Ionic Liquid-Based Non-Aqueous Electrolytes for Nickel/Metal Hydride Batteries
Abstract
:1. Introduction
2. Experimental Setup
3. Results and Discussion
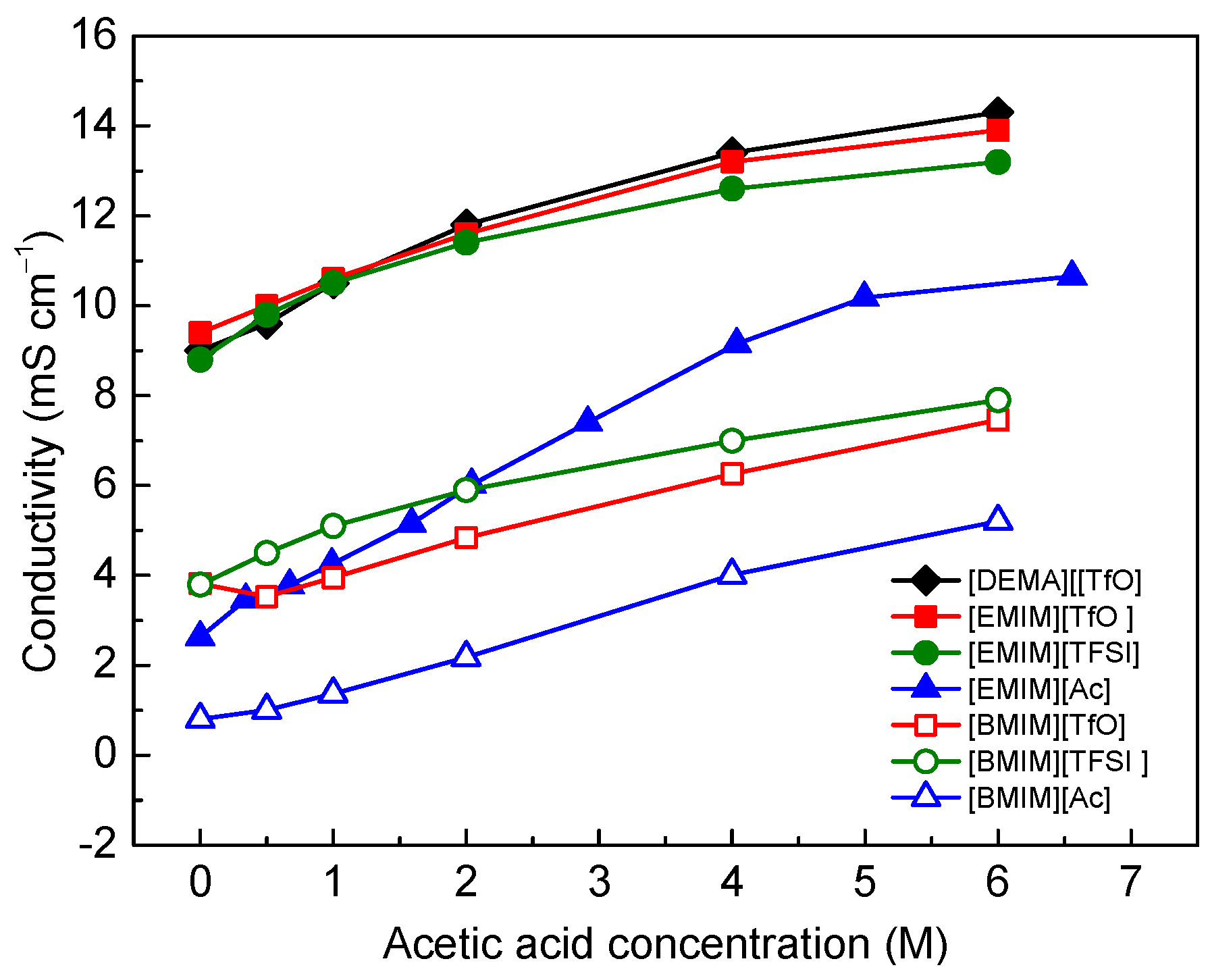
3.1. Ionic Conductivity of Ionic Liquid/Acetic Acid Electrolytes
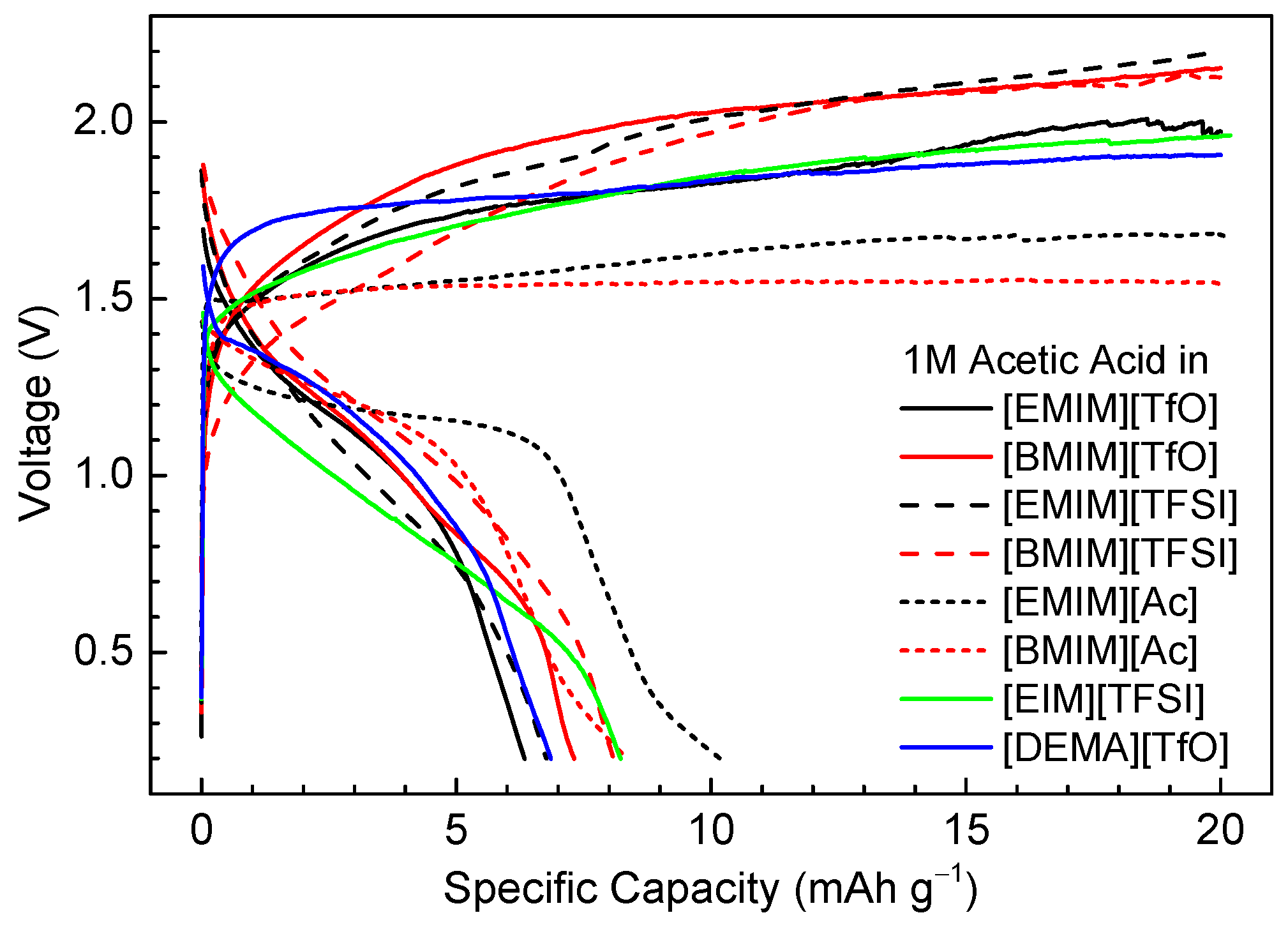
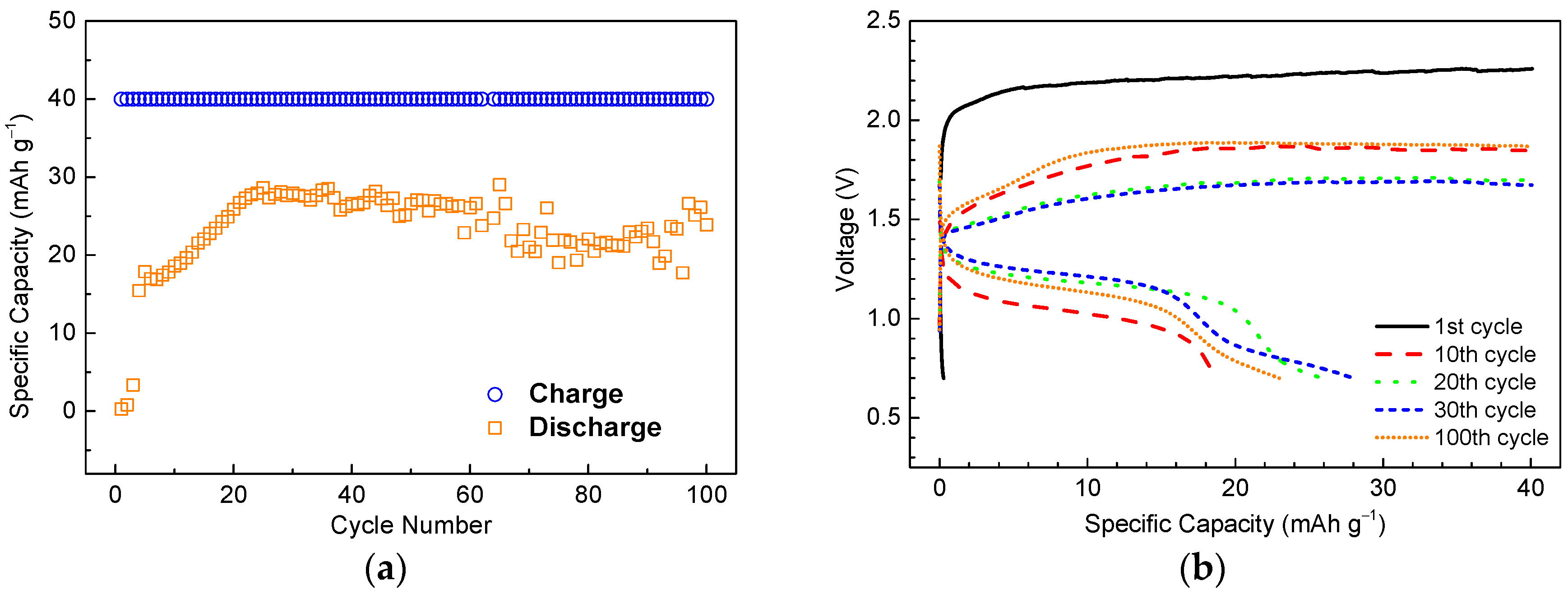
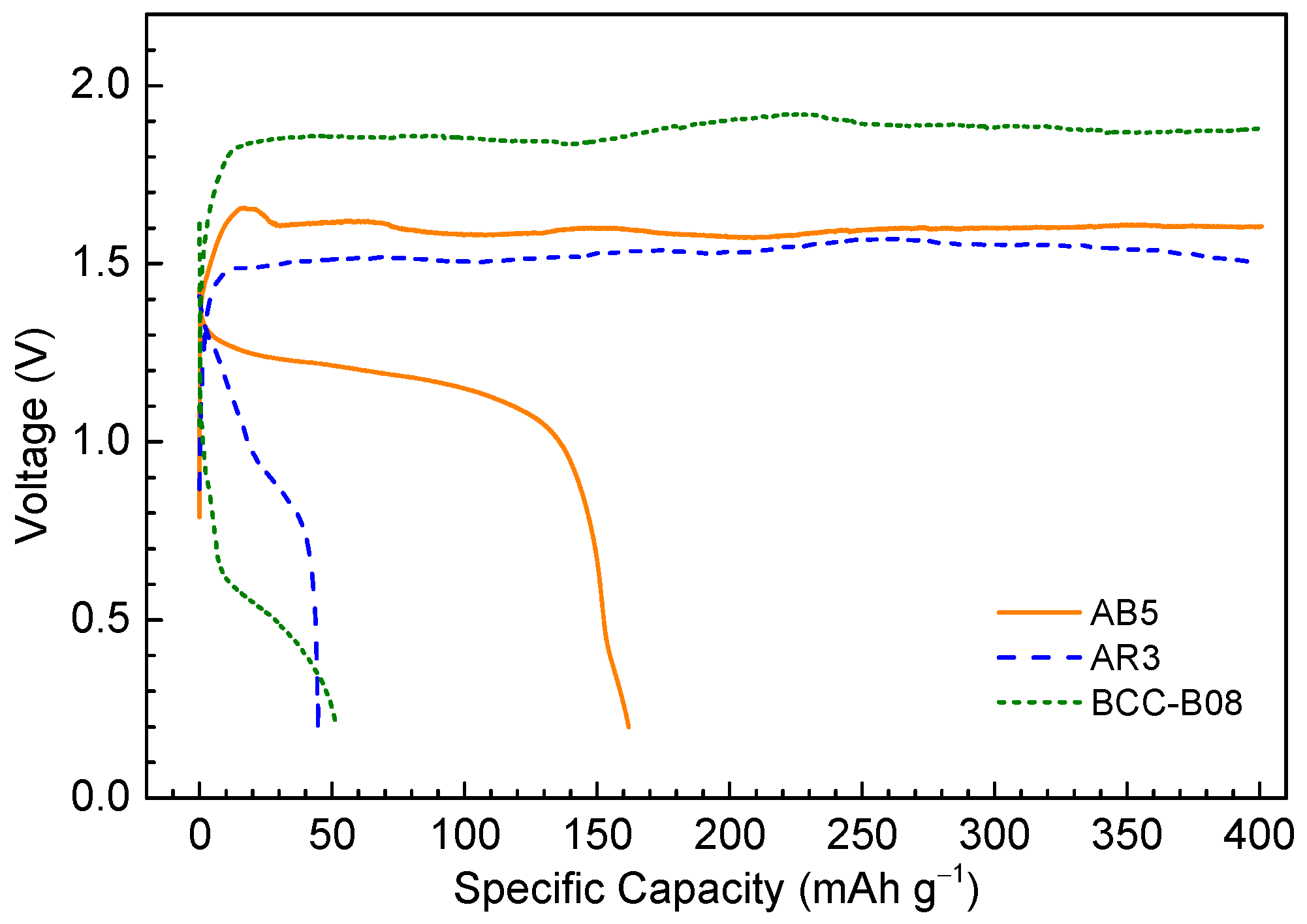
3.2. Half-Cell Electrochemical Tests
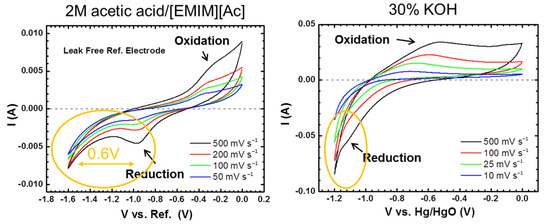
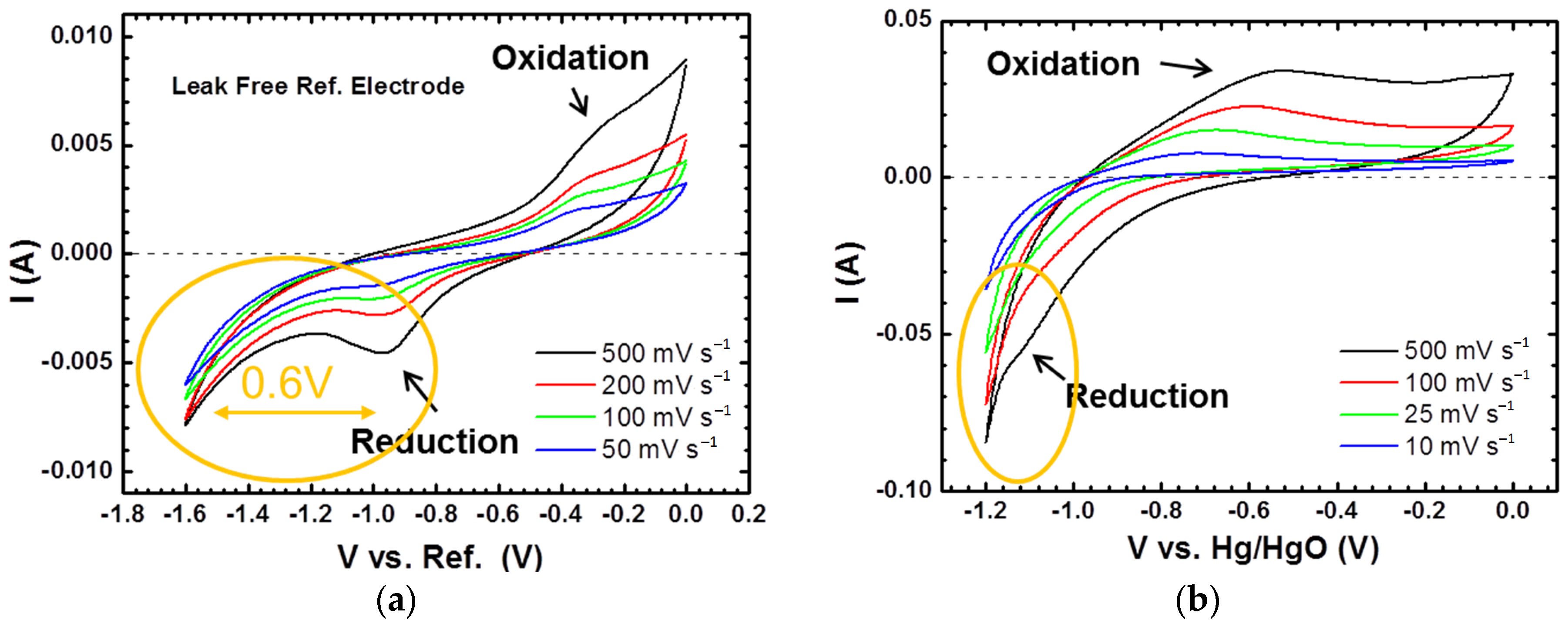
3.3. Cyclic Voltammetry
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IL | Ionic liquid |
| [DEMA][TfO] | Diethylmethylammonium trifluoromethanesulfonate |
| [Im][TFSI] | Imidazolium bis(trifluoromethylsulfonyl)imide |
| PIL | Protic ionic liquid |
| AIL | Aprotic ionic liquid |
| Ni/MH | Nickel/metal hydride |
| [EMIM][TfO] | 1-ethyl-3-methylimidazolium trifluoromethanesulfonate |
| [BMIM][TfO] | 1-butyl-3-methylimidazolium trifluoromethanesulfonate |
| [EMIM][TFSI] | 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| [BMIM][TFSI] | 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| [EMIM][Ac] | 1-ethyl-3-methylimidazolium acetate |
| [BMIM][Ac] | 1-butyl-3-methylimidazolium acetate |
| [EIM][TFSI] | 1-ethylimidazolium bis(trifluoromethylsulfonyl)imide |
| CV | Cyclic voltammetry |
| σ | Conductivity |
| Λ | Molar conductivity |
| η | Viscosity |
| C | Temperature dependent constant |
| r | Effective radius of spherical particles |
| z | Valence of the charge carrier |
| e | Elementary charge |
| V | Number of charge carriers |
| N | Volume |
| NMR | Nuclear magnetic resonance |
| M | Hydrogen storage metal alloy |
| MH | Metal hydride |
| ads | Adsorbed |
| abs | Absorbed |
| SEI | Solid electrolyte interface |
References
- Yamamoto, T.; Nohira, T.; Hagiwara, R.; Fukunaga, A.; Sakai, S.; Nitta, K.; Inazawa, S. Charge–discharge behavior of tin negative electrode for a sodium secondary battery using intermediate temperature ionic liquid sodium bis(fluorosulfonyl)amide–potassium bis(fluorosulfonyl)amide. J. Power Sources 2012, 217, 479–484. [Google Scholar] [CrossRef]
- Nohira, T.; Ishibashi, T.; Hagiwara, R. Properties of an intermediate temperature ionic liquid NaTFSA–CsTFSA and charge–discharge properties of NaCrO2 positive electrode at 423 K for a sodium secondary battery. J. Power Sources 2012, 205, 506–509. [Google Scholar] [CrossRef]
- Khoo, T.; Somers, A.; Torriero, A.A.J.; MacFarlane, D.R.; Howlett, P.C.; Forsyth, M. Discharge behaviour and interfacial properties of a magnesium battery incorporating trihexyl(tetradecyl)phosphonium based ionic liquid electrolytes. Electrochim. Acta 2013, 87, 701–708. [Google Scholar] [CrossRef]
- Kakibe, T.; Hishii, J.Y.; Yoshimoto, N.; Egashira, M.; Morita, M. Binary ionic liquid electrolytes containing organo-magnesium complex for rechargeable magnesium batteries. J. Power Sources 2012, 203, 195–200. [Google Scholar] [CrossRef]
- Simons, T.J.; Howlett, P.C.; Torriero, A.A.J.; MacFarlane, D.R.; Forsyth, M. Electrochemical, transport, and spectroscopic properties of 1-ethyl-3-methylimidazolium ionic liquid electrolytes containing zinc dicyanamide. J. Phys. Chem. C 2013, 117, 2662–2669. [Google Scholar] [CrossRef]
- Simons, T.J.; Torriero, A.A.J.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. High current density, efficient cycling of Zn2+ in 1-ethyl-3-methylimidazolium dicyanamide ionic liquid: The effect of Zn2+ salt and water concentration. Electrochem. Commun. 2012, 18, 119–122. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Ogawa, A.; Kanno, M.; Nakamoto, H.; Yasuda, T.; Watanabe, M. Nonhumidified intermediate temperature fuel cells using protic ionic liquids. J. Am. Chem. Soc. 2010, 132, 9764–9773. [Google Scholar] [CrossRef] [PubMed]
- Susan, M.A.B.H.; Noda, A.; Mitsushima, S.; Watanabe, M. Brønsted acid–base ionic liquids and their use as new materials for anhydrous proton conductors. Chem. Commun. 2003, 8, 938–939. [Google Scholar] [CrossRef]
- Nakamoto, H.; Watanabe, M. Brønsted acid-base ionic liquids for fuel cell electrolytes. Chem. Commun. 2007, 24, 2539–2541. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Nakamura, S.-I.; Honda, Y.; Kinugawa, K.; Lee, S.-Y.; Watanabe, M. Effects of polymer structure on properties of sulfonated polyimide/protic ionic liquid composite membranes for nonhumidified fuel cell applications. ACS Appl. Mater. Interfaces 2012, 4, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Sakaebe, H.; Tatsumi, K.; Kikuta, M.; Ishiko, E.; Kono, M. Fast cycling of Li/LiCoO2 cell with low-viscosity ionic liquids based on bis(fluorosulfonyl)imide [FSI]−. J. Power Sources 2006, 160, 1308–1313. [Google Scholar] [CrossRef]
- Ishikawa, M.; Sugimoto, T.; Kikuta, M.; Ishiko, E.; Kono, M. Pure ionic liquid electrolytes compatible with a graphitized carbon negative electrode in rechargeable lithium-ion batteries. J. Power Sources 2006, 162, 658–662. [Google Scholar] [CrossRef]
- Tsai, W.Y.; Lin, R.Y.; Murali, S.; Zhang, L.L.; McDonough, J.K.; Ruoff, R.S.; Taberna, P.L.; Gogotsi, Y.; Simon, P. Outstanding performance of activated graphene based supercapacitors in ionic liquid electrolyte from −50 to 80 °C. Nano Energy 2013, 2, 403–411. [Google Scholar] [CrossRef]
- Lin, R.Y.; Taberna, P.L.; Fantini, S.; Presser, V.; Perez, C.R.; Malbosc, F.; Rupesinghe, N.L.; Teo, K.B.K.; Gogotsi, Y.; Simon, P. Capacitive energy storage from −50 to 100 °C using an ionic liquid electrolyte. J. Phys. Chem. Lett. 2011, 2, 2396–2401. [Google Scholar] [CrossRef]
- Lin, Z.; Taberna, P.-L.; Simon, P. Graphene-based supercapacitors using eutectic ionic liquid mixture electrolyte. Electrochim. Acta 2016, 206, 446–451. [Google Scholar] [CrossRef]
- Balducci, A.; Bardi, U.; Caporali, S.; Mastragostino, M.; Soavi, F. Ionic liquids for hybrid supercapacitors. Electrochem. Commun. 2004, 6, 566–570. [Google Scholar] [CrossRef]
- Bai, Y.; Cao, Y.; Zhang, J.; Wang, M.; Li, R.; Wang, P.; Zakeeruddin, S.M.; Gratzel, M. High-performance dye-sensitized solar cells based on solvent-free electrolytes produced from eutectic melts. Nat. Mater. 2008, 7, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhang, J.; Wang, Y.; Zhang, M.; Wang, P. Lithium-modulated conduction band edge shifts and charge-transfer dynamics in dye-sensitized solar cells based on a dicyanamide ionic liquid. Langmuir 2011, 27, 4749–4755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, J.; Bai, Y.; Wang, Y.; Su, M.; Wang, P. Anion-correlated conduction band edge shifts and charge transfer kinetics in dye-sensitized solar cells with ionic liquid electrolytes. Phys. Chem. Chem. Phys. 2011, 13, 3788–3794. [Google Scholar] [CrossRef] [PubMed]
- Kawano, R.; Watanabe, M. Equilibrium potentials and charge transport of an I−/I3− redox couple in an ionic liquid. Chem. Commun. 2003, 3, 330–331. [Google Scholar] [CrossRef]
- Meng, T.; Young, K.; Beglau, D.; Yan, S.; Zeng, P.; Cheng, M.M.-C. Hydrogenated amorphous silicon thin film anode for proton conducting batteries. J. Power Sources 2016, 302, 31–38. [Google Scholar] [CrossRef]
- Kreuer, K.-D.; Paddison, S.J.; Spohr, E.; Schuster, M. Transport in proton conductors for fuel-cell applications: Simulations, elementary reactions, and phenomenology. Chem. Rev. 2004, 104, 4637–4678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuda, T.; Watanabe, M. Protic ionic liquids: Fuel cell applications. MRS Bull. 2013, 38, 560–566. [Google Scholar] [CrossRef]
- Hoarfrost, M.L.; Tyagi, M.; Segalman, R.A.; Reimer, J.A. Proton hopping and long-range transport in the protic ionic liquid [Im][TFSI], probed by pulsed-field gradient NMR and quasi-elastic neutron scattering. J. Phys. Chem. B 2012, 116, 8201–8209. [Google Scholar] [CrossRef] [PubMed]
- Galinski, M.; Lewandowski, A.; Stepniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- ILCO Chemikalien GmbH. Ionic Liquid. Available online: http://www.ilco-chemie.de/downloads/Ionic%20Liquid.pdf (accessed on 24 October 2016).
- Walsh, D.A.; Ejigu, A.; Smith, J.; Licence, P. Kinetics and mechanism of oxygen reduction in a protic ionic liquid. Phys. Chem. Chem. Phys. 2013, 15, 7548–7554. [Google Scholar] [CrossRef] [PubMed]
- Nei, J.; Young, K.; Rotarov, D. Studies on MgNi-based metal hydride electrode with aqueous electrolytes composed of various hydroxides. Batteries 2016, 2, 27. [Google Scholar] [CrossRef]
- Liao, X.; Yin, Z.; Young, K.; Nei, J. Studies in molybdenum/manganese content in the dual body-centered-cubic phases metal hydride alloys. Int. J. Hydrog. Energy 2016, 41, 15277–15286. [Google Scholar] [CrossRef]
- Xu, W.; Angell, C.A. Solvent-free electrolytes with aqueous solution-like conductivities. Science 2003, 302, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, M.; Xu, W.; Angell, C.A. Ionic liquids by proton transfer: Vapor pressure, conductivity, and the relevance of ΔpKa from aqueous solutions. J. Am. Chem. Soc. 2003, 50, 15411–15419. [Google Scholar] [CrossRef] [PubMed]
- Fraser, K.J.; Izgorodina, E.I.; Forsyth, M.; Scott, J.L.; Macfarlane, D.R. Liquids intermediate between “molecular” and “ionic” liquids: Liquid ion pairs? Chem. Commun. 2007, 37, 3817–3819. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Forsyth, M.; Izgorodina, E.I.; Abbott, A.P.; Annat, G.; Fraser, K. On the concept of ionicity in ionic liquids. Phys. Chem. Chem. Phys. 2009, 11, 4962–4967. [Google Scholar] [CrossRef] [PubMed]
- Bonhote, P.; Dias, A.; Papageorgiou, N.; Kalyanasundaram, K.; Gratzel, M. Hydrophobic, highly conductive ambient-temperature molten salts. Inorg. Chem. 1996, 35, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhao, D.; Wen, L.; Yang, S.; Chen, X. Viscosity of ionic liquids: Database, observation, and quantitative structure-property relationship analysis. AIChE J. 2012, 58, 2885–2899. [Google Scholar] [CrossRef]
- Rocha, M.A.A.; Neves, C.M.S.S.; Freire, M.G.; Russina, O.; Triolo, A.; Coutinho, J.A.P.; Santos, L.M.N.B.F. Alkylimidazolium based ionic liquids: Impact of cation symmetry on their nanoscale structural organization. J. Phys. Chem. B 2013, 117, 10889–10897. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Voth, G.A. Unique spatial heterogeneity in ionic liquids. J. Am. Chem. Soc. 2005, 127, 12192–12193. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Kumar, P.; Samanta, A. On the optical properties of the imidazolium ionic liquids. J. Phys. Chem. B 2005, 109, 9148–9153. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.N.A.C.; Padua, A.A.H. Nanostructural organization in ionic liquids. J. Phys. Chem. B 2006, 110, 3330–3335. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Wang, J.; Han, L.; Fan, M. Measurements and correlation of viscosities and conductivities for the mixtures of imidazolium ionic liquids with molecular solutes. Chem. Eng. J. 2009, 147, 27–35. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, N. Determination of hydrogen diffusion coefficient in metal hydride electrode by cyclic voltammetry. J. Alloy. Compd. 2001, 316, 113–117. [Google Scholar] [CrossRef]
- Dippel, T.; Kreuer, K.D.; Lassegues, J.C.; Rodriguez, D. Proton conductivity in fused phosphoric acid; A 1H/31P PFG-NMR and QNS study. Solid State Ion. 1993, 61, 41–46. [Google Scholar] [CrossRef]
- Tliha, M.; Mathlouthi, H.; Khaldi, C.; Lamloumi, J.; Percheron-guegan, A. Electrochemical properties of the LaNi3.55Mn0.4Al0.3Co0.4Fe0.35 hydrogen storage alloy. J. Power Sources 2006, 160, 1391–1394. [Google Scholar] [CrossRef]
- Geng, M.; Feng, F.; Gamboa, S.A.; Sebastian, P.J.; Matchett, A.J.; Northwood, D.O. Electrocatalytic characteristics of the metal hydride electrode for advanced Ni/MH batteries. J. Power Sources 2001, 96, 90–93. [Google Scholar] [CrossRef]
- Li, X.; Dong, H.; Zhang, A.; Wei, Y. Electrochemical impedance and cyclic voltammetry characterization of a metal hydride electrode in alkaline electrolytes. J. Alloy. Compd. 2006, 426, 93–96. [Google Scholar] [CrossRef]
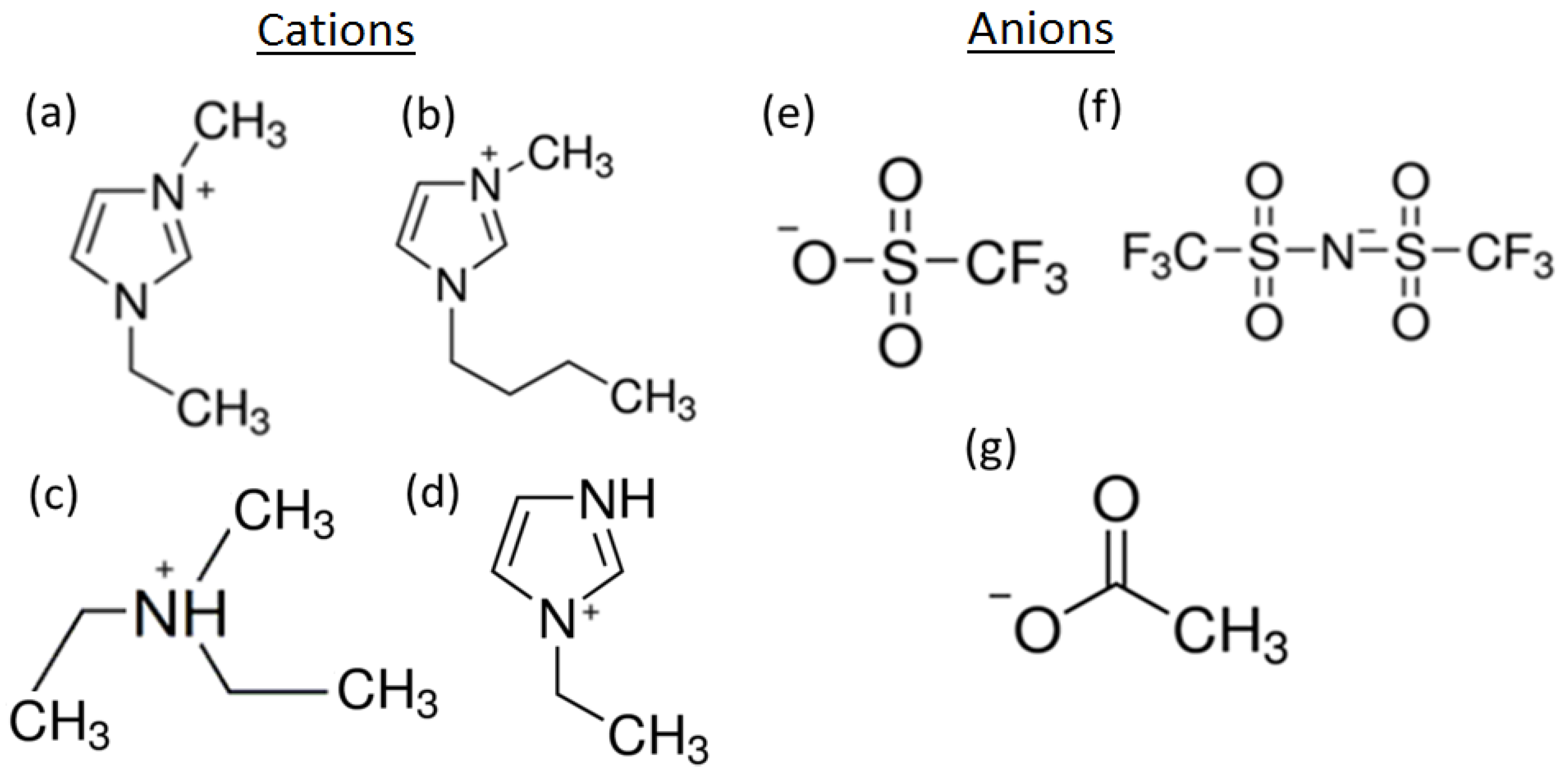

| IL’s Abbreviation | Tmelt (°C) | Viscosity (cP) | Electrochemical Window (V) | Conductivity at 25 °C (mS·cm−1) | References |
|---|---|---|---|---|---|
| [EMIM][TfO] | −9 | 45 | 4.1 | 8.6–11 | [25] |
| [BMIM][TfO] | 16 | 90 | - | 3.7 | [25] |
| [EMIM][TFSI] | −21~−3 | 28–34 | 4.0–4.5 | 5.7–8.8 | [25] |
| [BMIM][TFSI] | −4 | 52 | 4.6 | 3.9 | [25] |
| [EMIM][Ac] | <−20 | 93 | 3.2 | 2.5 | [26] |
| [BMIM][Ac] | <−20 | 554 | 3.1 | 1.1 | [26] |
| [EIM][TFSI] | - | 54 | - | 4 | [8] |
| [DEMA][TfO] | −6 | 19.4 | - | 55 * | [23,27] |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, T.; Young, K.-H.; Wong, D.F.; Nei, J. Ionic Liquid-Based Non-Aqueous Electrolytes for Nickel/Metal Hydride Batteries. Batteries 2017, 3, 4. https://doi.org/10.3390/batteries3010004
Meng T, Young K-H, Wong DF, Nei J. Ionic Liquid-Based Non-Aqueous Electrolytes for Nickel/Metal Hydride Batteries. Batteries. 2017; 3(1):4. https://doi.org/10.3390/batteries3010004
Chicago/Turabian StyleMeng, Tiejun, Kwo-Hsiung Young, Diana F. Wong, and Jean Nei. 2017. "Ionic Liquid-Based Non-Aqueous Electrolytes for Nickel/Metal Hydride Batteries" Batteries 3, no. 1: 4. https://doi.org/10.3390/batteries3010004