Influence of Using Metallic Na on the Interfacial and Transport Properties of Na-Ion Batteries
Abstract
:1. Introduction
2. Results and Discussion
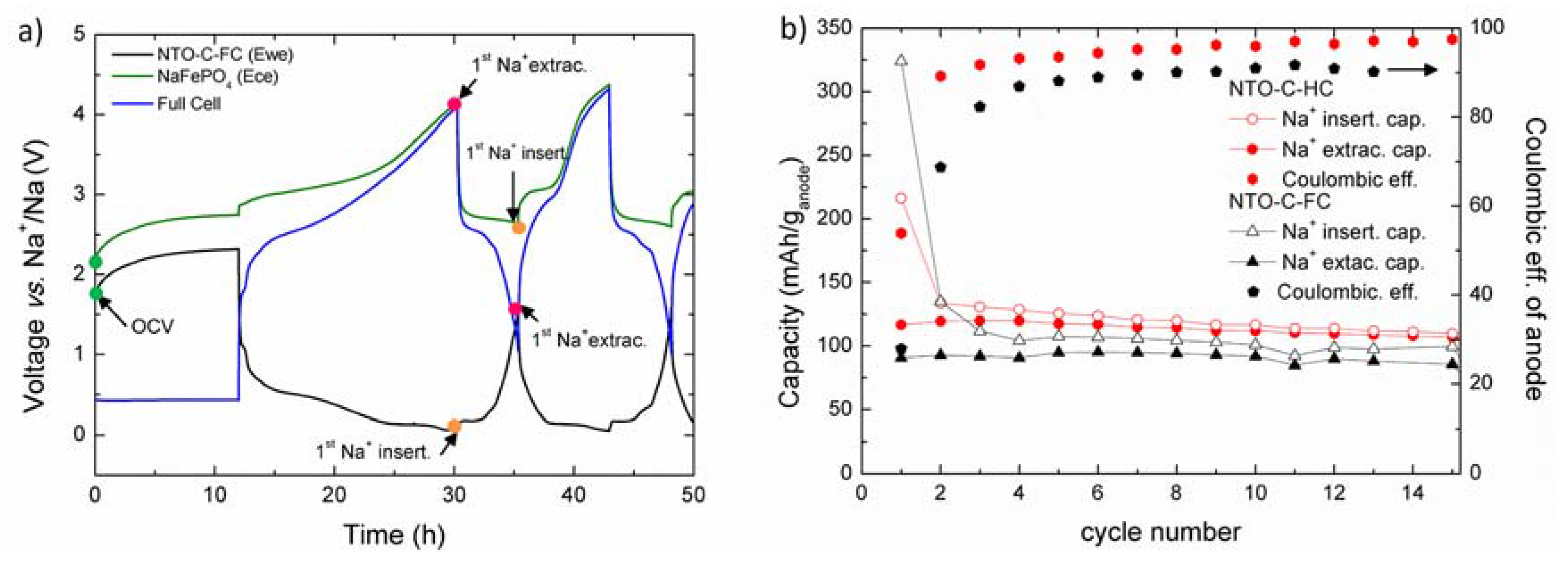
2.1. Galvanostatic Experiments in a Full-Cell
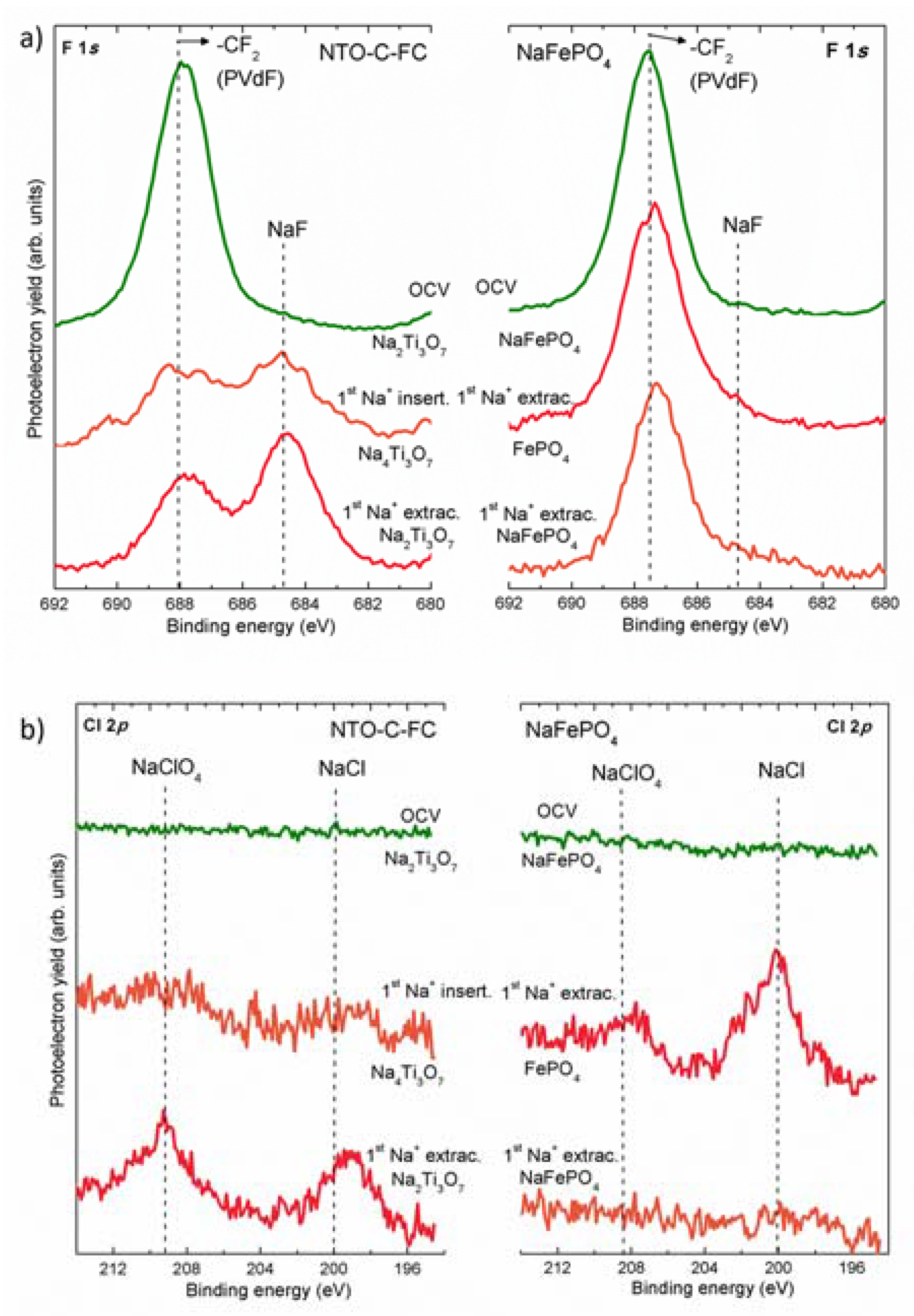
2.2. Study of the SEI/SPI Layers by Conventional XPS Experiments
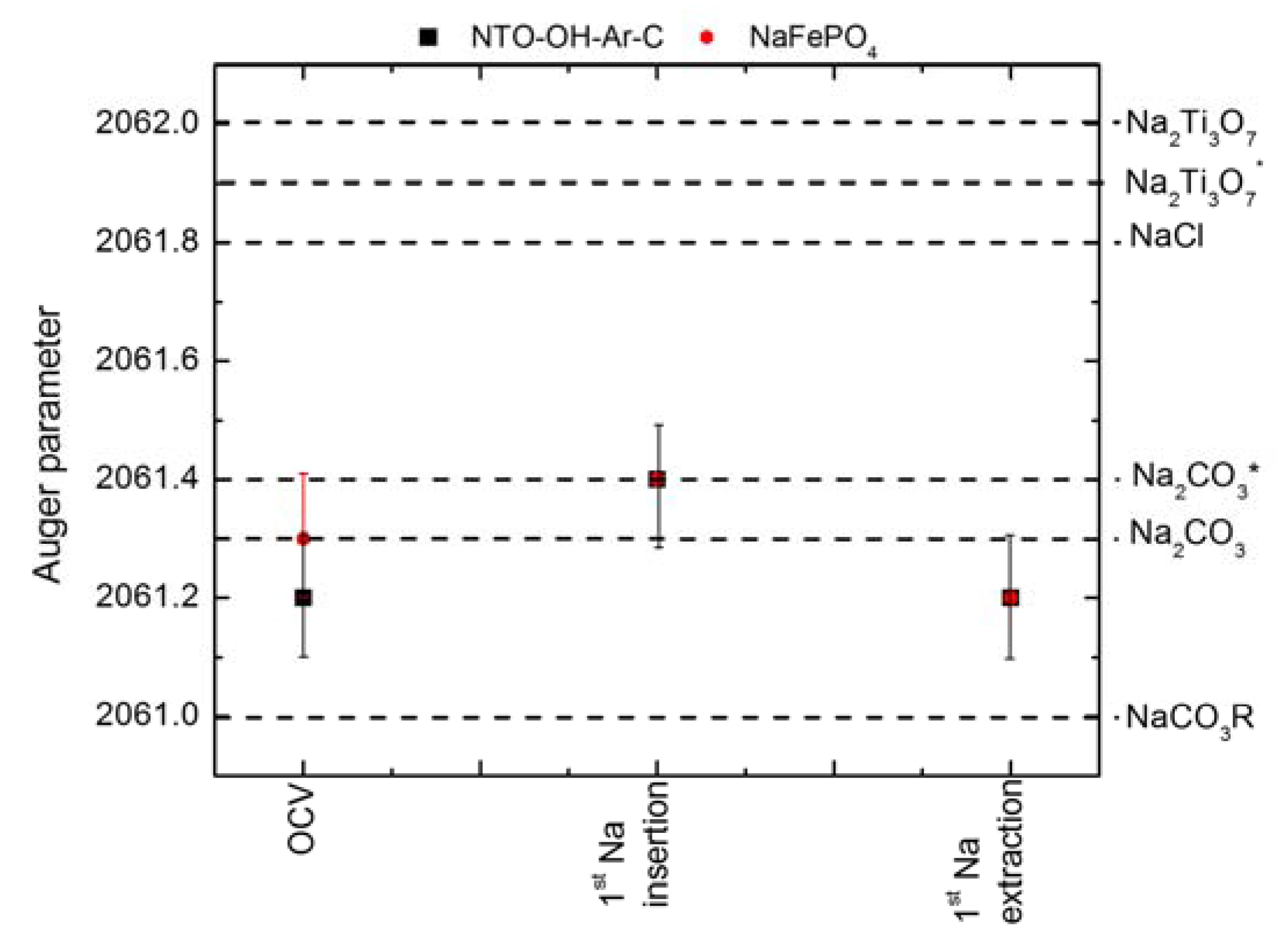
2.3. Auger Parameter Determination
2.4. Study of the Ionic/Electronic Properties by EIS
3. Materials and Methods
3.1. Synthesis of C-Coated Na2Ti3O7 and NaFePO4
3.2. Electrochemical Experiments
3.3. XPS Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A

| OCV (at. %) | 1st Na+ Extraction (at. %) | 1st Na+ Insertion (at. %) | |
|---|---|---|---|
| Graphite | 41.2 | 32.25 | 36.2 |
| Hydrocarbons | 20.3 | 23.3 | 28.7 |
| PEO | 16.7 | 18.0 | 18.6 |
| Na2-xCO3R + PVdF | 16.3 | 10.6 | 8.9 |
| NaCO3R | 5.5 | 12.8 | 7.6 |

| OCV (at. %) | 1st Na+ insertion (at. %) | 1st Na+ extraction (at. %) | |
|---|---|---|---|
| Na2Ti3O7 | 12.7 | 12.5 | 12.6 |
| Na2CO3 | 43.6 | 45.0 | 47.8 |
| NaCO3R | 38.0 | 37.6 | 36.3 |
| PEO | 5.7 | 4.9 | 3.3 |
References
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Palomares, V.; Casas-Cabanas, M.; Castillo-Martínez, E.; Han, M.H.; Rojo, T. Update on Na-based battery materials. A growing research path. Energy Environ. Sci. 2013, 6, 2312–2337. [Google Scholar] [CrossRef]
- Luo, W.; Shen, F.; Bommier, C.; Zhu, H.; Ji, X.; Hu, L. Na-ion battery anodes: materials and electrochemistry. Acc. Chem. Res. 2016, 49, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B.L.; Nazar, L.F. Sodium and sodium-ion energy storage batteries. Curr. Opin. Solid St. M. 2012, 16, 168–177. [Google Scholar] [CrossRef]
- Kang, H.; Liu, Y.; Cao, K.; Zhao, Y.; Jiao, L.; Wang, Y.; Yuan, H. Update on anode materials for Na-ion batteries. J. Mater. Chem. A 2015, 3, 17899–17913. [Google Scholar] [CrossRef]
- Kim, Y.; Ha, K.H.; Oh, S.M.; Lee, T. High-capacity anode materials for sodium-ion batteries. Chem. Eur. J. 2014, 20, 11980–11992. [Google Scholar] [CrossRef] [PubMed]
- Senguttuvan, P.; Rousse, G.; Seznec, V.; Tarascon, J.M.; Palacín, M.R. Na2Ti3O7: Lowest voltaje ever reported oxide insertion electrode for sodium ion batteries. Chem. Mater. 2011, 23, 4109–4111. [Google Scholar] [CrossRef]
- Doeff, M.M.; Cabana, J.; Shirpour, M. Titanate anodes for sodium ion batteries. J. Inorg. Organomet. Polym. 2014, 24, 5–14. [Google Scholar] [CrossRef]
- Rudola, A.; Saravanan, K.; Mason, C.W.; Balaya, P. Na2Ti3O7: An intercalation based anode for sodium-ion battery applications. J. Mater. Chem. A 2013, 1, 2653–2662. [Google Scholar] [CrossRef]
- Pan, H.; Lu, X.; Yu, X.; Hu, Y.S.; Li, H.; Yang, X.Q.; Chen, L. Sodium storage and transport properties in layered Na2Ti3O7 for room-temperature sodium-ion batteries. Adv. Energy Mater. 2013, 3, 1186–1194. [Google Scholar] [CrossRef]
- Nava-Avendaño, J.; Morales-García, A.; Ponrouch, A.; Rousse, G.; Frontera, C.; Senguttuvan, P.; Tarascon, J.M.; Arroyo-de Dompablo, M.E.; Palacín, M.R. Taking steps forward in understanding the electrochemical behavior of Na2Ti3O7. J. Mater. Chem. A 2015, 3, 22280–22286. [Google Scholar] [CrossRef]
- Zarrabeitia, M.; Castillo-Martínez, E.; López Del Amo, J.M.; Eguía-Barrio, A.; Muñoz-Márquez, M.A.; Rojo, T.; Casas-Cabanas, M. Identification of the critical synthesis parameters for enhanced cycling stability of Na-ion anode material Na2Ti3O7. Acta Mater. 2016, 104, 125–130. [Google Scholar] [CrossRef]
- Muñoz-Márquez, M.A.; Zarrabeitia, M.; Castillo-Martínez, E.; Eguía-Barrio, A.; Rojo, T.; Casas-Cabanas, M. Composition and evolution of the Solid Electrolyte Interphase in Na2Ti3O7 electrodes for Na-ion batteries: XPS and Auger parameter analysis. ACS Appl. Mater. Interfaces 2015, 7, 7801–7808. [Google Scholar] [CrossRef] [PubMed]
- Zarrabeitia, M.; Nobili, F.; Muñoz-Márquez, M.A.; Rojo, T.; Casas-Cabanas, M. Direct observation of electronic conductivity transitions and solid electrolyte interphase stability of Na2Ti3O7 electrodes for Na-ion batteries. J. Power Sources 2016, 330, 78–83. [Google Scholar] [CrossRef]
- Iermakova, D.I.; Dugas, R.; Palacín, M.R.; Ponrouch, A. On the comparative stability of Li and Na metal anode interfaces in conventional alkyl carbonate electrolytes. J. Electrochem. Soc. 2015, 162, A7060–A7066. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Komaba, S.; Murata, W.; Ishikawa, T.; Yabuuchi, N.; Ozeki, T.; Nakayama, T.; Ogata, A.; Gotoh, K.; Fujiware, K. Electrochemical Na insertion and Solid Electrolyte Interphase for Hard-Carbon electrodes and application to Na-ion batteries. Adv. Funct. Mater. 2011, 21, 3859–3867. [Google Scholar] [CrossRef]
- Kim, D.; Lee, E.; Slater, M.; Lu, W.; Rood, S.; Johnson, C.S. Layered Na[Ni1/3Fe1/3Mn1/3]O2 cathodes for Na-ion battery application. Electrochem. Commun. 2012, 18, 66–69. [Google Scholar] [CrossRef]
- Jian, Z.; Han, W.; Yang, H.; Hu, Y.S.; Zhou, J.; Zhou, Z.; Li, J.; Chen, W.; Chem, D.; Chen, L. Superior electrochemical performance and storage mechanism of Na3V2(PO4)3 cathode for room-temperature sodium-ion batteries. Adv. Energy Mater. 2013, 3, 156–160. [Google Scholar] [CrossRef]
- Ponrouch, A.; Dedryvère, R.; Monti, D.; Demet, A.E.; Ateba Mba, J.M.; Croguennec, L.; Masquelier, C.; Johansson, P.; Palacín, M.R. Towards high energy density sodium ion batteries through electrolyte optimization. Energy Environ. Sci. 2013, 6, 2361–2369. [Google Scholar] [CrossRef]
- Nose, M.; Nakayama, H.; Nobuhara, K.; Yamarguchi, H.; Nakanishi, S.; Iba, H. Na4Co3(PO4)2P2O7: a novel storage material for sodium-ion batteries. J. Power Sources 2013, 234, 175–179. [Google Scholar] [CrossRef]
- Xu, J.; Ma, C.; Balasubramanian, M.; Meng, Y.S. Understanding Na2Ti3O7 as an ultra-low voltaje anode material for a Na-ion battery. Chem. Commun. 2014, 50, 12564–12567. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Bates, A.; Schuppert, N.; Son, B.; Kim, J.G.; Choi, J.S.; Choi, M.J.; Lee, D.H.; Kwon, O.; Jasinski, J.; et al. A study of a novel NA ion battery and its anodic degradation using sodium rich Prussian blue cathode coupled with different titanium based oxide anodes. J. Power Sources 2015, 286, 276–289. [Google Scholar] [CrossRef]
- Blyth, R.I.R.; Buqa, H.; Netzer, F.P.; Ramset, M.G.; Besenhard, J.O.; Golob, P.; Winter, M. XPS studied of graphite electrode materials for lithium ion batteries. App. Surf. Sci. 2000, 167, 99–106. [Google Scholar] [CrossRef]
- Andersson, A.M.; Henningson, A.; Siegbahn, H.; Jansson, U.; Edström, K. Electrochemically lithiated graphite characterized by photoelectron spectroscopy. J. Power Sources 2003, 119, 522–527. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; John Wiley & Sons, Ltd: Chichester, UK, 1992. [Google Scholar]
- Bhide, A.; Hofmann, J.; Katharina Dürr, A.; Janek, J.; Adelhelm, P. Electrochemical stability of non-aqueous electrolytes for sodium-ion batteries and their compatibility with Na0.7CoO2. Phys. Chem. Chem. Phys. 2014, 16, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Membreño, N.; Park, K.; Goodenogh, J.B.; Stevenson, K.J. Electrode/electrolyte interface of composite α-Li3V2(PO4)3 cathodes in a nonaqueous electrolyte for lithium ion batteries and the role of the carbon additive. Chem. Mater. 2015, 27, 3332–3340. [Google Scholar] [CrossRef]
- Tanuma, S. Calculations of electron inelastic mean free paths. Surf. Interface Anal. 1991, 17, 911–926. [Google Scholar] [CrossRef]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of electron inelastic mean free paths. IX. Data for 41 elemental solids over the 50 eV to 30 KeV range. Surf. Interface Anal. 2011, 43, 689–713. [Google Scholar] [CrossRef]
- Malmgren, S.; Ciosek, K.; Hahlin, M.; Gustafsson, T.; Gorgoi, M.; Rensmo, H. Comparing anode and cathode electrode/electrolyte interface composition an morphology using soft and hard X-ray photoelectron spectroscopy. Electrochim. Acta 2013, 97, 23–32. [Google Scholar] [CrossRef]
- Ji, L.; Gu, M.; Shao, Y.; Li, X.; Engelhard, M.H.; Arey, B.W.; Wang, W.; Nie, Z.; Xiao, J.; Wang, C.; et al. Controlling SEI formation on SnSb-porous carbon nanofibres for improved Na ion storage. Adv. Mater. 2014, 26, 2901–2908. [Google Scholar] [CrossRef] [PubMed]
- Bodenes, L.; Darwiche, A.; Monconduit, L.; Martínez, H. The solid electrolyte interphase a key parameter of the high performance of Sb in sodium-ion batteries: comparative X-ray photoelectron spectroscopy study of Sb/Na-ion and Sb/Li-ion batteries. J. Power Sources 2015, 273, 14–24. [Google Scholar]
- Lahiri, A.; Olschewski, M.; Gustus, R.; Borisenko, N.; Endres, F. Surface modification of battery electrodes via electroless deposition with improved performance for Na-ion batteries. Phys. Chem. Chem. Phys. 2016, 18, 14782–14786. [Google Scholar] [CrossRef] [PubMed]
- Doubaji, S.; Philippe, B.; Saadoune, I.; Gorgoi, M.; Gustafsson, T.; Solhy, A.; Valvo, M.; Rensmo, H.; Edström, K. Passivation layer and cathodic redox reactions in sodium-ion batteries probed by HAXPES. ChemSusChem. 2016, 9, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Cherkashinin, G.; Nikolwski, K.; Ehrenberg, H.; Jacke, S.; Dimesso, L.; Jaegermann, W. The stability of the SEI layer, surface composition and the oxidation state of transition metals at the electrolyte-cathode interface impacted by electrochemical cycling: X-ray photoelectron spectroscopy investigation. Phys. Chem. Chem. Phys. 2012, 14, 12321–12331. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.D.; Joshi, A. The Auger parameter, its utility and advantages: A review. J. Electron. Spectrosc. 1988, 47, 283–313. [Google Scholar] [CrossRef]
- Walton, J.; Wincott, P.; Fairley, N.; Carrick, A. Peak Fitting with CasaXPS: A Casa Pocket Book; Accolyte Sicence: Knutsford, UK, 2010. [Google Scholar]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation, Physical Electronics Division: Eden Prairie, Minnesota, 1979; p. 170. [Google Scholar]
- NIST X-ray Photoelectron Spectroscopy Database. Available online: http://srdata.nist.gov/xps (accessed on 28 March 2017).
- Boukamp, B.A. A nonlinear least squares fit procedure for analysis of immittance data of electrochemical systems. Solid State Ion. 1986, 20, 31–44. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Batteries. In Impedance Spectroscopy Theory, Experiment and Application, 2nd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Casas-Cabanas, M.; Rodattis, V.V.; Saurel, D.; Kubiak, P.; Carretero-González, J.; Palomares, V.; Serras, P.; Rojo, T. Crystal chemistry of Na insertion/deinsertion in FePO4-NaFePO4. J. Mater. Chem. 2012, 22, 17421–171423. [Google Scholar] [CrossRef]
- Xia, X.; Obrovac, M.N.; Dahn, J.R. Comparison of the reactivity of NaxC6 and LixC6 with non-aqueous solvents and electrolytes. Electrochem. Solid-State Lett. 2011, 14, A130–A133. [Google Scholar] [CrossRef]
- Doeff, M.M.; Ma, Y.; Visco, S.J.; De Jonghe, L.C. Electrochemical insertion of sodium into carbon. J. Electrochem. Soc. 1993, 140, L169–L170. [Google Scholar] [CrossRef]
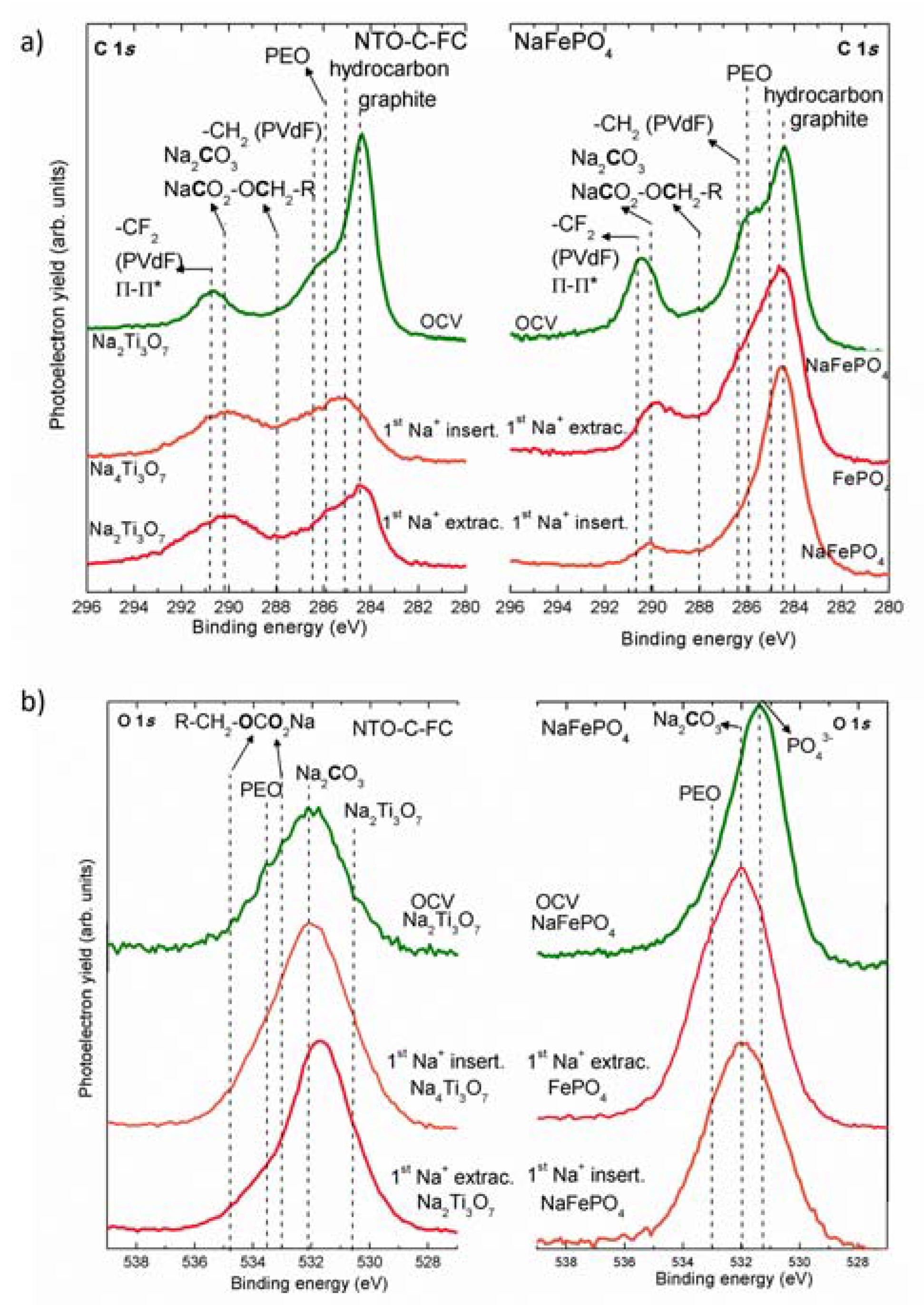

| Species | Binding Energy (eV) | Peak Evolution | |
|---|---|---|---|
| C 1s | O 1s | ||
| Graphitic-like | 284.4 | -- | Decreases during Na+ insertion |
| Na2Ti3O7 | -- | 531 | Constant at all states |
| PEO | 286 | 533 | More at Na+ insertion than at extraction |
| Na2CO3 | 290–291 | 532 | More formation upon Na+ insertion and remains constant during the extraction |
| NaCO3R | 288 & 290–291 | 534 & 532.5 | Increases upon Na+ insertion |
| –CF2 (PVdF) | 290–291 | -- | Observed at all states but probably decreases upon Na+ insertion |
| V vs. Na+/Na | Rele (Ohm/mg) | RCcoating (Ohm/mg) | RCT (Ohm/mg) | RSEI (Ohm/mg) | Rsol (Ohm/mg) | χ2 |
|---|---|---|---|---|---|---|
| 1.0 | 46,921.2 | 682.5 | 149.3 | 75.9 | 2.5 | 1.9 × 10−3 |
| 0.90 | 38,215.3 | 1372.5 | 143.0 | 69.3 | 2.4 | 2.23 × 10−3 |
| 0.77 | 14,096.1 | 1003.4 | 117.2 | 112.1 | 2.1 | 1.51 × 10−3 |
| 0.65 | 1108.4 | 572.6 | 114.9 | 126.9 | 2.3 | 1.72×10−3 |
| 0.52 | 885.0 | 396.8 | 140.2 | 86.7 | 2.5 | 1.97 × 10−3 |
| 0.40 | 579.9 | 409.8 | 124.2 | 105.1 | 2.5 | 1.27 × 10−3 |
| 0.27 | 237.6 | 309.8 | 108.9 | 79.8 | 2.5 | 8.7 × 10−4 |
| 0.15 | 123.3 | 189.7 | 201.8 | 57.8 | 2.3 | 5.9 × 10−5 |
| 0.05 | 132.0 | 135.7 | 217.8 | 89.5 | 2.8 | 4.5 × 10−3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarrabeitia, M.; Muñoz-Márquez, M.Á.; Nobili, F.; Rojo, T.; Casas-Cabanas, M. Influence of Using Metallic Na on the Interfacial and Transport Properties of Na-Ion Batteries. Batteries 2017, 3, 16. https://doi.org/10.3390/batteries3020016
Zarrabeitia M, Muñoz-Márquez MÁ, Nobili F, Rojo T, Casas-Cabanas M. Influence of Using Metallic Na on the Interfacial and Transport Properties of Na-Ion Batteries. Batteries. 2017; 3(2):16. https://doi.org/10.3390/batteries3020016
Chicago/Turabian StyleZarrabeitia, Maider, Miguel Ángel Muñoz-Márquez, Francesco Nobili, Teófilo Rojo, and Montse Casas-Cabanas. 2017. "Influence of Using Metallic Na on the Interfacial and Transport Properties of Na-Ion Batteries" Batteries 3, no. 2: 16. https://doi.org/10.3390/batteries3020016






