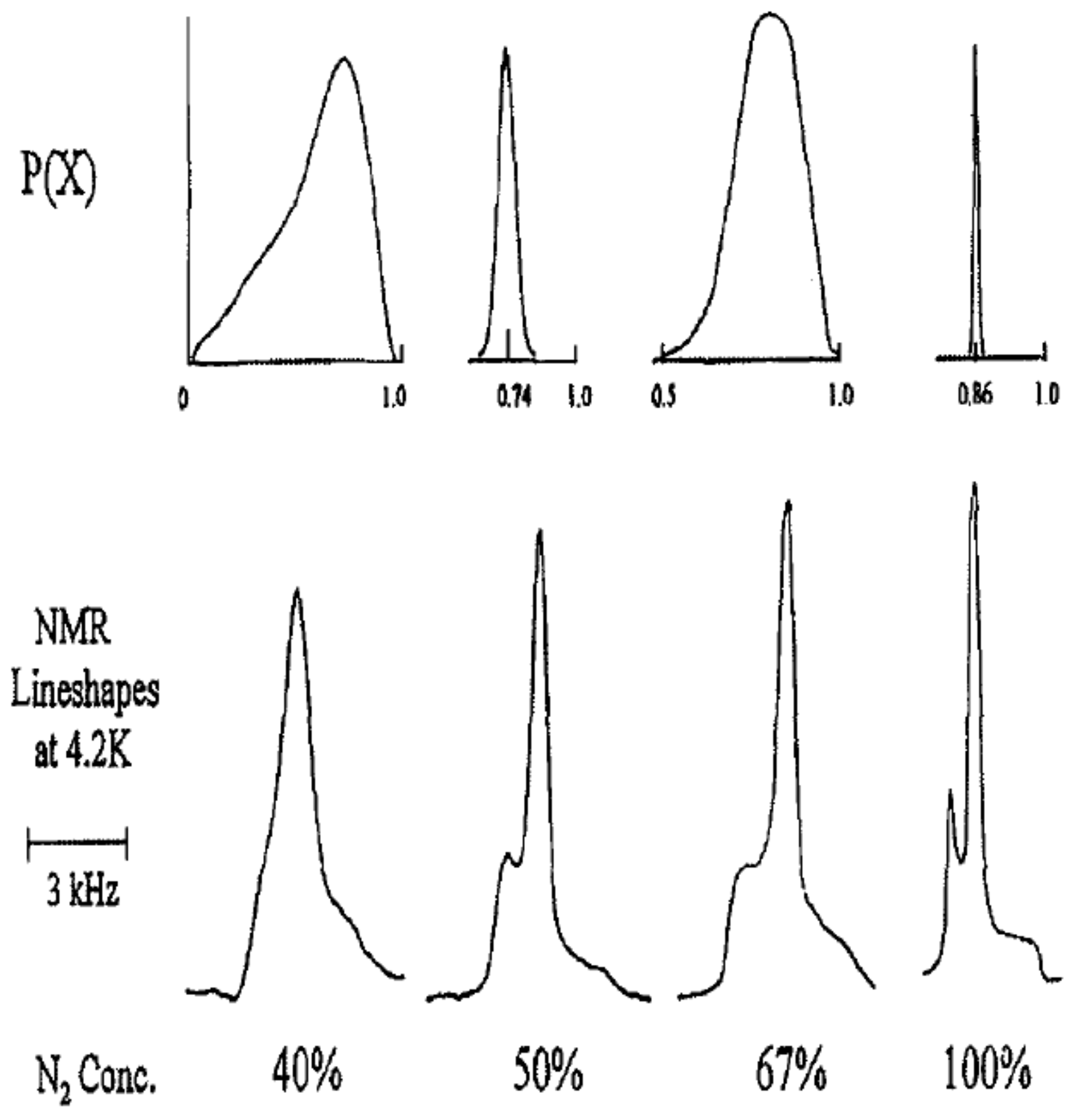
In order to calculate the line shape expected for a quadrupolar glass, we need to consider an approximate form for the distribution of local order parameters. If
is the probability of finding the value
in the glass state, then we find
from the distribution of the values of local entropies. For each molecule with local order parameter
, the contribution to the entropy is:
For small
,
is linear in
, and assuming the distribution of entropies,
is a constant, we have as a first approximation:
Using this approximation and the expressions for the line shapes for the doublets given by Equation (
7), the glass line shape is expected to be given by:
The calculated glass line shape using Equation (
10) is shown in
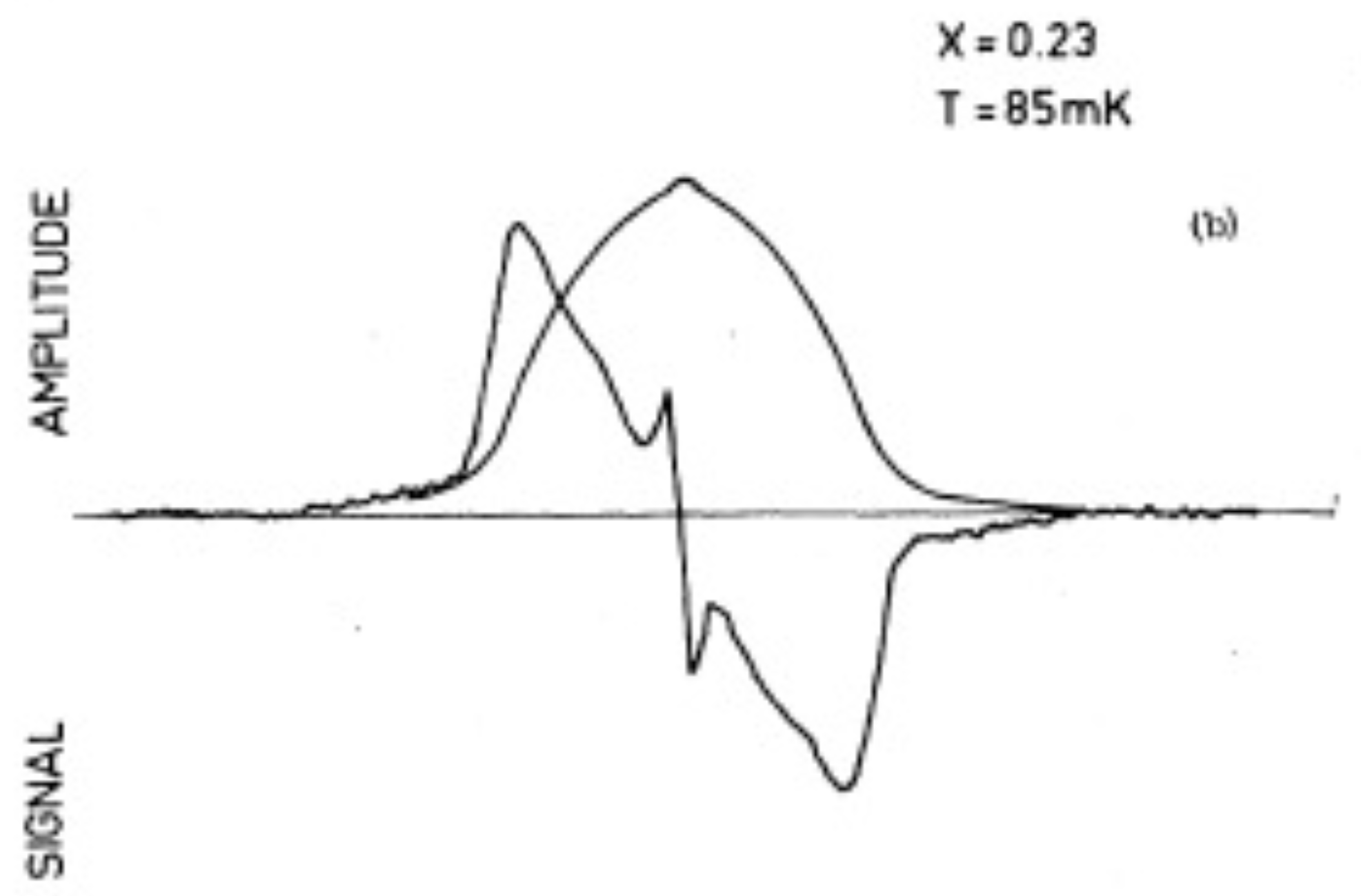
Figure 6. This shape is to be compared with the shape observed at low temperatures. An example is shown in
Figure 7 for an ortho-H
concentration of
and a temperature of 85 mK. The general form is in good agreement with the predictions.
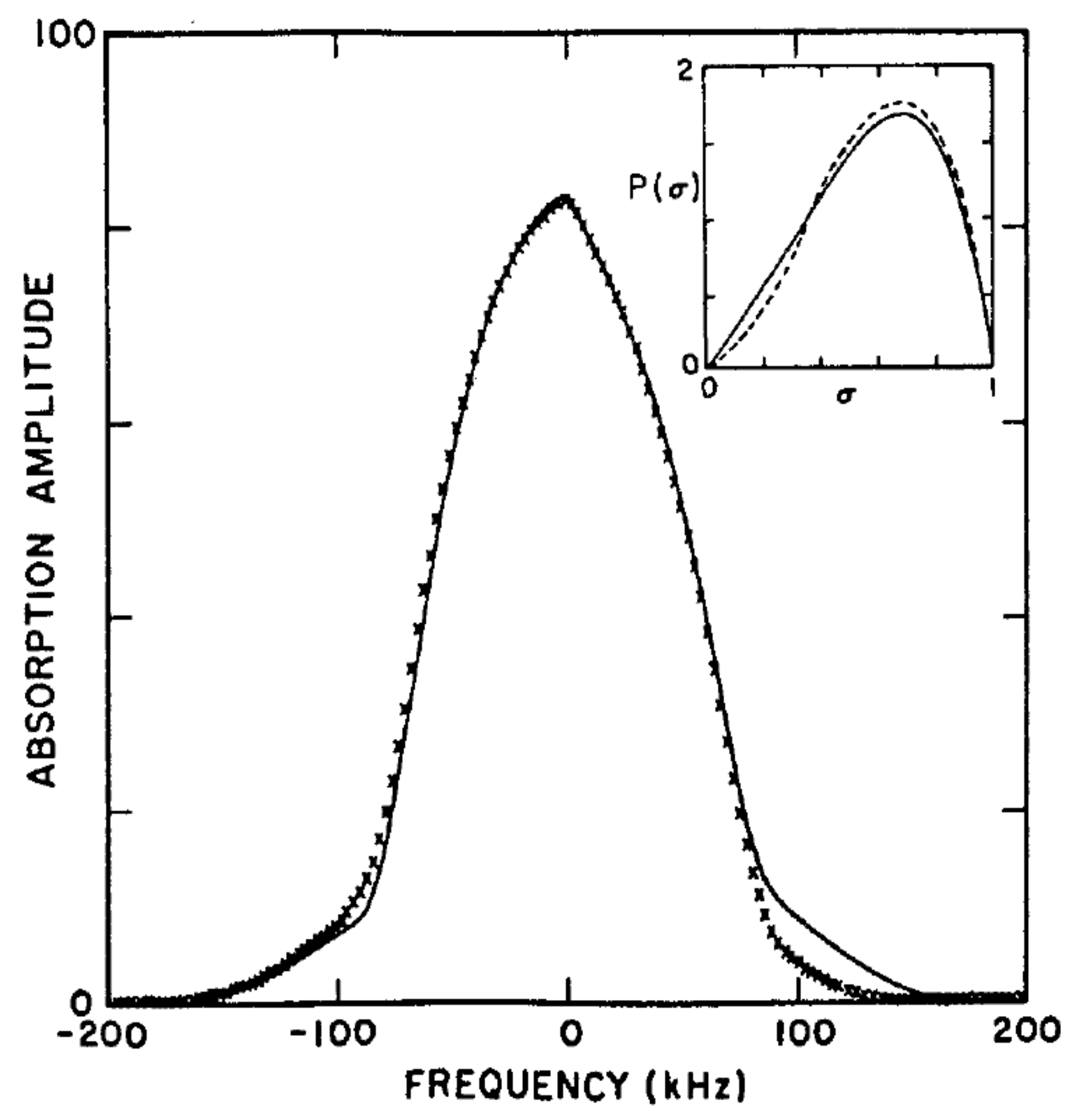
At lower temperatures and for high magnetic fields, the line shape shows a distinct asymmetry as shown in
Figure 8. This asymmetry is due to the finite nuclear spin polarization (16% in the case of
Figure 8). For non-trivial nuclear polarizations, the ratio of the amplitudes of the two branches of the Pake doublet is not unity. It is given by:
where
is the nuclear Larmor frequency and
T is the absolute temperature of the sample, obtained by a polynomial fit to the order parameter distribution function using
to find:
using
The order parameter distribution estimated from the best fits to the NMR line shapes is shown in
Figure 9 for ortho-para H
samples with 23% ortho hydrogen [
27]. There is a smooth variation with temperature with no discontinuity or other evidence of a sudden transition. The relevant parameter for a spin glass transition, however, is the Edwards–Anderson [
40] order parameter given by:
where
is the component of the spin at site
i,
is a thermodynamic average and
is an average over configurations
c. While the average
vanishes for paramagnetism and for a spin glass,
is non-zero for the spin glass.
For the quadrupolar glass, we therefore consider the glass order parameter:
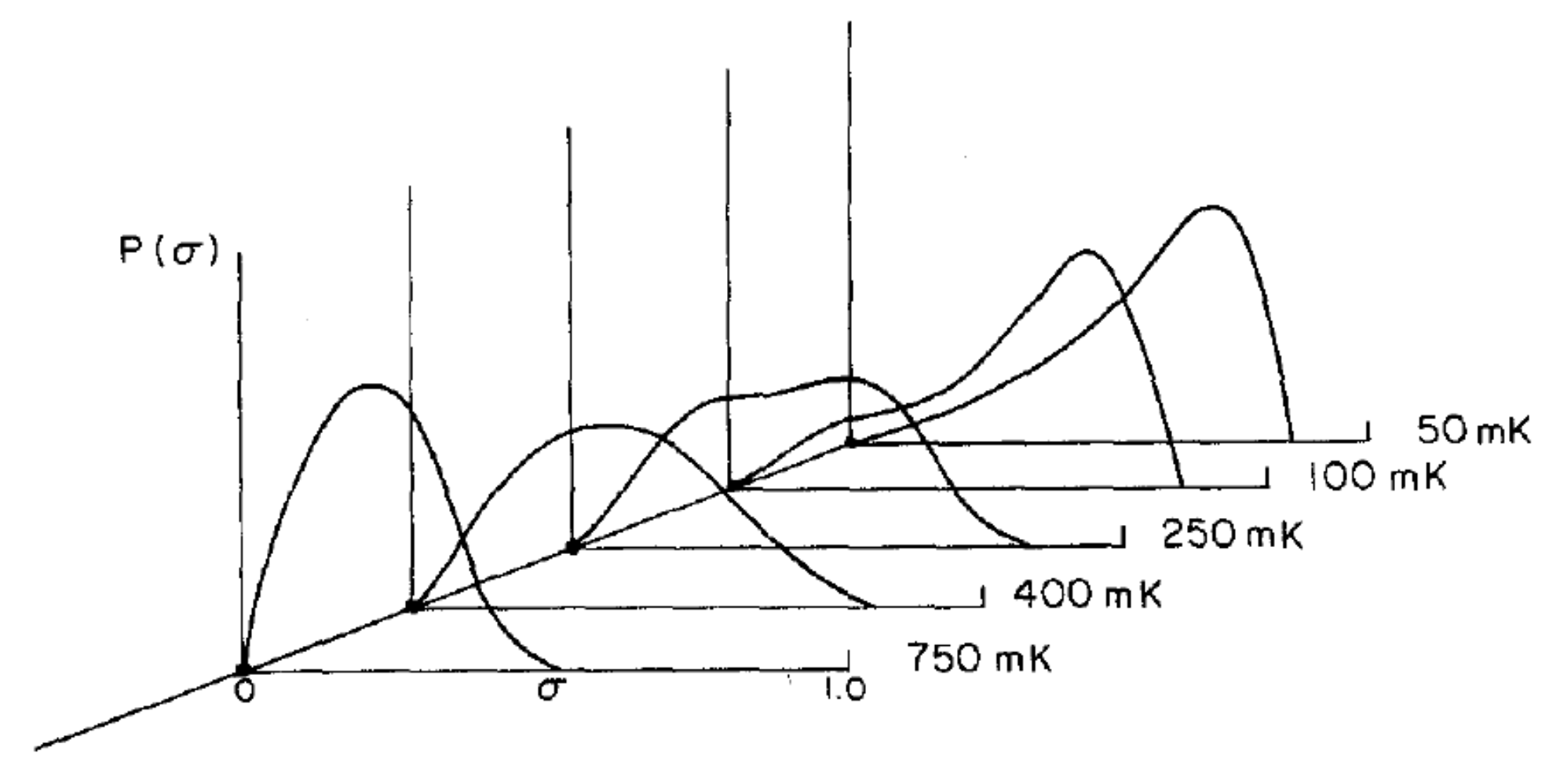
The temperature dependence of the quadrupolar glass order parameter
is calculated from the order parameter distribution functions
determined from the NMR line shapes shown in
Figure 10 [
41]. Although there is no evidence for an abrupt transition in the temperature variation of
, the observed temperature dependence is much stronger than that predicted for a non-collective simple random field model [
42,
43,
44] shown by the broken dashed line of
Figure 10. The observed variation is in good qualitative agreement with the model for a quadrupolar glass proposed by Lutchinskaia et al. [
45] consistent with a collective quadrupolar glass ordering at low temperatures.
Classical orientational glass ordering can be observed in solid N
-Ar mixtures using NMR techniques at low temperatures. Instead of the common isotope
N, experimenters use
N, which has nuclear spin
, and the large (∼3 MHz) nuclear quadrupole interaction for
N vanishes. For the diatomic molecule
N
, one can treat the total molecular nuclear spin as
and use a similar analysis of the NMR spectra as was used for diatomic H
. Because of the larger mass and large moment of inertia compared to H
molecules, the angular momentum is not a good quantum number, and the local order parameters are purely classical and given by:
where
is the angle between the instantaneous molecular orientation and the mean orientation. The NMR line shapes for local orientational ordering in solid N
-Ar mixtures have one major difference compared to those observed for solid hydrogen because there is an extra term in the nuclear spin Hamiltonian that depends on the orientational order parameter. That term is the anisotropic chemical shift resulting from the interaction between the nuclear spin and the magnetic fields of the molecular electron distribution given by:
. The
N
NMR line shapes in the presence of a finite orientational order parameter form doublets for a powered sample, but unlike those for solid H
, they are antisymmetric with Equation (
6) replaced by:
as illustrated in
Figure 11. A typical line shape is shown in
Figure 12 and compared with a calculated line shape for a fixed order parameter
that includes a small broadening, as shown by the inset to
Figure 12. The agreement is very satisfactory.
Narrow lines consistent with long-range ordering in a Pa3 structure are only observed for N
concentrations above 76%, where the lattice structure remains fcc (Part III of
Figure 3b). In the region where the lattice remains hcp for
(Part II of
Figure 3b), only broad NMR line shapes characteristic of quadrupolar glass-like ordering are observed. The remarkable observation is that in Region IB of the phase diagram (see
Figure 3b), one once again observes a relatively narrow line shape (see
Figure 13) for
[
38]. Because of the presence of high substitutional disorder, this narrow line shape is believed to be a true orientational glass (analogous to that depicted in
Figure 1a) with a broad distribution of alignment angles, but a relatively narrow distribution of order parameters about those angles and with a mean
. On further dilution of
to 40%, only very broad NMR line shapes are observed in Region IA of
Figure 3b.
While the NMR line shapes of solid ortho-para hydrogen mixtures and N-Ar mixtures are consistent with quadrupolar and orientational glass states at low temperature and the growth of the quadrupolar order parameter as the temperature is lowered suggests that the local ordering is collective in nature and not driven by fixed local fields, it is important that the dynamics be investigated to determine whether the characteristic rapid freezing of the orientational fluctuations occurs at the onset of glassiness. This is discussed in the next section.
4. Dynamics
NMR methods can be used to investigate the dynamics of molecular fluctuations and in particular the orientational or quadrupolar fluctuations over a wide frequency range [
25,
26,
37]. The sensitivity of NMR methods to motions arises because fluctuations of the molecular alignments or local order parameters render the intra-molecular nuclear dipole-dipole interactions time dependent [
46]. Specifically, the nuclear spin-lattice relaxation time,
, due to the modulation of the dipole-dipole interactions, is given by:
D measures the strength of the intra-molecular dipole-dipole interaction, and
is the nuclear Larmor frequency.
is the spectral weight of the fluctuations at frequency
.
The
are the tensorial operators defined in Equation (
2).
The expectation value
must be calculated with a quantization axis parallel to the magnetic field. The order parameters, however, are calculated with respect to the local molecular symmetry axis. In order to carryout this evaluation, we therefore consider the rotation:
The
are the rotation matrix elements, and the angles
are polar angles defining the orientation of the magnetic field in a local reference frame aligned along the molecular equilibrium orientation. One then assumes [
47] that the time dependence can be separated from the order parameter variations as:
The original data shown in
Figure 14 [
48] were interpreted in terms a single correlation time
. The result, a strong almost exponential temperature dependence, resembles, but is not identical to a Fulcher–Vogel temperature dependence. The data in
Figure 14 along with the results observed for the very slow dynamics determined by NMR stimulated echo studies [
49] are among the most convincing experimental evidence for traditional glassy dynamics for the quadrupolar glass state of solid ortho-para hydrogen mixtures.
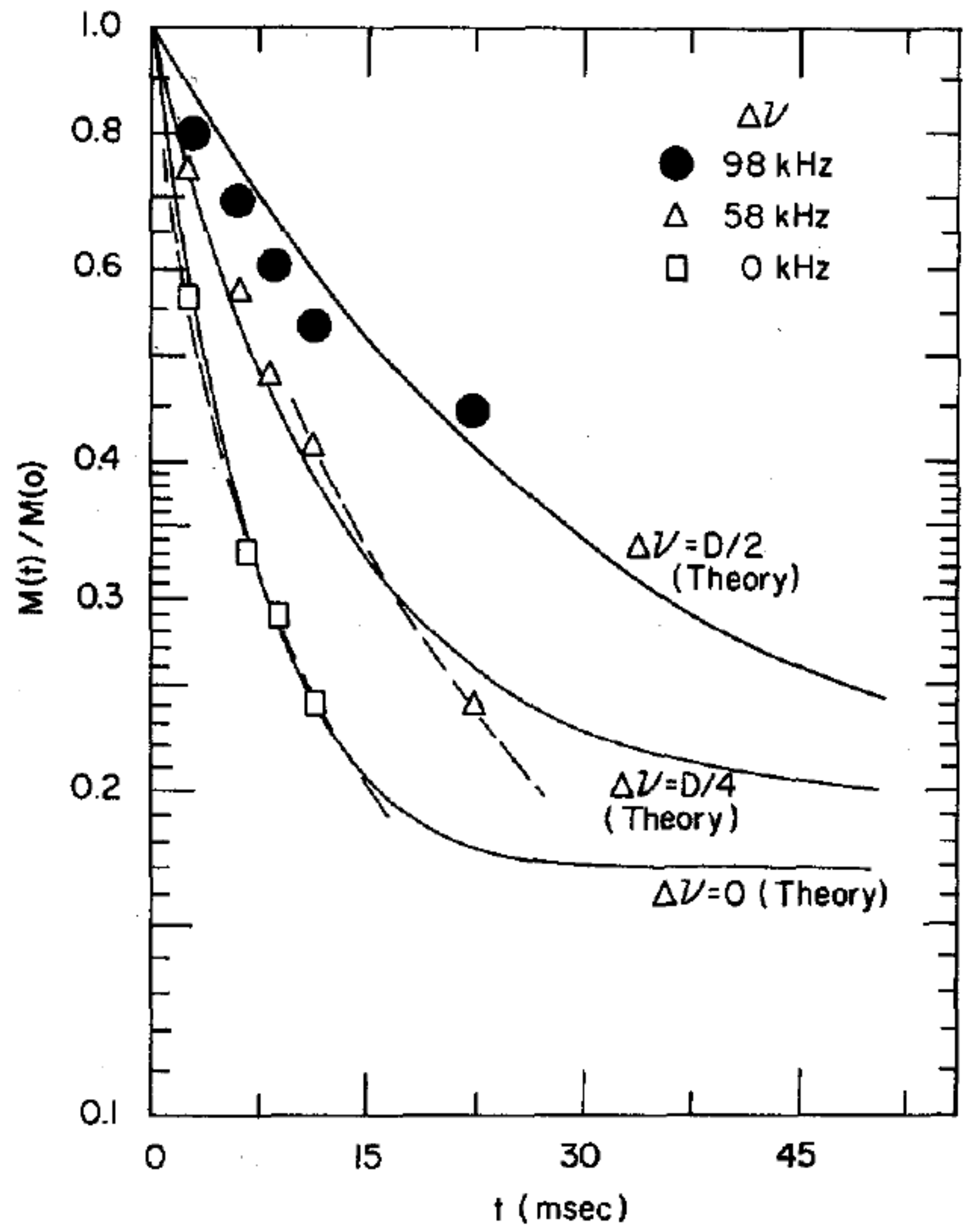
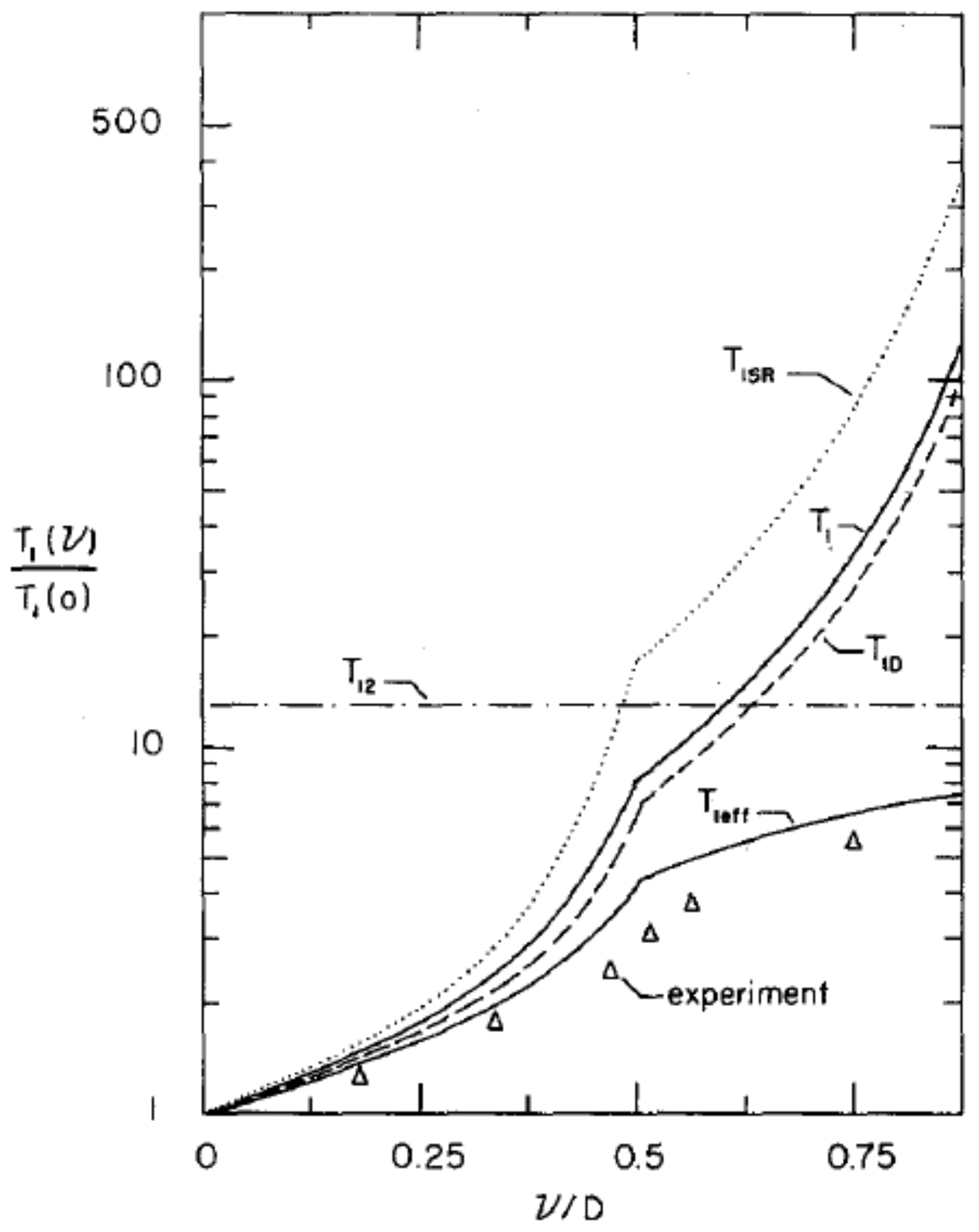
Further studies, however, showed that the nuclear spin relaxation was not a simple exponential, but rather a sum of different exponentials associated with different parts of the NMR line shape, as illustrated in
Figure 15.
This spectral inhomogeneity of the nuclear spin-lattice relaxation can be expected from Equation (
22). If one assumes that the relaxation is dominated by the fluctuations of the axial order parameter,
, then Equation (
22) leads to:
for the contribution to the relaxation by the dipole terms. The factor
is the mean square variation of the operator
. The angular terms are associated by different isochromats of the NMR line shape, and if one assumes Lorentzian forms with correlation time
for the spectral densities
, we have [
47]:
is the frequency of an isochromat with respect to the center of the NMR absorption line. The spin-rotational (SR) interaction also contributes to the relaxation rate:
where
kHz for a hydrogen molecule. These two contributions to the relaxation rate from the intra-molecular dipole-dipole and spin-rotation interactions are additive, and the overall relaxation given by
is shown in
Figure 16. The agreement with experiment is satisfactory. Yu et al. [
50] also noted that cross-relaxation,
, between different isochromats will tend to smooth out the inhomogeneities in the relaxation rates, and the observed relaxation will be determined by
. The resulting variation of the relaxation as a function of
is shown in
Figure 16.
5. Thermodynamic Measurements
Measurements of the heat capacities of solid ortho-para hydrogen [
51,
52] and N
-Ar mixtures [
53,
54] showed rather featureless temperature variations and were at first sight disappointing, but studies of the very slow dynamics of solid hydrogen with dilute ortho-H
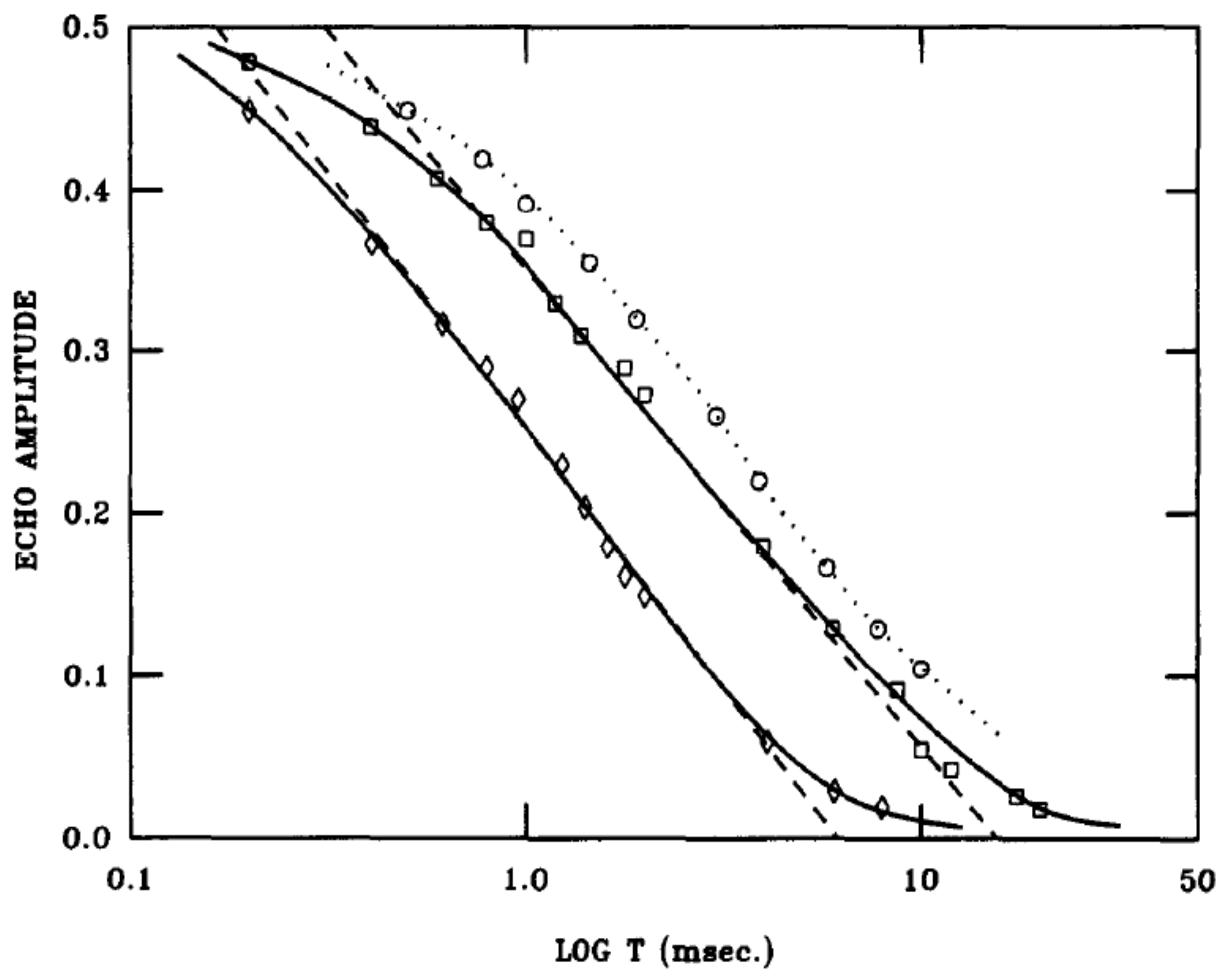
concentrations were able to provide a uniform picture for the very slow motions and the thermodynamic behavior. The low frequency dynamics was investigated using NMR stimulated echo techniques employing three RF pulses [
49]. After a first pulse, the nuclear magnetization is rotated to a transverse axis (x-axis), where it evolves under the intra-molecular dipole interactions, which depend on the local order parameter. After a time
, a second pulse stores the transverse magnetization along the original z-axis. The value stored depends on the original value of the order parameter. After a waiting time
, a third pulse returns the stored magnetization back to the x-axis, and after a further time
, a stimulated echo is formed, provided that the order parameter varies only very slowly in the waiting time
. A variety of different pulse sequences can be considered but the simplest (
) about the same axes produces an echo signal:
designates a configurational average over sites
i. The dependence on
allows one to explore slow temporal fluctuations of the molecular order parameters over time
. The results (
Figure 17) were surprising, as they showed a unique logarithmic decay.
The logarithmic decay can be understood in terms of the droplet model proposed by Fisher and Huse [
55,
56]. Fisher and Huse considered the density of low energy states for connected clusters and argued that because the clusters are rare, they could be treated as “non-interacting two level systems”. For a free energy barrier
, the tunneling rate at temperature
T is:
where
is an attempt frequency. For long times,
is well defined because it is associated with a characteristic cluster size. The relaxation is hierarchical as low-energy barriers must be overcome before larger barriers can be crossed. The dynamics of of the faster degrees of freedom therefore constrain the slow motions. As a result, in time
t, the barriers overcome will be those with energy less than
where:
For all energy barriers traversed in time
t, the order parameters change significantly, and the correlation functions
will vanish after the barriers are crossed. If the density of low energy excitations is
, the stimulated echo amplitude will be given by:
At low temperatures, we only need consider the density of states near
, and the echo amplitude becomes:
is determined by the attempt frequency
. Since the order parameter changes by the order of unity in time
:
The decay of the stimulated echos is therefore directly related to the density of states at low energy,
, which is expected to be constant and can be related to the thermodynamic measurements. From
Figure 17, we find
.
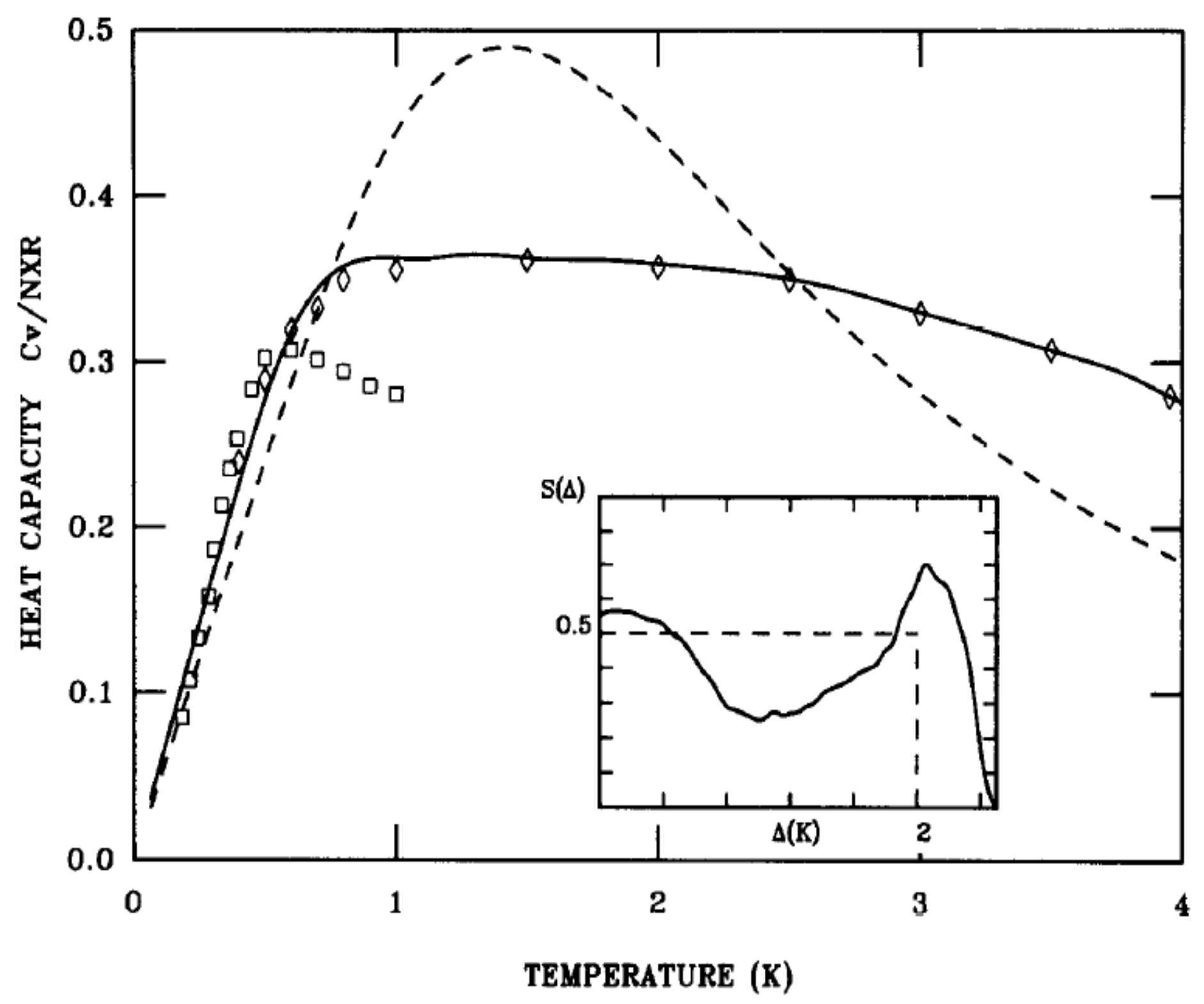
Knowledge of the density of energy levels
allows one to calculate the heat capacity and test the interpretation of the dynamics against the experimental results for heat capacity studies [
51,
52,
53,
54]. Using the energy scheme of
Figure 18, the heat capacity is calculated from the sum over the distribution of energy states
as:
with
. X is the ortho-H
concentration. The result of the calculation shown in
Figure 19 gives a good description of the characteristic linear temperature dependence at low temperatures, and an excellent fit is obtained using small deviations from a constant density of energy states shown by the solid line of
Figure 19.
6. Dielectric Measurements
While the analysis of the NMR line shapes of solid ortho-para H
and solid N
-Ar mixtures with high disorder (that is, low concentrations of the active quadrupole bearing molecule (ortho-H
or N
)) show clear evidence of the formation of glassy states at low temperatures and the temperature dependence of the dynamics cannot be understood except in terms of local collective ordering, the characteristic hysteresis of the susceptibility (the parameter conjugate to the local order parameters) remained elusive until the studies of Pilla et al. [
57,
58] using electric susceptibility measurements. These measurements were carried out on dilute solid N
-Ar mixtures for concentrations where the lattice remained cubic. In this case, the concern that there exist local electric field gradients due to the introduction of substitutional disorder is greatly lessened, and the system is much closer to the class of the familiar spin glasses.
The use of a high sensitivity capacitance bridge [
59] to measure dielectric susceptibilities
showed distinct hystereses in the observed values of the dielectric susceptibility on thermal cycling [
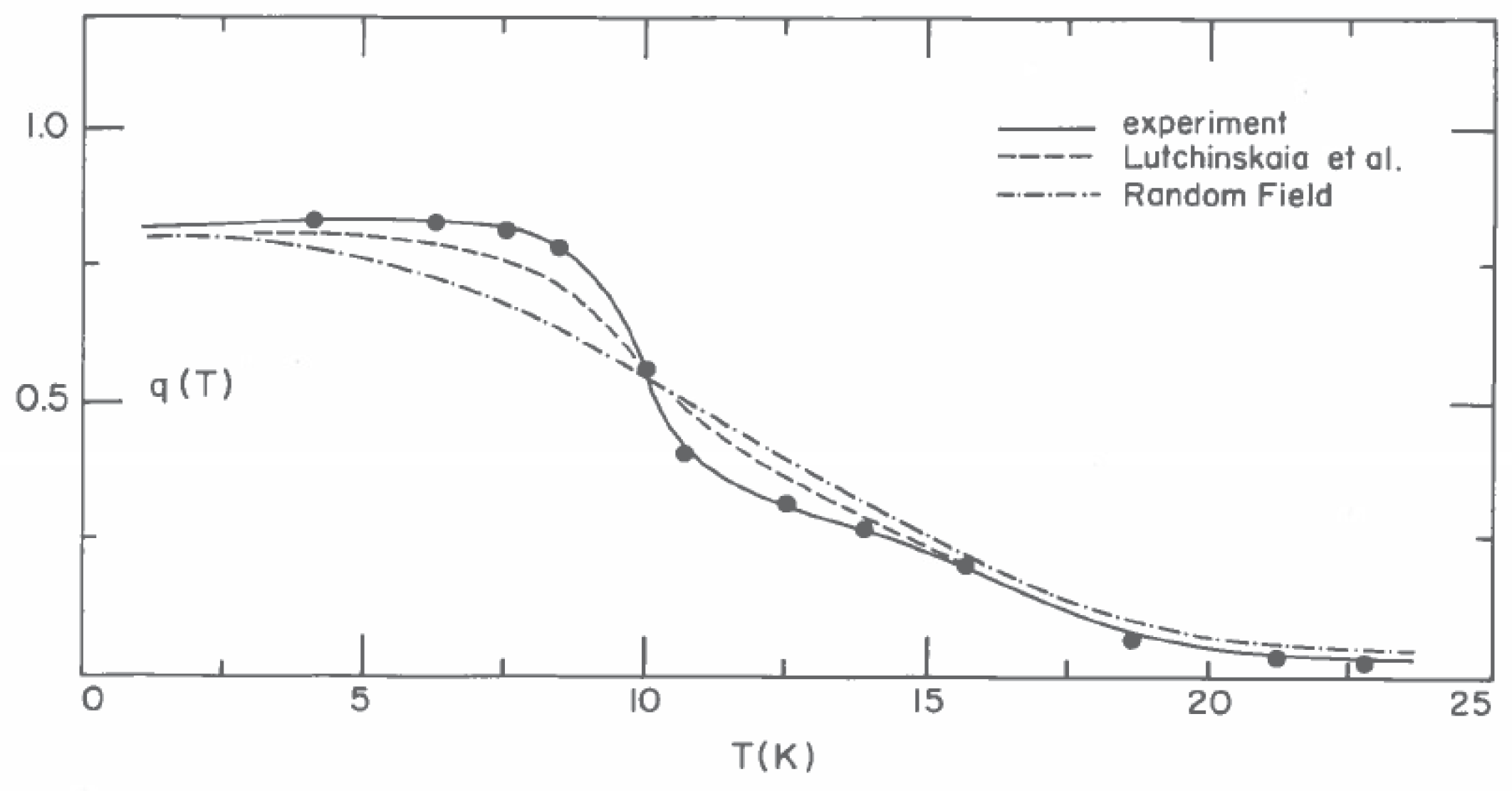
57]. The break in the observed values of
at 9.8 ± 0.1 K (
Figure 20) clearly marks a well-defined dynamical transition for solid N
-Ar mixtures with 51% N
that was absent in the NMR measurements.
The really significant consequence of the dielectric susceptibility measurements for the molecular glasses is that they can be used to test the applicability of replica symmetry breaking theories to this class of glass states [
14,
60]. For simple replica symmetry breaking, Cugliandolo and Kurchan [
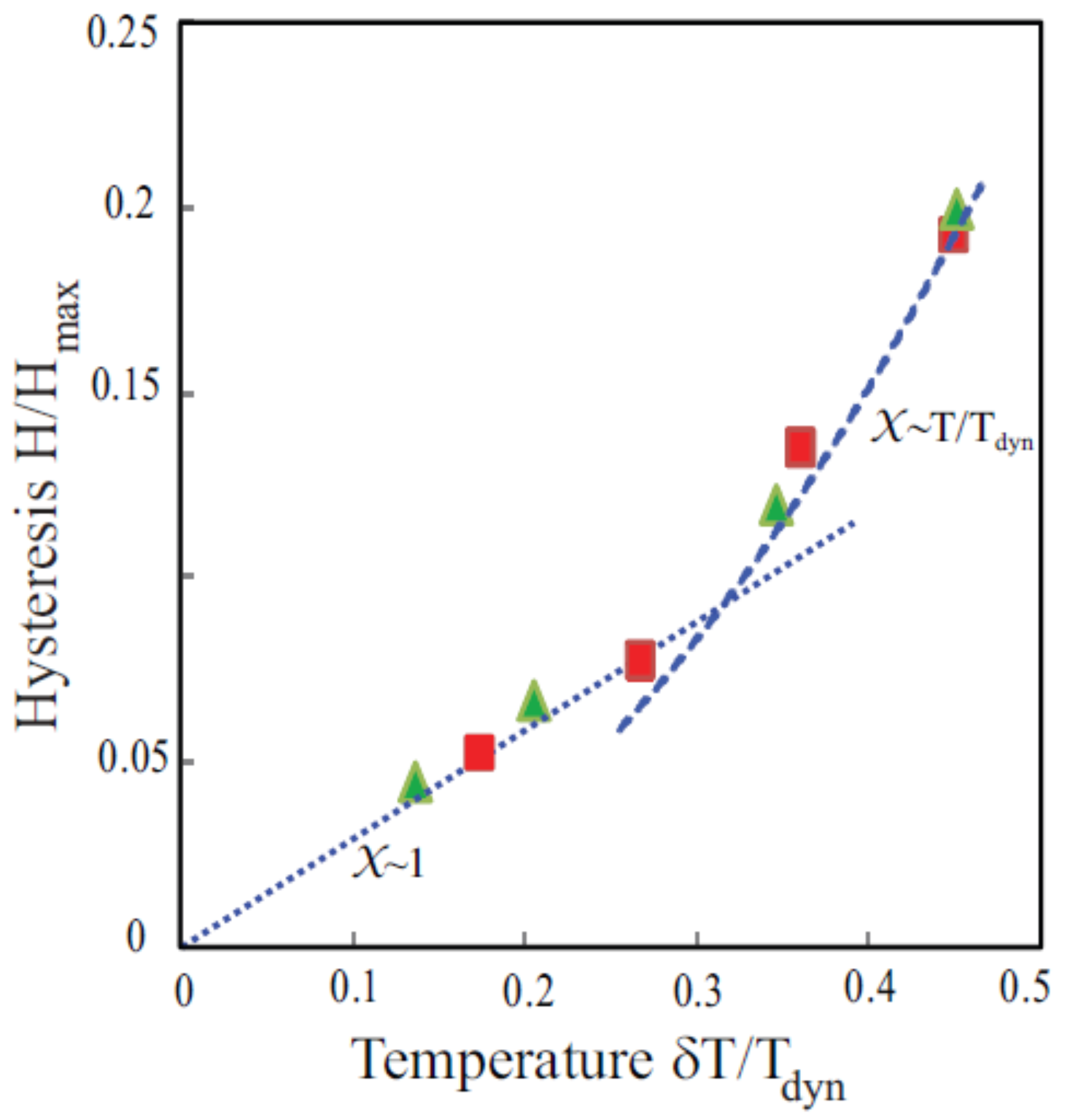
18] developed a generalized fluctuation dissipation theorem for the generalized susceptibility:
where
is a generalized coordinate. The essential result is that below the characteristic glass freezing temperature of the dynamics,
, the change in the response function depends on the waiting time
because of the changes in the landscape of free energy barriers that occur in the time
. The response function is defined by:
where
is the relevant susceptibility. Pilla et al. [
23] showed that as a consequence of this generalized fluctuation dissipation theorem,
for large excursions compared to
1 (the classical result) for small
. The results are shown in
Figure 21.