Performance Comparison between AB5 and Superlattice Metal Hydride Alloys in Sealed Cells
Abstract
:1. Introduction
2. Experimental Setup
3. Results and Discussion
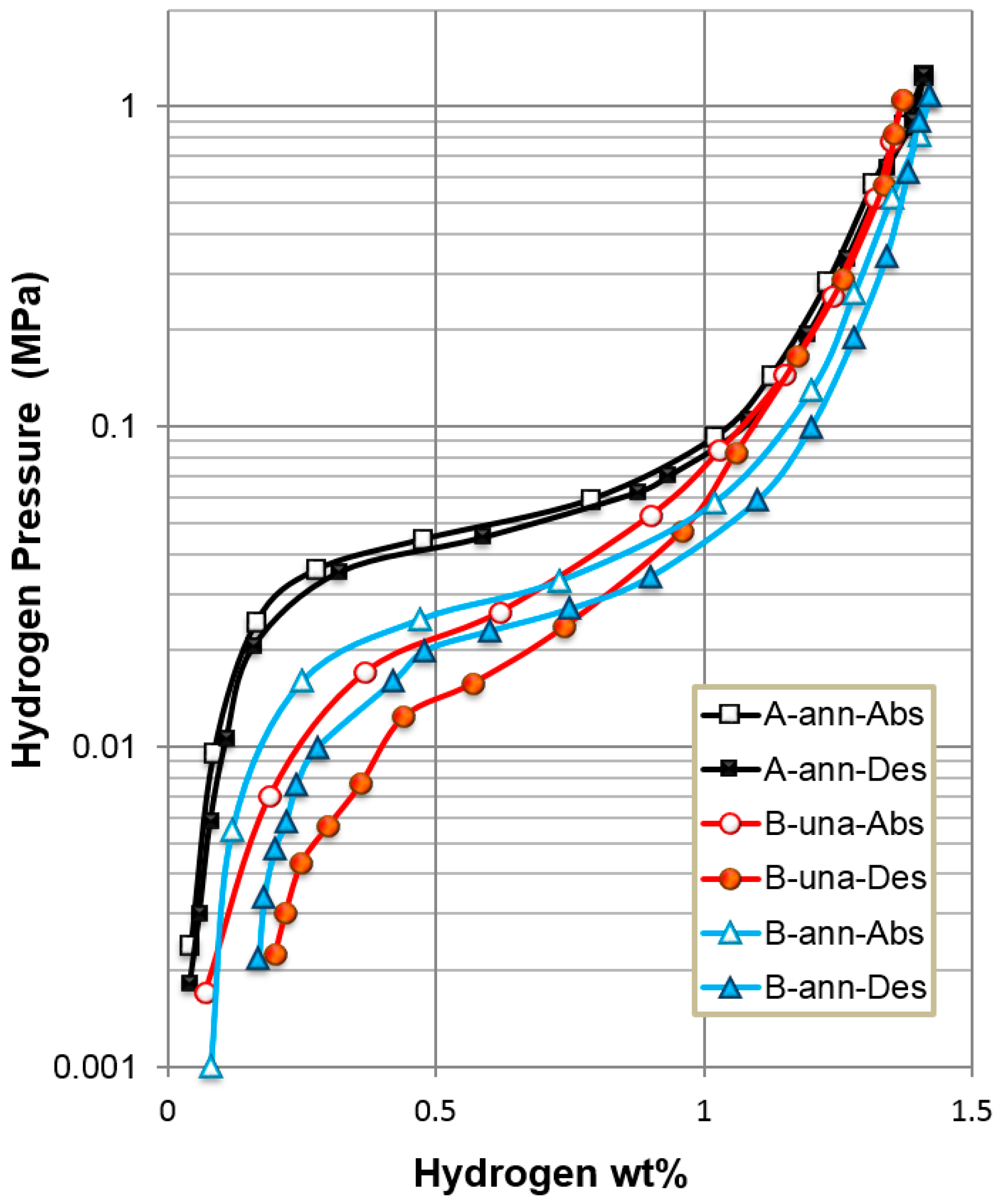
3.1. Alloy Properties Comparison
3.2. Sealed-Cell Performance
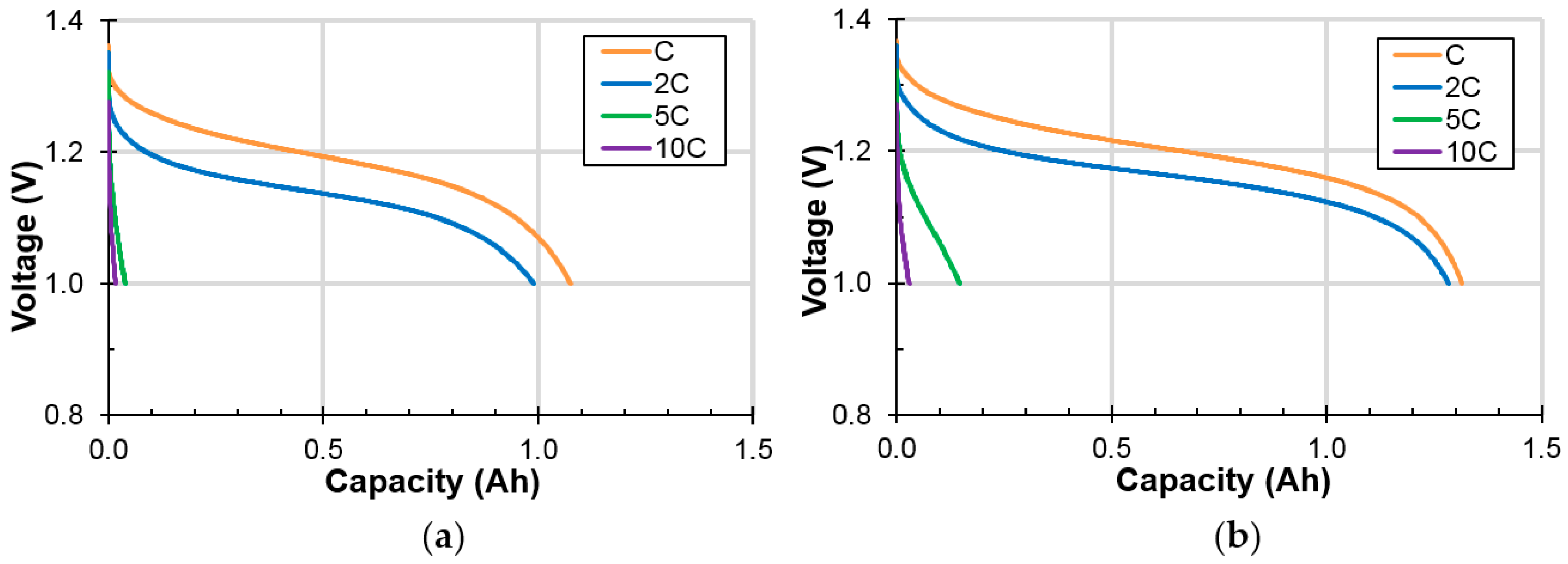
3.2.1. High-Rate
3.2.2. Low Temperature
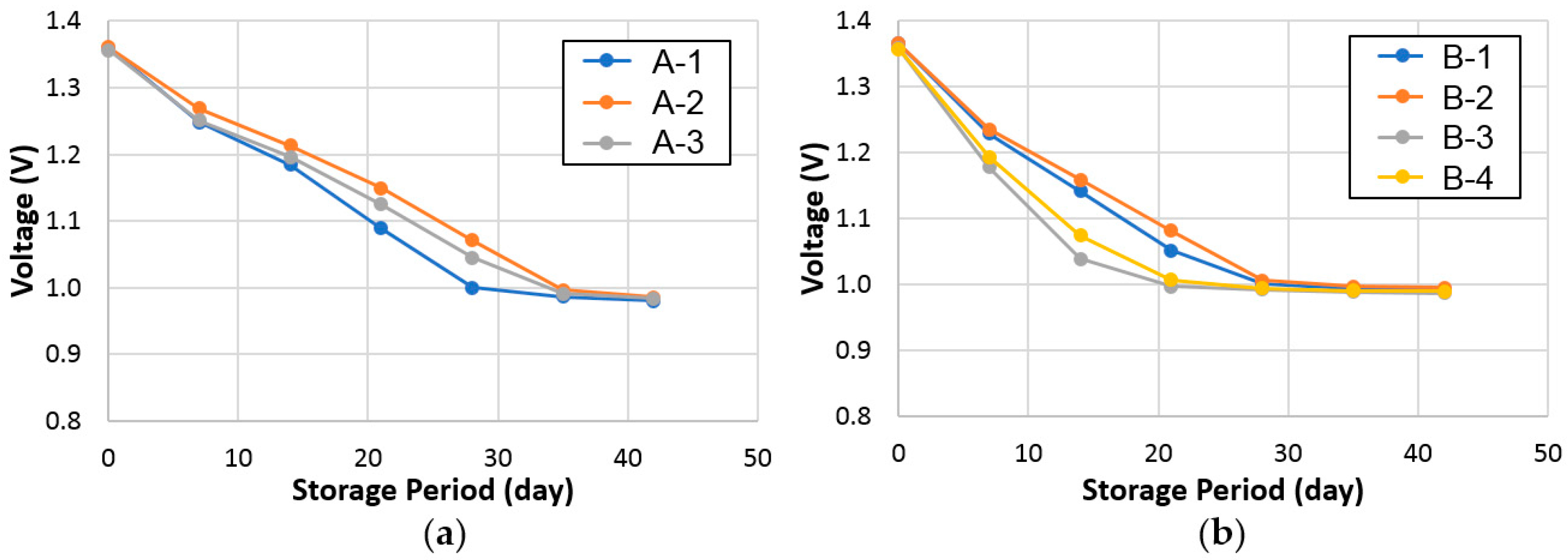
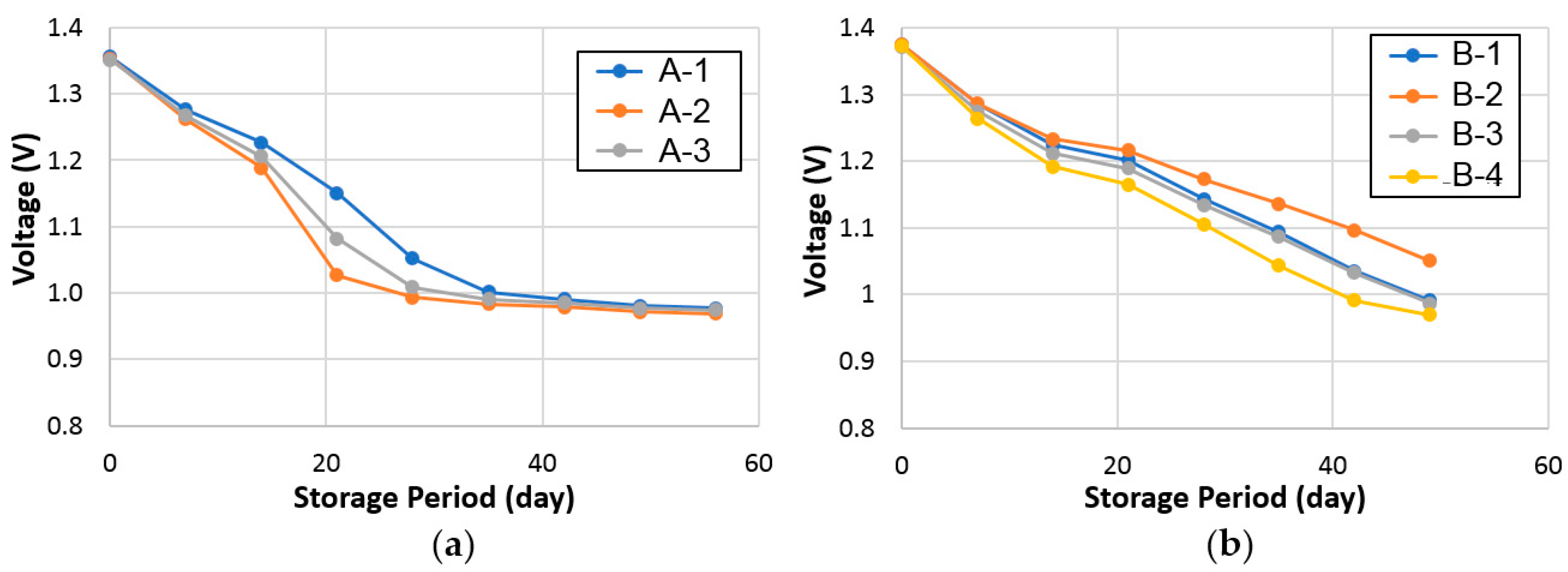
3.2.3. Charge Retention
3.2.4. Internal Resistance
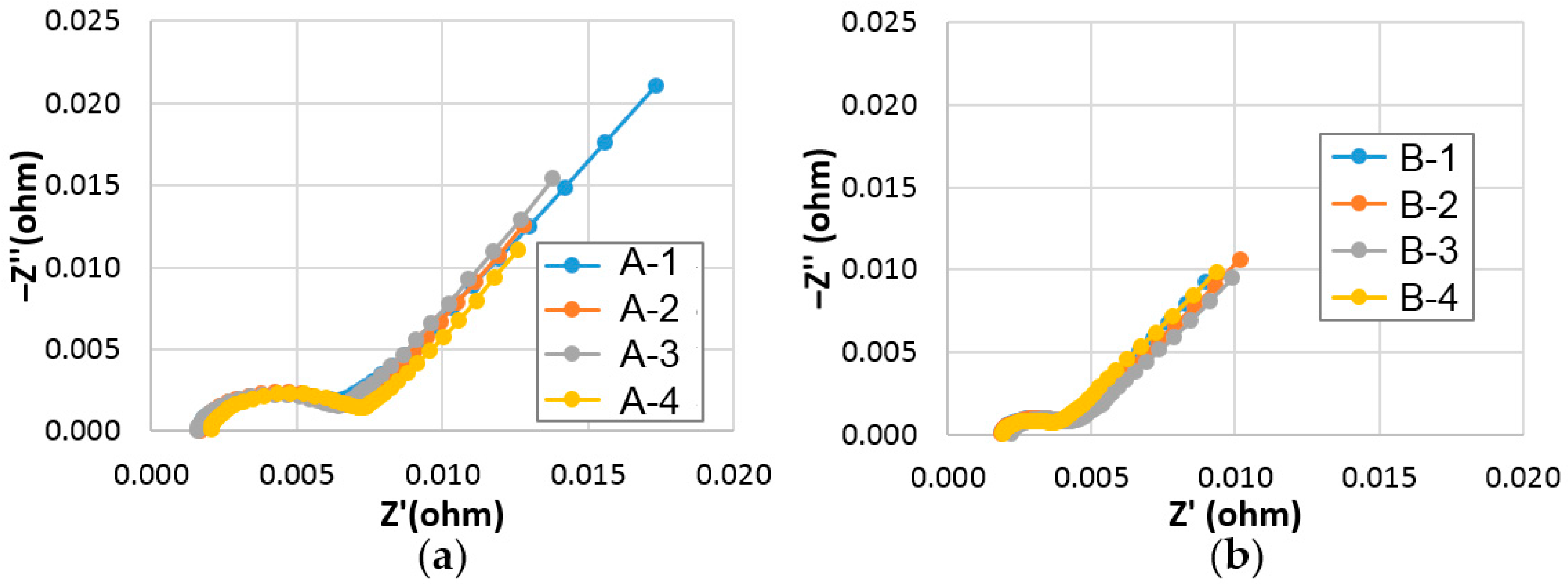
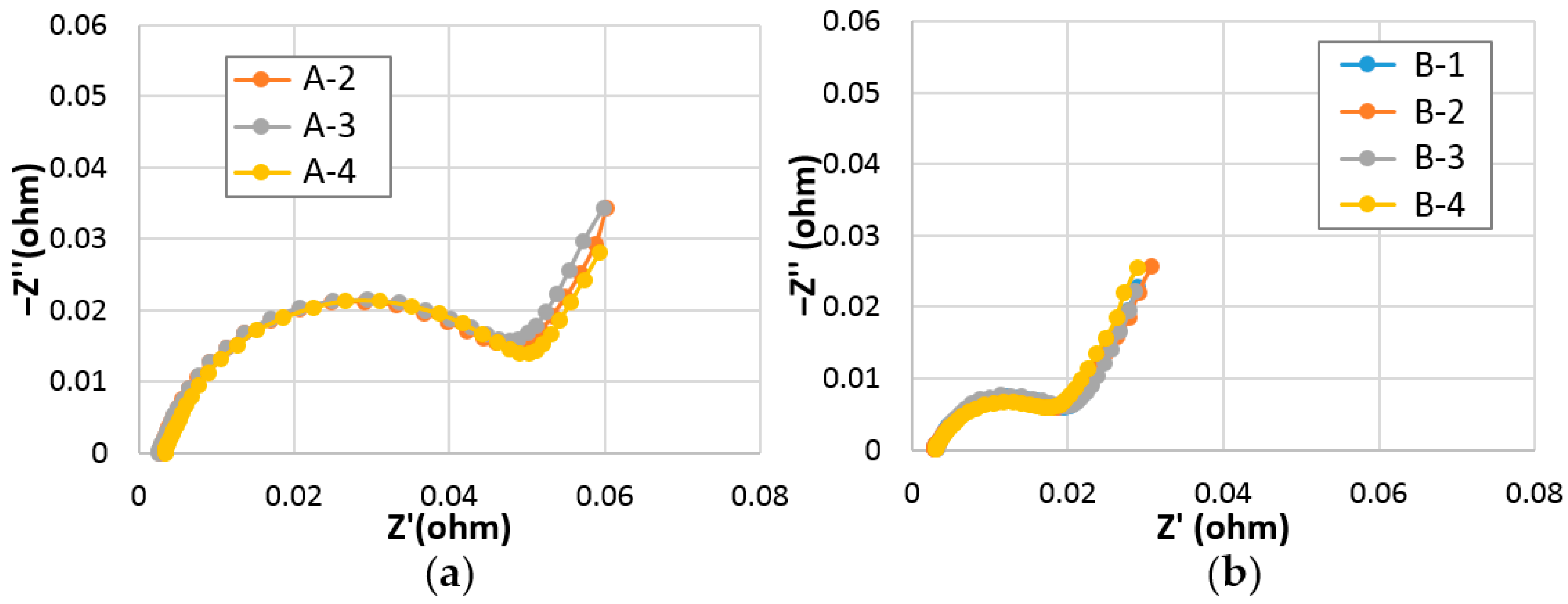
3.2.5. Surface Charge-Transfer Resistance
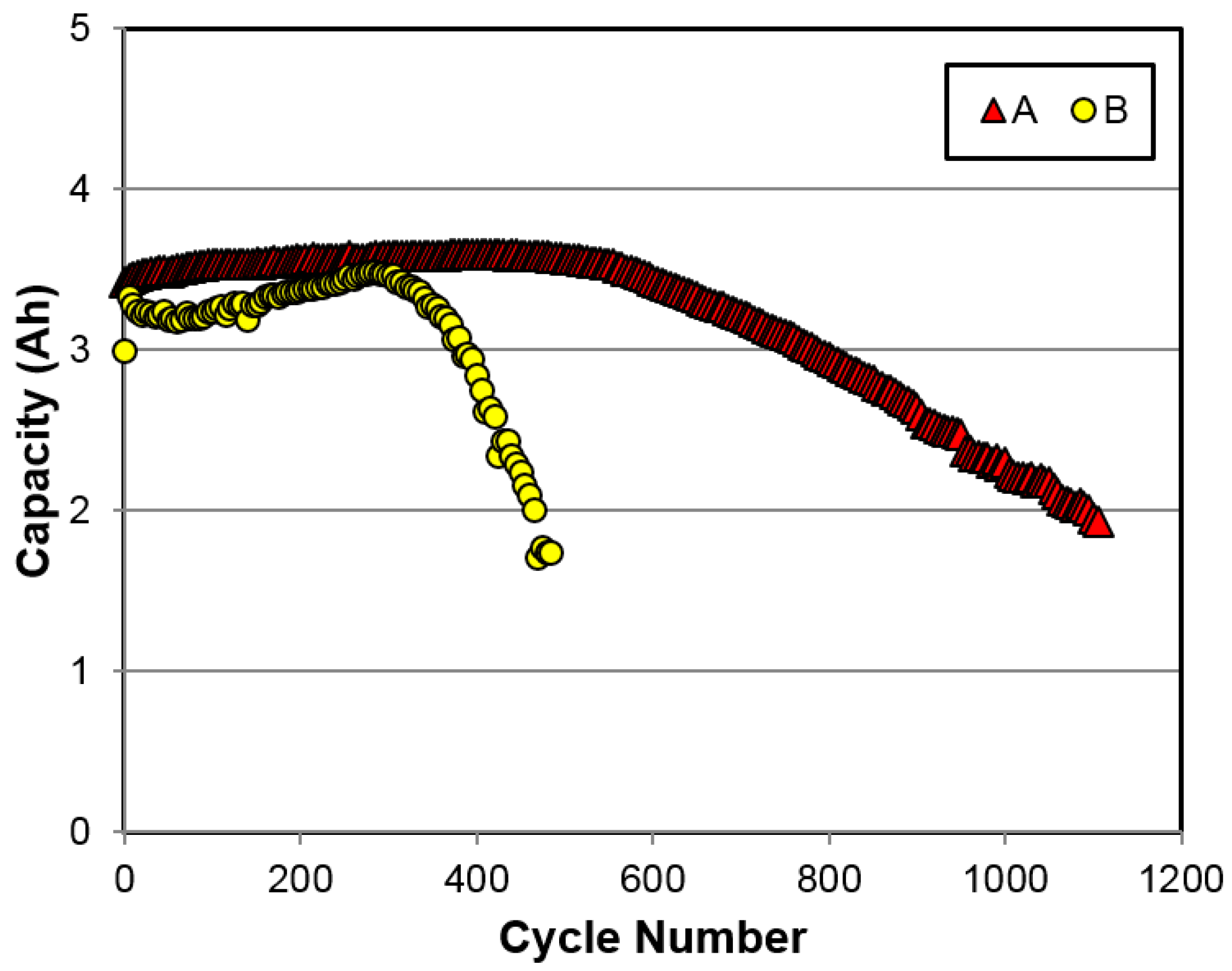
3.2.6. Cycle Life
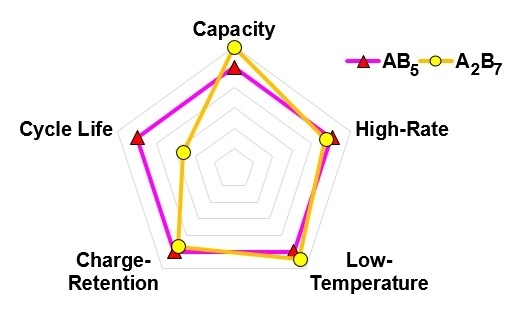
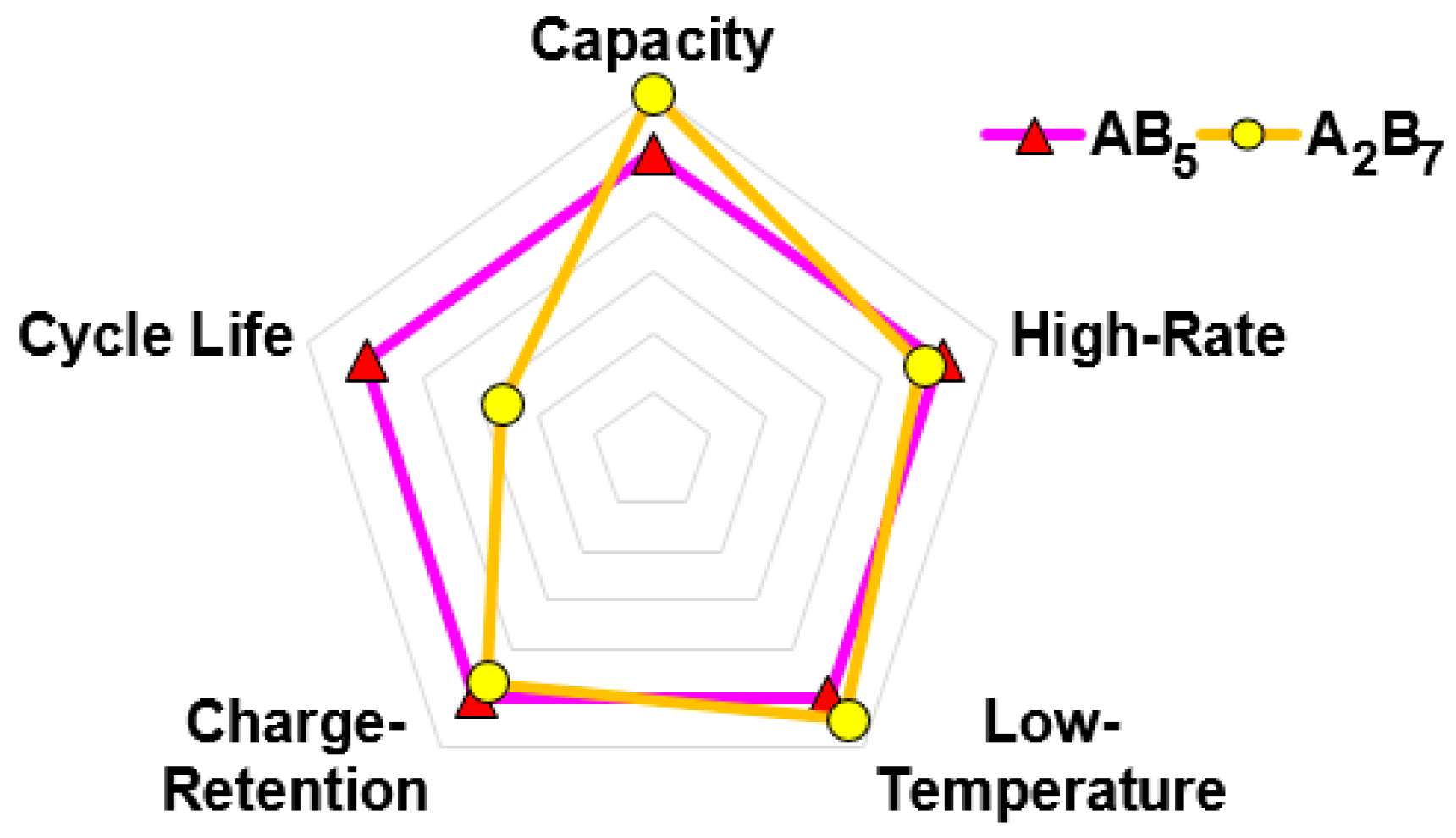
3.2.7. Comparison
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Ni/MH | Nickel/metal hydride |
| Mm | Misch metal |
| MH | Metal hydride |
| RE | Rare-earth |
| XRD | X-ray diffractometer |
| SEM | Scanning electron microscope |
| PCT | Pressure–concentration–temperature |
| EDS | Energy-dispersive spectroscopy |
| HEX | Hexagonal |
| CUB | Cubic |
| RHO | Rhombohedral |
| Abs | Absorption |
| Des | Desorption |
| RT | Room temperature |
| V | Cell voltage |
| i | Current |
| Voc | Open-circuit voltage |
| Rint | Internal resistance |
| R0 | Ohmic resistance |
| Rct | Charge-transfer resistance |
| C | Double-layer capacitance |
| A | Surface reactive area |
| ε | Dielectric constant of electrolyte |
| d | Alloy surface dipole thickness |
References
- Zelinsky, M.A.; Koch, J.M.; Young, K. Performance comparison of rechargeable batteries for stationary applications (Ni/MH vs. Ni-Cd and VRLA). Tatteries 2017. submitted. [Google Scholar]
- Young, K.; Cai, X.; Chang, S. Reviews on Chinese Patents regarding the nickel/metal hydride battery. Batteries 2017, 3, 24. [Google Scholar] [CrossRef]
- Yasuoka, S.; Magari, Y.; Murata, T.; Tanaka, T.; Ishida, J.; Nakamura, H.; Nohma, T.; Kihara, M.; Baba, Y.; Teraoka, H. Development of high-capacity nickel-metal hydride batteries using superlattice hydrogen-absorbing alloys. J. Power Sour. 2006, 156, 662–666. [Google Scholar] [CrossRef]
- Teraoka, H. Development of Low Self-Discharge Nickel-Metal Hydride Battery. Available online: http://www.scribd.com/doc/9704685/Teraoka-Article-En (accessed on 9 April 2016).
- Kai, T.; Ishida, J.; Yasuoka, S.; Takeno, K. The effect of nickel-metal hydride battery’s characteristics with structure of the alloy. In Proceedings of the 54th Battery Symposium in Japan, Osaka, Japan, 6–9 October 2013; p. 210. [Google Scholar]
- Teraoka, H. Development of Ni-MH EThSS with Lifetime and Performance Estimation Technology. In Proceedings of the 34th International Battery Seminar & Exhibit, Fort Lauderdale, FL, USA, 20–23 March 2017. [Google Scholar]
- Teraoka, H. Ni-MH Stationary Energy Storage: Extreme Temperature & Long Life Developments. In Proceedings of the 33th International Battery Seminar & Exhibit, Fort Lauderdale, FL, USA, 21–24 March 2016. [Google Scholar]
- Teraoka, H. Development of Highly Durable and Long Life Ni-MH Batteries for Energy Storage Systems. In Proceedings of the 32th International Battery Seminar & Exhibit, Fort Lauderdale, FL, USA, 9–12 March 2015. [Google Scholar]
- Liu, Y.; Cao, Y.; Huang, L.; Gao, M.; Pan, H. Rare earth-Mg-Ni-based hydrogen storage alloys as negative electrode materials for Ni/MH batteries. J. Alloy. Compd. 2011, 509, 675–686. [Google Scholar] [CrossRef]
- Young, K.; Nei, J. The current status of hydrogen storage alloy development for electrochemical applications. Materials 2013, 6, 4574–4608. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, S.; Li, Y.; Zhang, L.; Zhao, Y.; Yang, S.; Liu, B. Phase structures and electrochemical properties of La–Mg–Ni-based hydrogen storage alloys with superlattice structure. Int. J. Hydrogen Energy 2016, 41, 20261–20275. [Google Scholar] [CrossRef]
- Takasaki, T.; Nishimura, K.; Saito, M.; Fukunaga, H.; Iwaki, T.; Sakai, T. Cobalt-free nickel-metal hydride battery for industrial applications. J. Alloy. Compd. 2013, 580, S378–S381. [Google Scholar] [CrossRef]
- Yasuoka, S.; Ishida, J.; Kai, T.; Kajiwara, T.; Doi, S.; Yamazaki, T.; Kishida, K.; Inui, H. Function of aluminum in crystal structure of rare earth-Mg-Ni hydrogen-absorbing alloy and deterioration mechanism of Nd0.9Mg0.1Ni3.4 and Nd0.9Mg0.1Ni3.3Al0.2 alloys. Int. J. Hydrogen Energy 2017, 42, 11574–11583. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.F.; Wang, L.; Nei, J.; Ouchi, T.; Yasuoka, S. Mn in misch-metal based superlattice metal hydride alloy—Part 1 Structural, hydrogen storage and electrochemical properties. J. Power Sour. 2015, 277, 426–432. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.F.; Wang, L.; Nei, J.; Ouchi, T.; Yasuoka, S. Mn in misch-metal based superlattice metal hydride alloy—Part 2 Ni/MH battery performance and failure mechanism. J. Power Sour. 2015, 277, 433–442. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Nei, J.; Yasuoka, S. Fe-substitution for Ni in misch metal-based superlattice hydrogen absorbing alloys—Part 1. Structural, hydrogen storage, and electrochemical properties. Batteries 2016, 2, 34. [Google Scholar] [CrossRef]
- Meng, T.; Young, K.; Nei, J.; Koch, J.M.; Yasuoka, S. Fe-substitution for Ni in misch metal-based superlattice hydrogen absorbing alloys—Part 2. Ni/MH battery performance and failure mechanisms. Batteries 2017, 3, 28. [Google Scholar] [CrossRef]
- Wang, L.; Young, K.; Meng, T.; Ouchi, T.; Yasuoka, S. Partial substitution of cobalt for nickel in mixed rare earth metal based superlattice hydrogen absorbing alloy—Part 1 structural, hydrogen storage and electrochemical properties. J. Alloy. Compd. 2016, 660, 407–415. [Google Scholar] [CrossRef]
- Wang, L.; Young, K.; Meng, T.; English, N.; Yasuoka, S. Partial substitution of cobalt for nickel in mixed rare earth metal based superlattice hydrogen absorbing alloy—Part 2 battery performance and failure mechanism. J. Alloy. Compd. 2016, 664, 417–427. [Google Scholar] [CrossRef]
- Meng, T.; Young, K.; Koch, J.; Ouchi, T.; Yasuoka, S. Failure mechanisms of nickel/metal hydride batteries with cobalt-substituted superlattice hydrogen-absorbing alloy anodes at 50 °C. Batteries 2016, 2, 20. [Google Scholar] [CrossRef]
- Yasuoka, S.; Ishida, J.; Kishida, K.; Inui, H. Effects of cerium on the hydrogen absorption-desorption properties of rare earth-Mg-Ni hydrogen-absorbing alloys. J. Power Sour. 2017, 346, 56–62. [Google Scholar] [CrossRef]
- Young, K.; Chang, S.; Lin, X. C14 Laves phase metal hydride alloys for Ni/MH batteries applications. Batteries 2017, 3, 27. [Google Scholar] [CrossRef]
- Chang, S.; Young, K.; Nei, J.; Fierro, C. Reviews on the U.S. Patents regarding nickel/metal hydride batteries. Batteries 2016, 2, 10. [Google Scholar] [CrossRef]
- Young, K.; Wang, L.; Yan, S.; Liao, X.; Meng, T.; Shen, H.; May, W.C. Fabrications of high-capacity alpha-Ni(OH)2. Batteries 2017, 3, 6. [Google Scholar] [CrossRef]
- Young, K.; Wu, A.; Qiu, Z.; Tan, J.; Mays, W. Effects of H2O2 addition to the cell balance and self-discharge of Ni/MH batteries with AB5 and A2B7 alloys. Int. J. Hydrogen Energy 2012, 37, 9882–9891. [Google Scholar] [CrossRef]
- Young, K.; Koch, J.M.; Wan, C.; Denys, R.V.; Yartys, V.A. Cell performance comparison between C14- and C15-predominated AB2 metal hydride alloys. Batteries 2017, 3, 29. [Google Scholar] [CrossRef]
- Yan, S.; Meng, T.; Young, K.; Nei, J. A Ni/MH pouch cell with high-capacity Ni(OH)2. Tatteries 2017. submitted. [Google Scholar]
- Meng, T.; Young, K.; Hu, C.; Reichman, B. Effects of alkaline pre-etching to metal hydride alloys. Batteries 2017, 3, 30. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Koch, J.M.; Lien, Y. Comparison among constituent phases in superlattice metal hydride alloys for battery applications. Tatteries 2017. submitted. [Google Scholar] [CrossRef]
- Zhou, X.; Young, K.; West, J.; Regalado, J.; Cherisol, K. Degradation mechanisms of high-energy bipolar nickel metal hydride battery with AB5 and A2B7 alloys. J. Alloy. Compd. 2013, 580, S373–S377. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Huang, B. Effects of various annealing conditions on (Nd, Mg, Zr)(Ni, Al, Co)3.74 metal hydride alloys. J. Power Sour. 2014, 248, 147–153. [Google Scholar] [CrossRef]
- Ouchi, T.; Young, K.; Moghe, D. Reviews on the Japanese Patent Applications regarding nickel/metal hydride batteries. Batteries 2016, 2, 21. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Meng, T.; Wong, D.F. Studies on the synergetic effects in multi-phase metal hydride alloys. Batteries 2016, 2, 15. [Google Scholar] [CrossRef]
- Osumi, Y. Suiso Kyuzou Goukin; Agune Technology Center: Tokyo, Japan, 1999; p. 218. (In Japanese) [Google Scholar]
- Zhang, L. AC impedance studies on sealed nickel metal hydride batteries over cycle life in analog and digital operations. Electrochim. Acta 1998, 43, 3333–3342. [Google Scholar] [CrossRef]
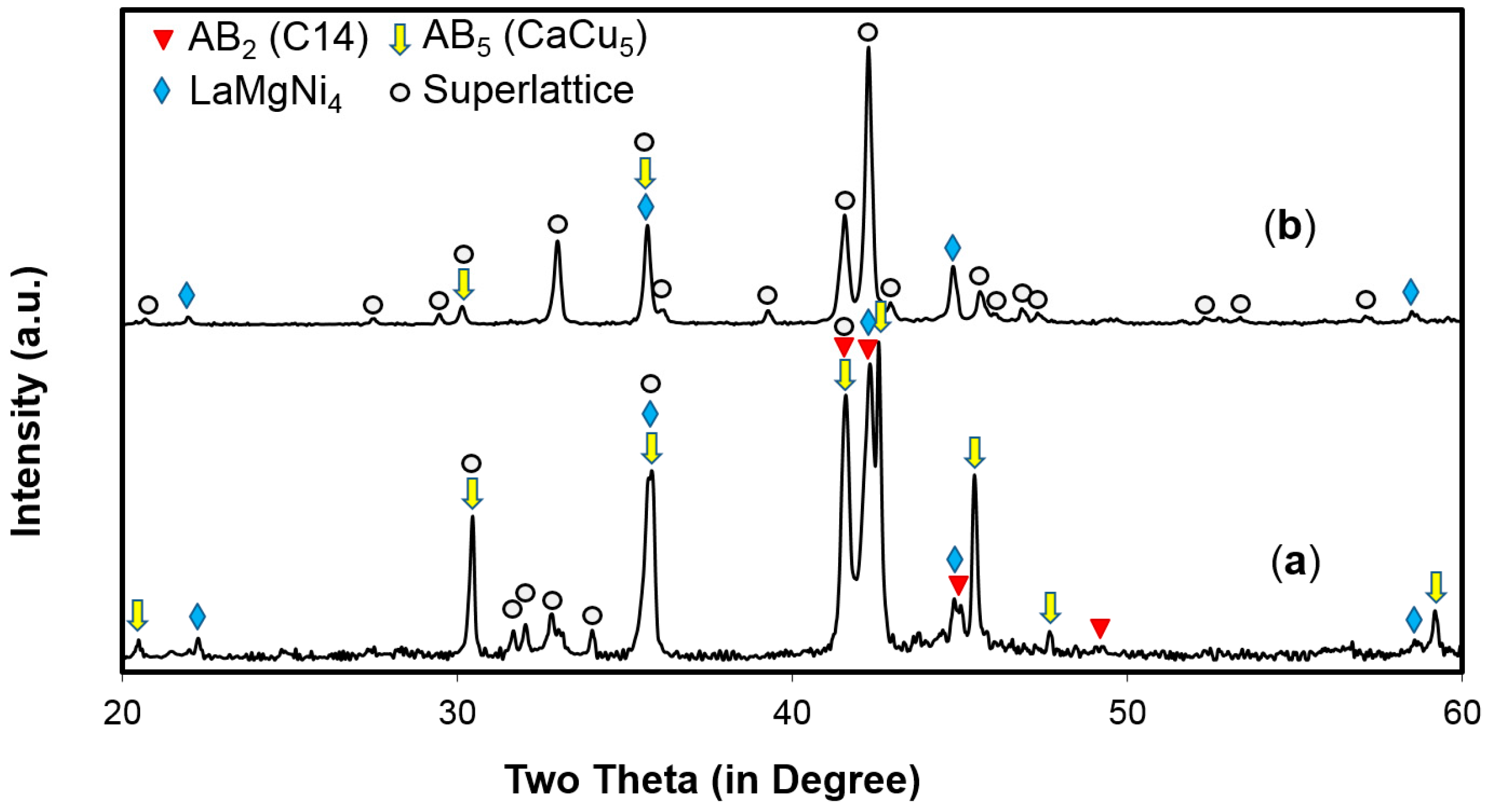


| Stoichiometry | AB2 | AB3 | A2B7 | A5B19 | AB5 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Structure | HEX | CUB | HEX | RHO | HEX | RHO | HEX | RHO | HEX |
| Phase | MgZn2 | LaMgNi4 | CeNi3 | NdNi3 | Nd2Ni7 | Pr2Ni7 | Sm5Ni19 | Nd5Co19 | CaCu5 |
| Pristine alloy B | 7.9 | 5.7 | 2.0 | 19.3 | 0.0 | 13.1 | 1.6 | 17.7 | 32.7 |
| Annealed alloy B | 0.0 | 3.2 | 7.5 | 7.4 | 56.9 | 0.0 | 6.4 | 17.0 | 1.6 |
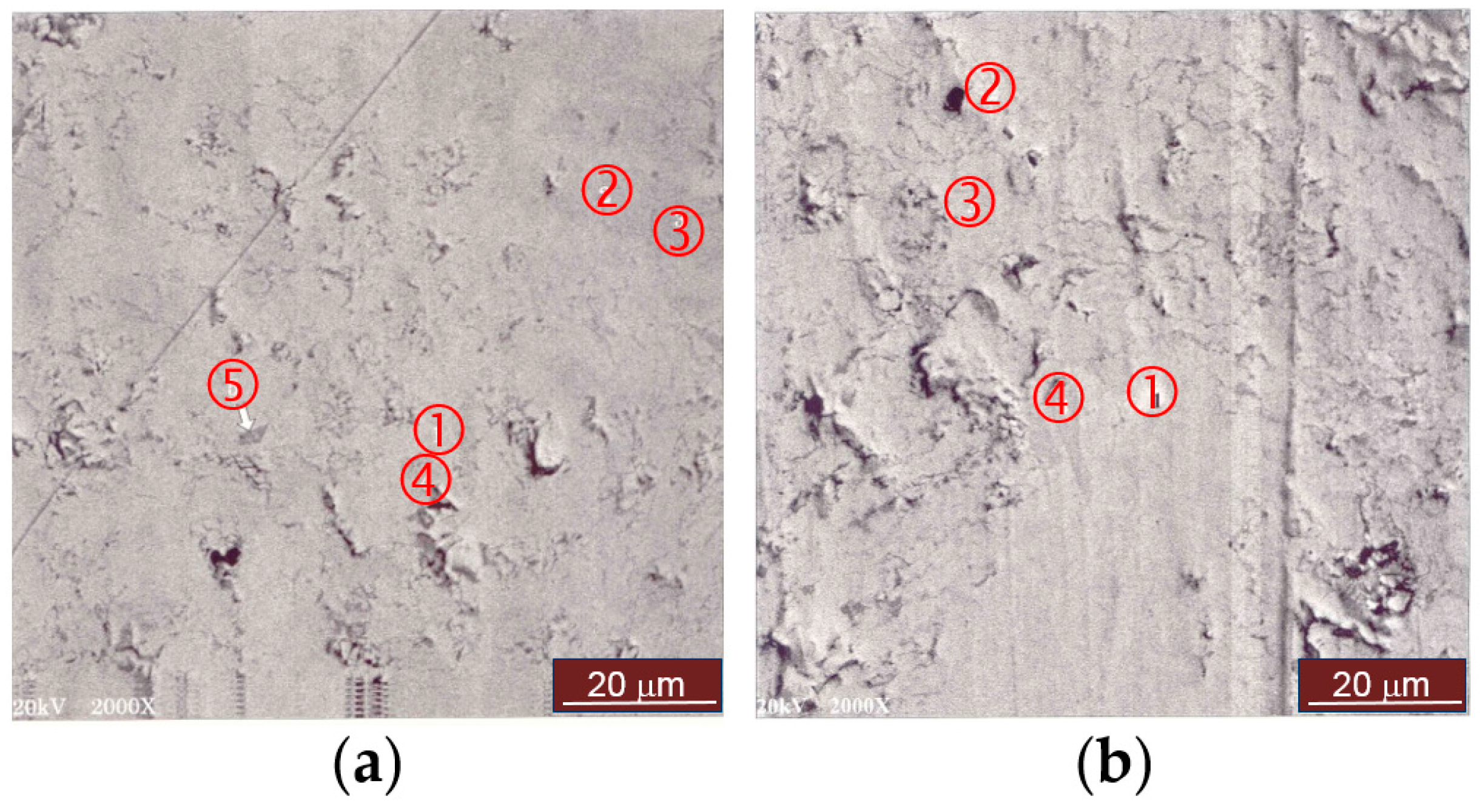
| Location | La | Pr | Nd | Mg | Ni | Co | Zr | B/A | Phase |
|---|---|---|---|---|---|---|---|---|---|
| Figure 2a-1 | 13.6 | 0.7 | 4.5 | 6.6 | 62.6 | 11.9 | 0.1 | 2.93 | Superlattice |
| Figure 2a-2 | 11.1 | 1.2 | 5.3 | 0.9 | 67.2 | 14.1 | 0.2 | 4.41 | AB5 |
| Figure 2a-3 | 9.5 | 0.9 | 5.6 | 0.4 | 69.1 | 14.4 | 0.1 | 5.10 | AB5 |
| Figure 2a-4 | 11.1 | 0.8 | 5.2 | 18.7 | 57.5 | 6.5 | 0.2 | 1.79 | LaMgNi4 |
| Figure 2a-5 | 5.5 | 0.4 | 3.5 | 0.0 | 17.8 | 3.2 | 69.6 | 9.64 | ZrO2 |
| Figure 2b-1 | 86.0 | 0.0 | 4.5 | 0.8 | 6.4 | 0.8 | 1.5 | 0.1 | La metal |
| Figure 2b-2 | 16.3 | 0.7 | 4.1 | 4.0 | 61.5 | 13.2 | 0.2 | 2.98 | Superlattice |
| Figure 2b-3 | 14.4 | 0.9 | 4.4 | 12.4 | 60.3 | 7.5 | 0.1 | 2.11 | LaMgNi4 |
| Figure 2b-4 | 13.2 | 0.7 | 4.7 | 14.2 | 60.4 | 6.7 | 0.1 | 2.04 | LaMgNi4 |
| Alloy | Full H-Storage | Reversible H-Storage | Plateau Pressure | PCT Hysteresis | Discharge Capacity |
|---|---|---|---|---|---|
| Annealed alloy A | 1.41% | 1.37% | 0.058 MPa | 0.10 | 310 mAh·g−1 |
| Annealed alloy B | 1.42% | 1.25% | 0.025 MPa | 0.23 | 370 mAh·g−1 |
| Condition | 1C | 2C | 5C | 10C |
|---|---|---|---|---|
| Cell A RT 1 s | 0.144 | 0.142 | 0.138 | 0.130 |
| Cell A RT 10 s | 0.194 | 0.192 | 0.182 | 0.168 |
| Cell A −10 °C 1 s | 0.405 | 0.369 | 0.289 | 0.244 |
| Cell A −10 °C 10 s | 0.516 | 0.492 | 0.499 | — |
| Cell B RT 1 s | 0.087 | 0.087 | 0.087 | 0.085 |
| Cell B RT 10 s | 0.127 | 0.127 | 0.124 | 0.118 |
| Cell B −10 °C 1 s | 0.334 | 0.312 | 0.262 | 0.219 |
| Cell B −10 °C 10 s | 0.468 | 0.444 | 0.453 | — |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, J.M.; Young, K.-H.; Nei, J.; Hu, C.; Reichman, B. Performance Comparison between AB5 and Superlattice Metal Hydride Alloys in Sealed Cells. Batteries 2017, 3, 35. https://doi.org/10.3390/batteries3040035
Koch JM, Young K-H, Nei J, Hu C, Reichman B. Performance Comparison between AB5 and Superlattice Metal Hydride Alloys in Sealed Cells. Batteries. 2017; 3(4):35. https://doi.org/10.3390/batteries3040035
Chicago/Turabian StyleKoch, John M., Kwo-Hsiung Young, Jean Nei, Chaolan Hu, and Benjamin Reichman. 2017. "Performance Comparison between AB5 and Superlattice Metal Hydride Alloys in Sealed Cells" Batteries 3, no. 4: 35. https://doi.org/10.3390/batteries3040035