Reviews of European Patents on Nickel/Metal Hydride Batteries
Abstract
:1. Introduction
2. Ni/MH Battery Manufacturer
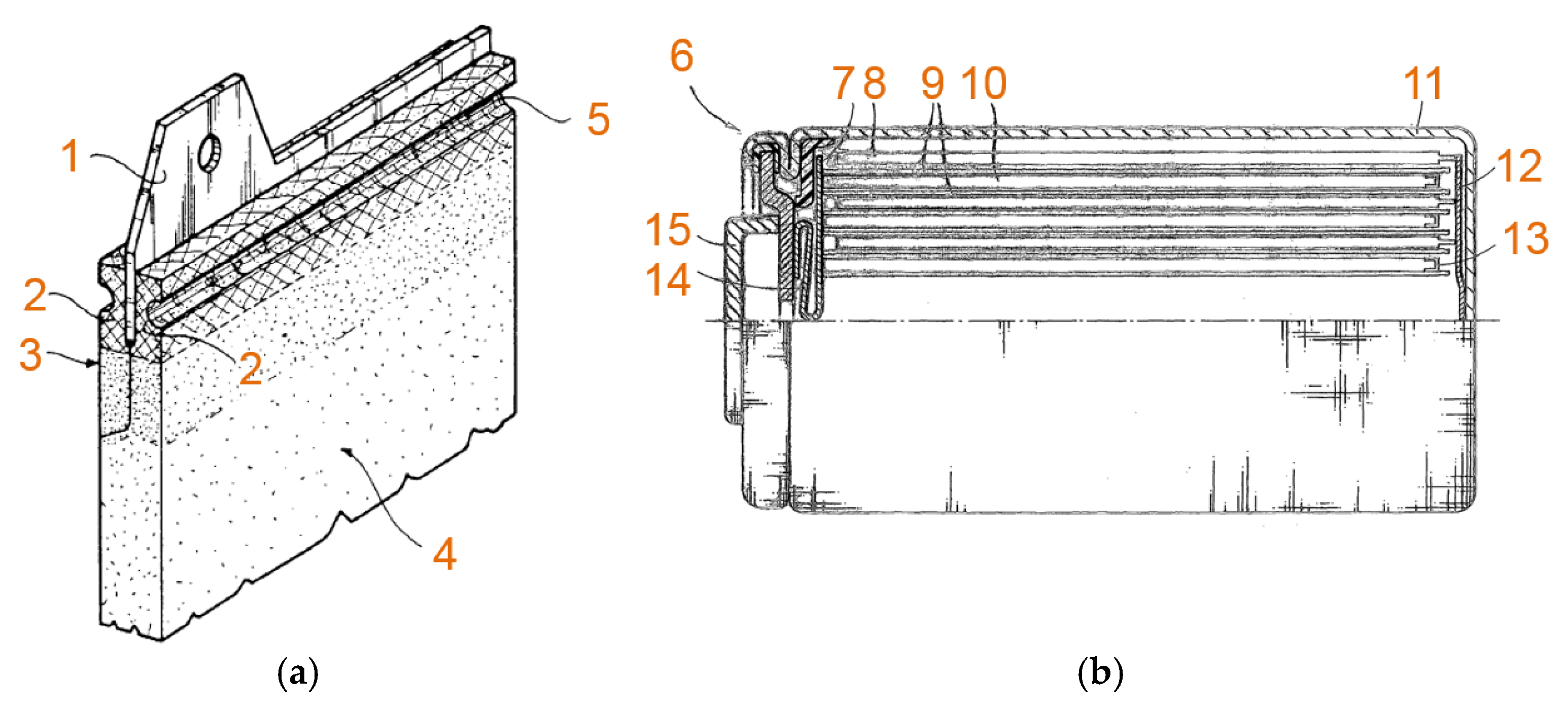
2.1. SAFT and Arts Energy
2.2. Varta and Varta Microbattery
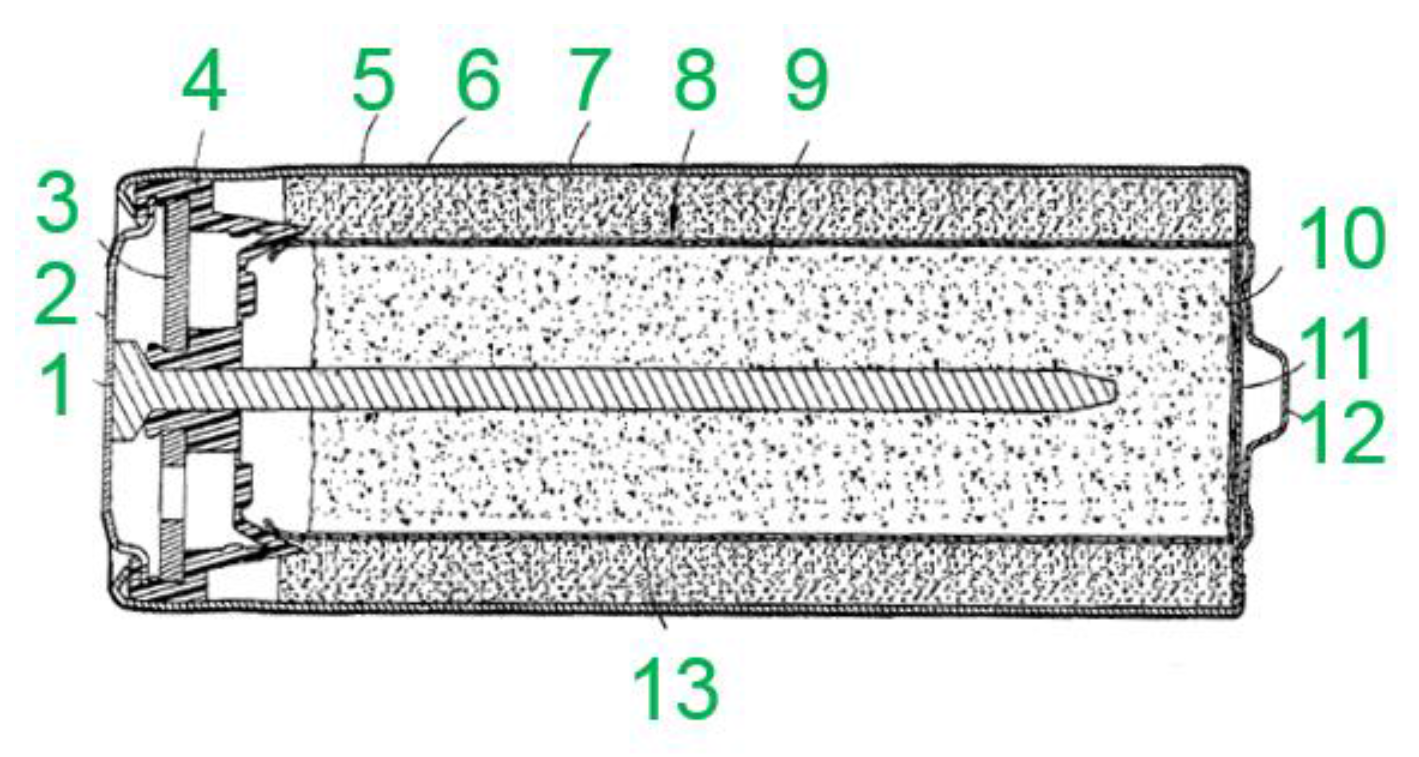
2.3. Alcatel
2.4. Nilar
2.5. Hoppecke
3. MH Alloy Suppliers
3.1. GfE
3.2. Treibacher
4. Separator Suppliers
4.1. Scimat
4.2. Freudenberg
5. Other Companies
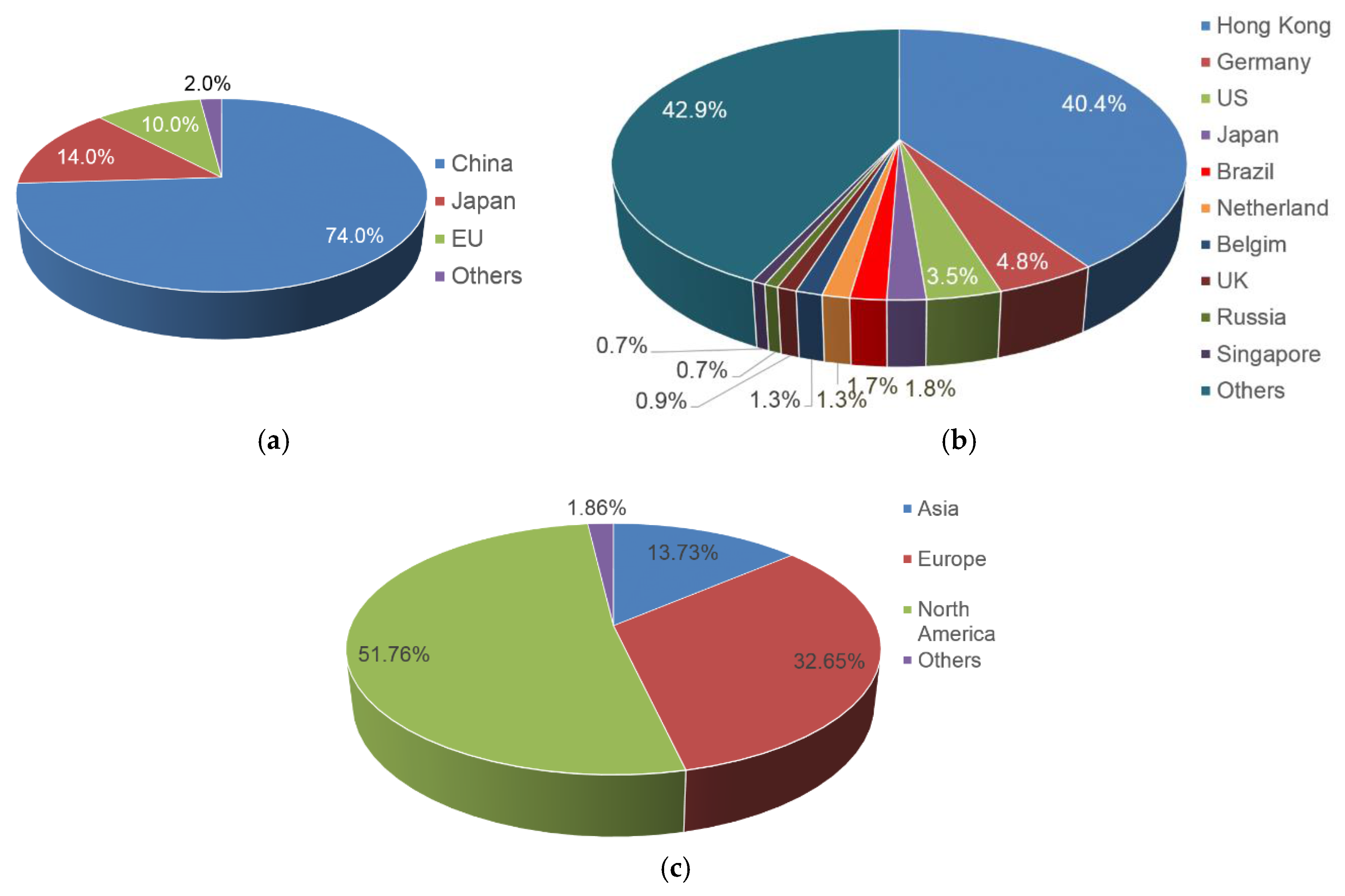
6. Comparisons to Other Countries
7. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| Ni/MH | Nickel/metal hydride |
| HEV | Hybrid electric vehicle |
| MH | Metal hydride |
| WIPO | World Intellectual Property Organization |
| EPA | European Patent Application |
| EV | Electric vehicle |
| PCT | Patent Cooperation Treaty |
| PTFE | Polytetrafluoroethylene |
| SOC | State-of-charge |
References
- Battery and Energy Technologies. Available online: http://www.mpoweruk.com/nimh.htm (accessed on 25 May 2017).
- Bai, W. The Current Status and Future Trends of Domestic and Foreign NiMH Battery Market. Available online: http://cbea.com/u/cms/www/201406/06163842rc0l.pdf (accessed on 25 May 2017).
- Beccu, K.-D. Electrical Accumulator with a Metal Hydride Serving as the Cathodic Reactive Material Arranged in Suspension in the Electrolyte. U.S. Patent 3,520,728, 14 July 1970. [Google Scholar]
- Percheron-Guégen, A.; Achard, J.C.; Loriers, J.; Bonnemay, M.; Bronoёl, G.; Sarradin, J.; Schlapbach, L. Electrode Materials Based on Lanthanum and Nickel, and Electrochemical Uses of Such Materials. U.S. Patent 4,107,405, 15 August 1978. [Google Scholar]
- Willems, J.J.G.S.A.; van Beek, J.R.G.C.M.; Buschow, K.H.J. Electrochemical cell comprising stable hydride-forming material. U.S. Patent 4,487,817, 11 December 1984. [Google Scholar]
- Chang, S.; Young, K.; Nei, J.; Fierro, C. Reviews on the U.S. Patents regarding nickel/metal hydride batteries. Batteries 2016, 2, 10. [Google Scholar] [CrossRef]
- Ouchi, T.; Young, K.; Moghe, D. Reviews on the Japanese Patent Applications regarding nickel/metal hydride batteries. Batteries 2016, 2, 21. [Google Scholar] [CrossRef]
- Young, K.; Cai, X.; Chang, S. Reviews on the Chinese Patent regarding Nickel/metal hydride battery. Batteries 2017, 3, 24. [Google Scholar] [CrossRef]
- World Intellectual Property Organization. Available online: https://patentscope.wipo.int/search/en/search.jsf (accessed on 23 May 2017).
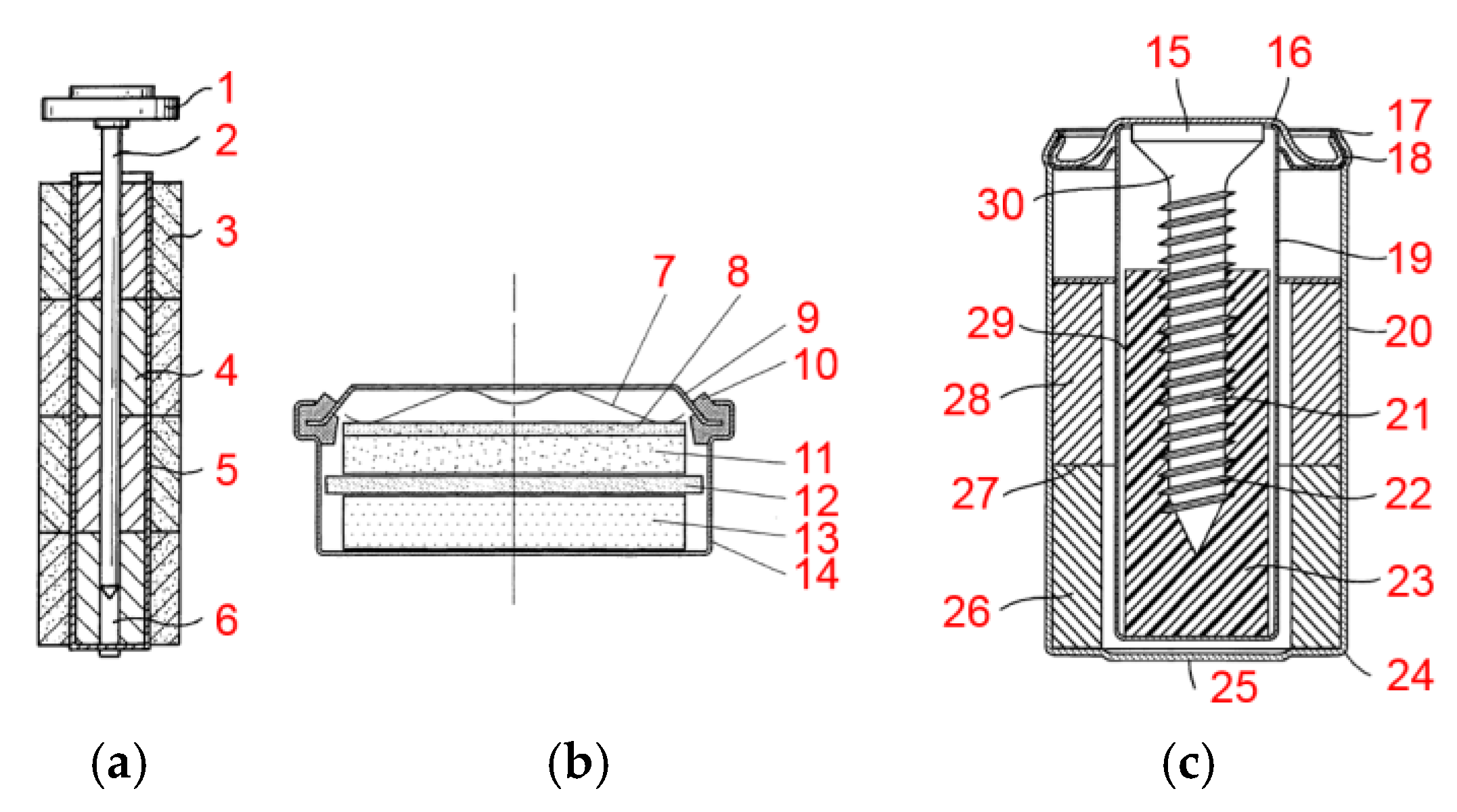
- Blanchard, P.; Klein, J.P. Process for the Preparation of a Metal Hydride Powder and the Powder Obtained. Eur. Pat. Appl. 91401640, 18 June 1991. [Google Scholar]
- Bernard, P.; Audry, C.; Lecerf, A.; Senyarich, S. Nickel Electrode for Alcaline Accumulator. Eur. Pat. Appl. 96400423, 28 February 1996. [Google Scholar]
- Bernard, P.; Dennig, C.; Cocciantelli, J.-M.; Coco, I.; Alcorta, J. Non-Sintered Nickel Electrode. Eur. Pat. Appl. 99400049, 11 January 1999. [Google Scholar]
- Bernard, P.; Simonneau, O.; Bertrand, F. Pasted Nickel Electrode. Eur. Pat. Appl. 97401011, 5 May 1997. [Google Scholar]
- Bernard, P.; Goubault, L.; Guiader, O. Positive Electrode for an Electrochemical Generator with an Alkaline Electrolyte. Eur. Pat. Appl. 08290869, 16 September 2008. [Google Scholar]
- Bernard, P.; Audry, C. Conductive Material for Electrode of Secondary Battery with Alkali Electrolyte. Eur. Pat. Appl. 01401672, 25 June 2001. [Google Scholar]
- Chevalier, S.; Debdary, M.; Desprez, P. Negative Electrode for an Asymmetric Supercapacitor with Nickel Hydroxide Positive Electrode and Alkali Electrolyte and Method for Manufacturing Same. Eur. Pat. Appl. 12167763, 11 May 2012. [Google Scholar]
- Bernard, P.; Baudry, M.; Jan, O.; Audry, C. Non-Sintered Nickel Electrode for Secondary Battery with Alkali Electrolyte. Eur. Pat. Appl. 99403260, 23 December 1999. [Google Scholar]
- Bernard, P.; Goubault, L.; Hezeque, T. Electrochemical Alkaline Generator with Improved Service Life. Eur. Pat. Appl. 05291300, 17 June 2005. [Google Scholar]
- Bernard, P.; Goubault, L.; Gillot, S. Electrode for Alkaline Storage Battery. Eur. Pat. Appl. 12175342, 6 July 2012. [Google Scholar]
- Bernard, P. Positive Electrode for Alkaline Battery. Eur. Pat. Appl. 06291402, 5 September 2006. [Google Scholar]
- Coco, I.; Cocciantelli, J.-M.; Villenave, J.-J. Electrode of non Sintered Type for Accumulator with Alkaline Electrolyte. Eur. Pat. Appl. 97401723, 17 July 1997. [Google Scholar]
- Bernard, P.; Goubault, L. Plastified Electrode for Alkaline Battery. Eur. Pat. Appl. 07848215, 11 September 2007. [Google Scholar]
- Bernard, P.; Goubault, L.; Gillot, S.; Feugnet, T. Plastic-Coated Electrode for Alkaline Storage Battery. Eur. Pat. Appl. 10162920, 17 May 2010. [Google Scholar]
- Bouet, J.; Knosp, B.; Percheron-Guegan, A.; Jordy, C. Hydridable Material for a Nickel-Hydride Battery Negative Electrode and Process for Production. Eur. Pat. Appl. 93401399, 2 June 1993. [Google Scholar]
- Bouet, J.; Knosp, B.; Percheron-Guegan, A.; Canet, O. Hydridable Material for a Nickel-Hydride Battery Negative Electrode. Eur. Pat. Appl. 93402198, 9 September 1993. [Google Scholar]
- Bouet, J.; Knosp, B.; Percheron-Guegan, A.; Cocciantelli, J.-M. Hydridable Material for Nickel-Hydride Accumulator Negative Electrode. Eur. Pat. Appl. 0608646, 3 August 1994. [Google Scholar]
- Bernard, P.; Knosp, B.; Baudry, M. Composition for Negative Electrode of Accumulator with Alkaline Electrolyte. Eur. Pat. Appl. 07291035, 23 August 2007. [Google Scholar]
- Lavaud, C.; Dillay, V.; Bernard, P. Negative Active Material for Hydride metal Nickel Accumulator. Eur. Pat. Appl. 08012162, 4 July 2008. [Google Scholar]
- Bernard, P.; Knosp, B.; Latroche, M.; Zhang, J.; Serin, V.; Hytch, M. Active Material for a Negative Electrode of a Nickel-Metal Hydride Alkaline Accumulator. Eur. Pat. Appl. 11188182, 8 November 2011. [Google Scholar]
- Bernard, P.; Knosp, B.; Baudry, M. Active Material Composition and Alkaline Electrolyte Accumulator. Eur. Pat. Appl. 06290808, 18 May 2006. [Google Scholar]
- Bernard, P.; Knosp, B.; Latroche, M.; Ferey, A. Hydridable Alloy for Alkaline Storage Battery. Eur. Pat. Appl. 07290197, 16 February 2007. [Google Scholar]
- Senyarich, S.; Cocciantelli, J.-M. Hydrophylic Electrode for Alkaline Accumulator and Method of Preparation. Eur. Pat. Appl. 97402510, 23 October 1997. [Google Scholar]
- Sjövall, R.; Greis, M. Low Maintenance Alkaline Electrochemical Cell. Eur. Pat. Appl. 12190882, 31 October 2012. [Google Scholar]
- Coco, I.; Cocciantelli, J.-M.; Villenave, J.-J. Negative Electrode for Ni-Metal Hydride Accumulator. Eur. Pat. Appl. 96400850, 22 April 1996. [Google Scholar]
- Grange-Cossou, M.; Torregrossa, W. Process of Manufacturing an Electrode with Spongiform Support for Electrochemical Generator and Electrode Obtained Thereby. Eur. Pat. Appl. 90117834, 17 September 1990. [Google Scholar]
- Caillon, G.; Lebarbier, C. Method for Covering an Electrode with a Sponge like Support for Electrochemical Generator and Electrode Obtained by This Process. Eur. Pat. Appl. 90121037, 2 November 1990. [Google Scholar]
- Guerinault, J.-M.; Brunarie, J. Method for Joining a Metallic Current Collector to an Electrode Comprising a Spongeous Supporting Structure for Electrochemical Generator and Electrode Obtained Thereby. Eur. Pat. Appl. 91121081, 9 December 1991. [Google Scholar]
- Grange-Cossou, M.; Guerinault, J.-M.; Carteau, B.; Brunarie, J. Method for Joining a Metallic Collector to an Electrode Comprising a Spongeous Supporting Structure for Electrochemical Generator and Electrode Obtained Thereby. Eur. Pat. Appl. 92401446, 26 May 1992. [Google Scholar]
- Bouet, J.; Pichon, B. Process for Making a Foam-Type Support for an Electrode of a Secondary Battery. Eur. Pat. Appl. 92401861, 30 June 1992. [Google Scholar]
- Fradin, J. Cylindrical Electrochemical Generator with Spirally Wounded Electrodes. Eur. Pat. Appl. 97401780, 24 July 1997. [Google Scholar]
- Goubault, L.; Bernard, P.; Gillot, S. Plastic-Coated Electrode for Alkaline Storage Battery. Eur. Pat. Appl. 14170069, 27 May 2014. [Google Scholar]
- Gineste, J.-L.; Pourcelly, G.; Brunea, J.; Perton, F.; Broussely, M. Grafted Microporous Separator for Electrochemical Generator and Its Manufacturing Process. Eur. Pat. Appl. 93402037, 10 August 1993. [Google Scholar]
- Caillon, G.; Crochepierre, B. Alkaline Open Battery Comprising a Microporous Membrane. Eur. Pat. Appl. 06290869, 30 May 2006. [Google Scholar]
- Green, A.; Champalle, P.; Liska, J.-L. Separator for Alkaline Accumulator. Eur. Pat. Appl. 91402721, 11 October 1991. [Google Scholar]
- Senyarich, S.; Leblanc, P. Separator for Accumulator with Spirally Wounded Electrodes and Alkaline Electrolyte. Eur. Pat. Appl. 97401667, 10 July 1997. [Google Scholar]
- Senyarich, S.; Viaud, P. Alkaline Electrolyte Secondary Battery. Eur. Pat. Appl. 97402264, 29 September 1997. [Google Scholar]
- Berlureau, T.; Liska, J.-L. Method for Charging Maintenance-Free Nickel Metal Hydride Batteries. Eur. Pat. Appl. 98400455, 26 February 1998. [Google Scholar]
- Berlureau, T.; Bariand, M.; Liska, J.-L. Method for Controlling the Rapid Charging of an Alkaline Electrolyte Industrial Accumulator. Eur. Pat. Appl. 99401903, 26 July 1999. [Google Scholar]
- Gardes, F.; Juan, A.; Maloizel, S. Method for Managing the Charge of a Battery. Eur. Pat. Appl. 08291208, 18 December 2008. [Google Scholar]
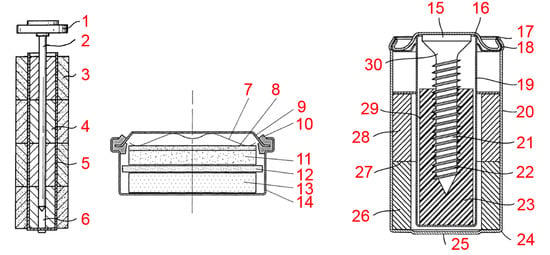
- Vasti, P.; Tridon, X. Leaktight Box for Alkaline Batteries. Eur. Pat. Appl. 13773814, 9 October 2013. [Google Scholar]
- Payen, S.; Raymond, A. A Resealable Valve and Electrochemical Generator Comprising Said Valve. Eur. Pat. Appl. 01402994, 22 November 2001. [Google Scholar]
- Vigier, N.; Besse, S. Electrochemical Generator with Surface of Revolution. Eur. Pat. Appl. 03290969, 18 April 2003. [Google Scholar]
- Arts Energy. Available online: http://www.arts-energy.com/ (accessed on 25 May 2017).
- Lichtenberg, F.; Kleinsorgen, K.; Hofmann, G. Nickel/Metallic Hydride Secondary Cell. Eur. Pat. Appl. 94116439, 19 October 1994. [Google Scholar]
- Lichtenberg, F.; Kleinsorgen, K.; Hofmann, G. Electrical Nickel-Metal Hydride Accumulator with Electrode of Nickelhydroxide Comprising Graphite. Eur. Pat. Appl. 94115951, 10 October 1994. [Google Scholar]
- Klaus, C. Gastight Alkaline Button-Type Accumulator. Eur. Pat. Appl. 94116622, 21 October 1994. [Google Scholar]
- Klaus, C.; Simon, G.; Koehler, U.; Kannler, H. Gastight Nickel/Hydride-Accumulation of Round Cell Type. Eur. Pat. Appl. 95194924, 18 March 1995. [Google Scholar]
- Koehler, U.; Chen, G.; Lindner, J. Gastight Metaloxide-Metalhydride Accumulator. Eur. Pat. Appl. 95106832, 5 May 1995. [Google Scholar]
- Koehler, U.; Klaus, C.; Hofmann, G.; Lichtenberg, F. Alkaline Gastight Accumulator in the Shape of a Button Cell. Eur. Pat. Appl. 95106833, 5 May 1995. [Google Scholar]
- Lichtenberg, F.; Koehler, U.; Kleinsorgen, K.; Foelzer, A.; Bouvier, A. Alkaline Metal Oxide, Metal Hydride Battery. Eur. Pat. Appl. 96102419, 17 February 1996. [Google Scholar]
- Lichtenberg, F. Alloys for Use as Active Material for the Negative Electrode of an Alkaline, Rechargeable, Nickel Metal-Hybride Battery and Its Method of Preparation. Eur. Pat. Appl. 96110281, 26 June 1996. [Google Scholar]
- Kleinsorgen, K.; Koehler, U.; Bouvier, A.; Foelzer, A. Process for Recovery of Metals from Used Nickel-Metal Hydride Accumulators. Eur. Pat. Appl. 95940265, 1 December 1995. [Google Scholar]
- Kleinsorgen, K.; Koehler, U.; Bouvier, A.; Foelzer, A. Process for Recovery of Metals from Used Nickel-Metal Hydride Accumulators. Eur. Pat. Appl. 95941642, 1 December 1995. [Google Scholar]
- Brenner, R. Electrochemical Cell with a Positive Electrode in the Form of a Hollow Cylinder and a Negative Electrode Assembled Therein. Eur. Pat. Appl. 13178468, 30 July 2013. [Google Scholar]
- Baeuerlein, P. Nickel-Metal Hydride Secondary Battery. Eur. Pat. Appl. 01116524, 7 July 2001. [Google Scholar]
- Knop, I. Charging Device Adapted for Charging NiMeH Batteries. Eur. Pat. Appl. 98102036, 6 February 1998. [Google Scholar]
- Schein, H.; Kron, N.; Ilic, D. Mobile Charger for Charging Secondary Batteries from Secondary Batteries. Eur. Pat. Appl. 06776545, 2 August 2006. [Google Scholar]
- Schula, C.; Scholz, S.; Pytlik, E.; Ensling, D. Secondary Electrochemical Cell and Charging Method. Eur. Pat. Appl. 15735963, 9 July 2015. [Google Scholar]
- Schula, C.; Scholz, S.; Pytlik, E.; Ensling, D. Secondary Electrochemical Cell and Charging Method. Eur. Pat. Appl. 15735964, 9 July 2015. [Google Scholar]
- VARTA. Available online: http://www.varta-microbattery.com (accessed on 25 May 2017).
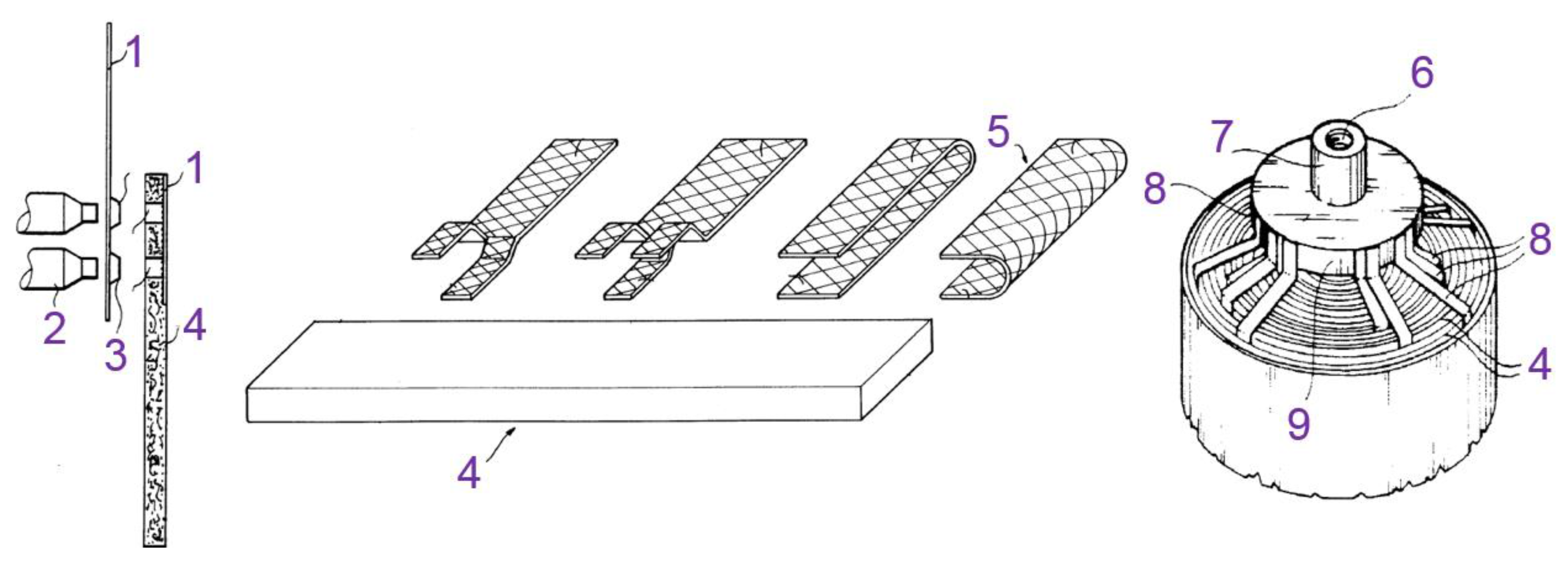
- Loustau, M.-T.; Verhoog, R.; Precigout, C. Method for Joining a Metallic Current Collector to an Electrode Comprising a Fibrous or a Spongeous Supporting Structure for Electrochemical Generator and Electrode Obtained Thereby. Eur. Pat. Appl. 94401155, 25 May 1994. [Google Scholar]
- Amiel, O.; Belkhir, I.; Freluche, J.-P.; Pineau, N.; Dupuy, C.; Babin, S. Non-Sintered Electrode with Tridimensional Support for Secondary Battery with Alcaline Electrolyte. Eur. Pat. Appl. 00403112, 9 November 2000. [Google Scholar]
- Cuesta, R.; Rouverand, C. Charge Control Method of a Sealed Nickel Storage Cell and Charger Therefor. Eur. Pat. Appl. 94401127, 20 May 1994. [Google Scholar]
- Knosp, B.; Mimoun, M.; Bouet, J.; Gicquel, D.; Jordy, C. Hydridable Material for Nickel-Hydride Accumulator Negative Electrode. Eur. Pat. Appl. 95400803, 10 April 1995. [Google Scholar]
- Cypel, L. Metal Hydride Negative Electrode Comprising a Coated Perforated Sheet. Eur. Pat. Appl. 98401150, 14 May 1998. [Google Scholar]
- Senyarich, S.; Viaud, P. Alkaline Electrolyte Secondary Battery, Particularly of the Type nickel-Cadmium or Nickel Metal Hydride. Eur. Pat. Appl. 98402291, 17 September 1998. [Google Scholar]
- Berlureau, T.; Liska, J.-L. Sealed Nickel-Metal Hydride Storage Battery. Eur. Pat. Appl. 00402019, 13 July 2000. [Google Scholar]
- Bernard, P.; Goubault, L.; Le, G.L. Electrochemically Active Material for Negative Electrode of an Alkaline Electrolyte Secondary Battery. Eur. Pat. Appl. 03290119, 17 January 2003. [Google Scholar]
- Alcatel-Lucent. Available online: https://en.wikipedia.org/wiki/Alcatel-Lucent (accessed on 29 July 2017).
- Fredriksson, L.; Puester, N. A Method for Manufacturing a Biplate Assembly, a Biplate Assembly and a Bipolar Battery. Eur. Pat. Appl. 02746283, 8 July 2002. [Google Scholar]
- Hug, L.; Puester, N.H.; Fredriksson, L. An Apparatus for Manufacturing an Electrode. Eur. Pat. Appl. 03770206, 7 November 2003. [Google Scholar]
- Fredriksson, L.; Puester, N. A Bipolar Battery and a Biplate assembly. Eur. Pat. Appl. 02798876, 13 September 2002. [Google Scholar]
- Fredriksson, L.; Puester, N. A Bipolar Battery, a Method for Manufacturing a Bipolar Battery and a Biplate Assembly. Eur. Pat. Appl. 02798875, 13 September 2002. [Google Scholar]
- Fredriksson, L.; Puester, N.H. An Electrode, a Method for Manufacturing an Electrode and a Bipolar Battery. Eur. Pat. Appl. 03810729, 7 November 2003. [Google Scholar]
- Fredriksson, L.; Puester, N.H. A Bipolar Battery and a Method for Manufacturing a Bipolar Battery. Eur. Pat. Appl. 03812398, 7 November 2003. [Google Scholar]
- Hock, D.; Puester, N.; Fredriksson, L. A Method for Manufacturing a Bipolar Battery with a Gasket. Eur. Pat. Appl. 04800257, 3 November 2004. [Google Scholar]
- Jensen, K.; Puester, N.; Hock, D. A Casing for a Sealed Battery. Eur. Pat. Appl. 06717032, 20 March 2006. [Google Scholar]
- Fredriksson, L.; Puester, N.H.; Howlett, R. A Battery Stack Arrangement. Eur. Pat. Appl. 07703860, 15 January 2007. [Google Scholar]
- Fredriksson, L.; Howlett, R. A Bipolar Battery Including a Pressure Sensor. Eur. Pat. Appl. 07712225, 15 February 2007. [Google Scholar]
- Fredriksson, L.; Puester, N.H. A System for Reducing the Power in a Battery Stack Arrangement. Eur. Pat. Appl. 07852114, 17 December 2007. [Google Scholar]
- Hock, D.; Fredriksson, L.; Puester, N.H. A Gasket, a Bipolar Battery and a Method for Manufacturing a Gasket. Eur. Pat. Appl. 08712719, 18 February 2008. [Google Scholar]
- Howlett, R. An Energy Management System. Eur. Pat. Appl. 12738289, 28 June 2012. [Google Scholar]
- Noréus, D. A Metal Hydride Battery with Added Hydrogen Gas, Oxygen Gas or Hydrogen Peroxide. International Application No. PCT/SE2016/051020, 27 April 2017. [Google Scholar]
- Nilar. Available online: http://www.nilar.com (accessed on 25 May 2017).
- Schaffrath, U.; Ohms, D.; Benczur-Uermoessy, G.; Markolf, R.; Schmelter, K. Method for Producing a Positive Nickel-Hydroxide Electrode for a Nickel-Metal Hydrid or Nickel-Cadmium Battery. Eur. Pat. Appl. 11007464, 14 September 2011. [Google Scholar]
- Ohms, D.; Schaedlich, G.R.N.; Kleinschnittger, B.D.-I.; Markolf, R.; Schmelter, K. Nickel-Metal Hydrid Battery. Eur. Pat. Appl. 11007465, 14 September 2011. [Google Scholar]
- Hoppecke Power from Innovation. Available online: https://www.hoppecke.com/ (accessed on 25 May 2017).
- Master Alloys. Available online: http://www.gfe.com/fileadmin/user_upload/pdfs/Produktspezifikationen_Alloys/HYDRALLOY_C_2004732_2005169_2019929_V4.pdf (accessed on 25 May 2017).
- Klassen, T.; Bormann, R.; Oelerich, W.; Guether, V.; Otto, A. Metalliferous Storage Material for Hydrogen and Method for Producing Same. Eur. Pat. Appl. 99955727, 17 September 1999. [Google Scholar]
- Non-Ferrous Metal Alloys. Available online: https://www.treibacher.com/en/products/non-ferrous-metal-alloys.html (accessed on 25 May 2017).
- Knosp, B.; Arnaud, O.; Hezeque, T.; Barbic, P.; Bouvier, A. Hydridable Alloy. Eur. Pat. Appl. 01402059, 30 July 2001. [Google Scholar]
- Hebenstreit, G.; Barbic, P. Metal Hydride Reservoir. International Application No. PCT/AT2006/000241, 28 December 2006. [Google Scholar]
- Dubrow, R.S.; Froix, M.F. Method of Making a Microporous Article. Eur. Pat. Appl. 85902861, 20 May 1985. [Google Scholar]
- Park, G.B.; Cook, J.A. Microporous Films. Eur. Pat. Appl. 90112666, 18 December 1985. [Google Scholar]
- Dorling, M.G.L.; Barker, D.J.; Mcloughlin, R.H. Microporous Films. Eur. Pat. Appl. 89906800, 13 June 1989. [Google Scholar]
- Singleton, R.W.; Cook, J.A.; Gargan, K. Polymer Membrane. Eur. Pat. Appl. 91916836, 19 September 1991. [Google Scholar]
- Gargan, K.; Singleton, R.W.; Cook, J.A. Polymeric Sheet. Eur. Pat. Appl. 92914965, 9 July 1992. [Google Scholar]
- Mcloughlin, R.H.; Gentilcore, G.; Cook, J.A. Non-Woven Fabric Treatment. Eur. Pat. Appl. 98925861, 8 June 1998. [Google Scholar]
- Gentilcore, G.; Lancaster, I.M. Non-Woven Fabric Laminate. Eur. Pat. Appl. 98925860, 8 June 1998. [Google Scholar]
- Hoffmann, H.; Schwoebel, R. Nonwoven Hydrophilic Material for Electrochemical Battery Separator and Method of Manufacturing. Eur. Pat. Appl. 93109577, 16 June 1993. [Google Scholar]
- Wenneis, W.; Hoffmann, H.; Severich, B.; Keul, H.R.N. Alkaline Cell or Battery. Eur. Pat. Appl. 02009348, 3 May 2002. [Google Scholar]
- Kritzer, P.; Trautmann, C. Hydrophylized Separator Material. Eur. Pat. Appl. 02023842, 24 October 2002. [Google Scholar]
- Kritzer, P.; Schwoebel, R.-P. Method for the Production of a Separator Material for Alkaline Batteries. Eur. Pat. Appl. 03003011, 12 February 2003. [Google Scholar]
- Kritzer, P.; Feistner, H.-J.; Schilling, H.; Kalbe, M. Nonwoven Fabric, Fiber and Electrochemical Cell. Eur. Pat. Appl. 05023520, 27 October 2005. [Google Scholar]
- Kritzer, P. Separator for Housing in Batteries and Battery. Eur. Pat. Appl. 07006211, 27 March 2007. [Google Scholar]
- Kritzer, P.; Schilling, H. Layer with Insulated Fibres and Electrochemical Cell. Eur. Pat. Appl. 07020102, 15 October 2007. [Google Scholar]
- Roth, M.; Weber, C.; Berg, M.; Geiger, S.; Hirn, K.; Waschinski, C.; Falusi, S.; Kasai, M. Separator with Increased Puncture Resistance. Eur. Pat. Appl. 10749599, 11 August 2010. [Google Scholar]
- Audebert, J.-F.; Feistner, H.-J.; Frey, G.; Farer, R.; Thrasher, G.L.; Weiss, M. Nonwoven Separator for Electrochemical Cell. Eur. Pat. Appl. 02789528, 8 November 2002. [Google Scholar]
- Benczur-Uermoessy, G. Gastight Prismatic Nickel-Metal Hydride cell. Eur. Pat. Appl. 00942051, 10 June 2000. [Google Scholar]
- Benczur-Uermoessy, G.; Ohms, D.; Waidelich, D. Electrode Capable of Storing Hydrogen and a Method for the Production of the Same. Eur. Pat. Appl. 00942050, 10 June 2000. [Google Scholar]
- Benczur-Uermoessy, G.; Gensierich, M.; Ohms, D.; Wiesener, K. Battery in Bipolar Stacked Configuration and Method for the Production Thereof. Eur. Pat. Appl. 00927157, 6 May 2000. [Google Scholar]
- Bartlett, P.N.; Owen, J.R.; Nelson, P.A. Electrochemical Cell. Eur. Pat. Appl. 03767996, 12 December 2003. [Google Scholar]
- Bartlett, P.N.; Owen, J.R.; Nelson, P.A. Electrochemical Cell. International Application No. PCT/GB2003/005441, 28 October 2004. [Google Scholar]
- Bartlett, P.N.; Owen, J.R.; Nelson, P.A. Electrochemical Cell Suitable for Use in Electronic Device. International Application No. PCT/GB2003/005442, 24 July 2004. [Google Scholar]
- Bruchmann, B.; Klok, H.; Scholl, M.T. Preparation and Use of Modified Polylysines. Eur. Pat. Appl. 10152919, 15 November 2006. [Google Scholar]
- Arvidsson, J.; Carlström, R.; Hallen, H.; Löfgren, S. Powder Composition and Process for the Preparation Thereof. Eur. Pat. Appl. 98928768, 5 June 1998. [Google Scholar]
- Popall, M.; Olsowski, B.; Cochet, S. Particles Coated with an Organically Modified (Hetero) Silicic Acid Polycondensate and Containing a Metal Core Suited for Storing Hydrogen, Batteries Produced Therewith and Method for the Production Thereof Using the Particles. Eur. Pat. Appl. 10726978, 29 June 2010. [Google Scholar]
- Maugy, C.; Porcellato, D.; Tarascon, J.-M.; Sainton, P. Method and System for Charging a Battery Wherein the Internal Pressure Varies Depending on State of Charge and Temperature. Eur. Pat. Appl. 04292653, 9 November 2004. [Google Scholar]
- Bothe, M.; Schröder, G.B.R.; Breuch, G. Battery Charger with State of Charge Determination on the Primary Side. Eur. Pat. Appl. 05012135, 6 June 2005. [Google Scholar]
- Nicolai, J. Quick Charge Method for Battery and Integrated Circuit for Performing the Method. Eur. Pat. Appl. 94400575, 16 March 1994. [Google Scholar]
- Yuen, T.K. Battery Charger. Eur. Pat. Appl. 94300655, 28 January 1994. [Google Scholar]
- Lang, O. Method for Regulating Charging of Nickel Cadmium and Nickel Metal Hydride Batteries, and Power Supply Unit. Eur. Pat. Appl. 06762920, 26 July 2006. [Google Scholar]
- Umicore. Battery Recycling in the EU. Presented in the NaatBatt Battery Recycling Workshop, Ann Arbor, MI, USA. 30 November 2016. Available online: http://naatbatt.org/wp-content/uploads/2016/12/What%E2%80%99s-the-Rest-of-the-World-Doing_Caffarey_A.pdf (accessed on 24 July 2017).
- About the Colabats Project. Available online: http://www.colabats.eu/about-the-colabats-project (accessed on 27 July 2017).
- Cheret, D.; Santén, S. Battery Recycling. Eur. Pat. Appl. 05075736, 30 March 2005. [Google Scholar]
- Luidold, S.; Antrekowitsch, H. Recovery of Rare Earth Metals from Waste Material by Leaching in Non-Oxidizing Acid and by Precipitation Using Sulphates. Eur. Pat. Appl. 10188264, 20 October 2010. [Google Scholar]
- Pudas, J.; Erkkila, A.; Viljamaa, J. Battery Recycling Method. Eur. Pat. Appl. 11708474, 16 March 2011. [Google Scholar]
- Hanulik, J. Battery Recycling Process, in Particular for Dry Batteries. Eur. Pat. Appl. 95920722, 16 June 1995. [Google Scholar]
- Wiaux, J.; Lazouni, A.; Indaco, A. Used Battery and Cell Sorting Method and Apparatus. Eur. Pat. Appl. 94906358, 24 February 1994. [Google Scholar]
- Alavi, K.; Salami, B. Method of Treating Nickel-Cadmium of Nickel-Hydride Cells. Eur. Pat. Appl. 93113054, 14 August 1993. [Google Scholar]
- Traverse, J.-P.; Bressolles, J.-C.; Archier, P. Method for Processing Scrap Containing One or More Alloys that React to Form Hydrides, to Enable Recycling Thereof. Eur. Pat. Appl. 96926454, 24 July 1996. [Google Scholar]
- Toro, L.; Veglio, F.; Beolchini, F.; Pagnanelli, F.; Furlani, G.; Granata, G.; Moscardini, E. Plant and Process for the Treatment of Exhausted Accumulators and Batteries. Eur. Pat. Appl. 11185532, 18 October 2011. [Google Scholar]
- Laucournet, R.; Billy, E. Method for Recovering Metals Contained in an Ni-MH Battery. International Application No. PCT/IB2014/066008, 21 May 2015. [Google Scholar]
- Black, V. Soap Dispenser Conversion Kit. Eur. Pat. Appl. 15156912, 27 February 2015. [Google Scholar]
- Young, K. Metal Hydride. In Reference Module in Chemistry, Molecular Science and Chemical Engineering; Reedijk, J., Ed.; Elsevier: Waltham, MA, USA, 2013. [Google Scholar] [CrossRef]





| Company Name | Headquarter | Products Related to Ni/MH | Trademark | Website |
|---|---|---|---|---|
| Saft | Bagnolet, France | C, D, F-size cells |  | http://saftbatteries.com/ |
| Arts Energy | Nersac, France | C, D, F-size cells |  | http://www.arts-energy.com |
| Varta | Ellwangen, Germany | AAA to FA, button cell |  | http://www.varta-microbattery.be/ |
| Nilar | Täby, Sweden | Prismatic, bipolar cell |  | http://www.nilar.com/ |
| Alcatel | Paris, France | Cell phone battery |  | http://www5.alcatel-lucent.com |
| Hoppecke | Brilon-Hoppecke, Germany | Prismatic cell |  | https://www.hoppecke.com/ |
| GfE | Nuremberg, Germany | MH alloy powder |  | http://www.gfe.com |
| Treibacher | Althofen, Austria | MH alloy powder |  | https://www.treibacher.com |
| SciMat | Swindon, UK | Separator |  | Not available now |
| Freudenberg | Weinheim, Germany | Separator |  | http://www.freudenberg.com |
| Country | EU | USA | Japan | China |
|---|---|---|---|---|
| Number of patents (application) related to Ni/MH | 126 | 371 | 275 | 692 |
| Number of patents in negative electrode | 22 | 58 | 128 | 136 |
| Number of patents in positive electrode | 18 | 54 | 27 | 83 |
| Number of patents in manufacturing technology | 10 | 33 | 28 | 104 |
| Number of companies (institutes) filed patents | 29 | 18 * | 46 | 99 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.; Young, K.-H.; Lien, Y.-L. Reviews of European Patents on Nickel/Metal Hydride Batteries. Batteries 2017, 3, 25. https://doi.org/10.3390/batteries3030025
Chang S, Young K-H, Lien Y-L. Reviews of European Patents on Nickel/Metal Hydride Batteries. Batteries. 2017; 3(3):25. https://doi.org/10.3390/batteries3030025
Chicago/Turabian StyleChang, Shiuan, Kwo-Hsiung Young, and Yu-Ling Lien. 2017. "Reviews of European Patents on Nickel/Metal Hydride Batteries" Batteries 3, no. 3: 25. https://doi.org/10.3390/batteries3030025