Towards Li-Ion Batteries Operating at 80 °C: Ionic Liquid versus Conventional Liquid Electrolytes
Abstract
:1. Introduction
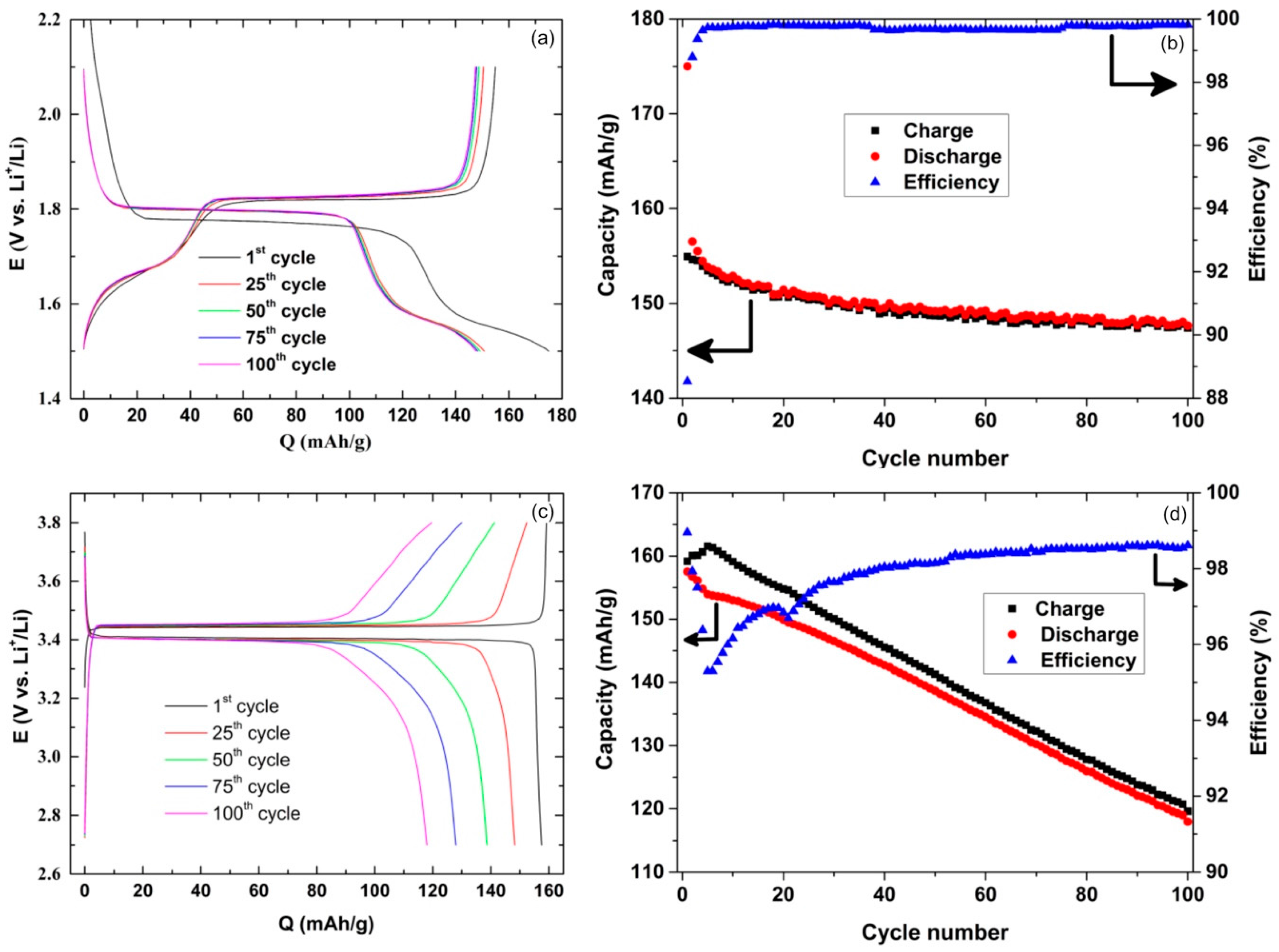
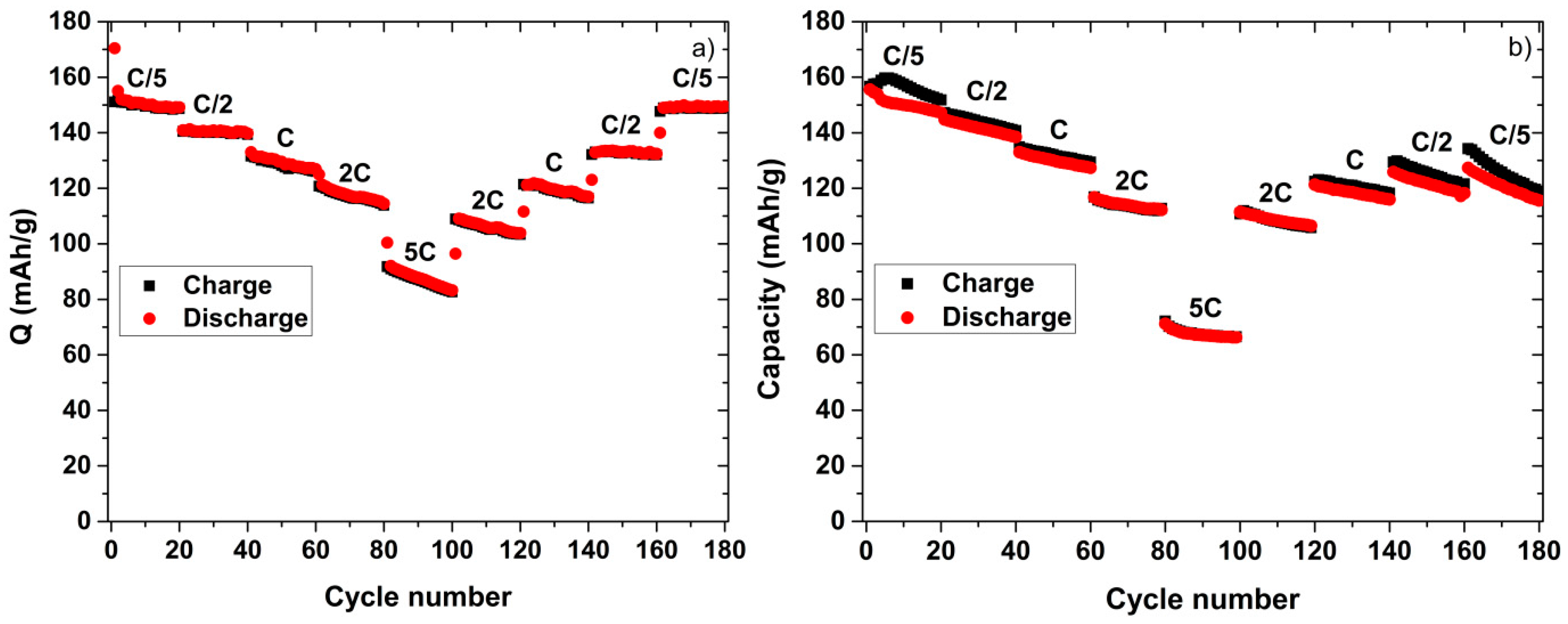
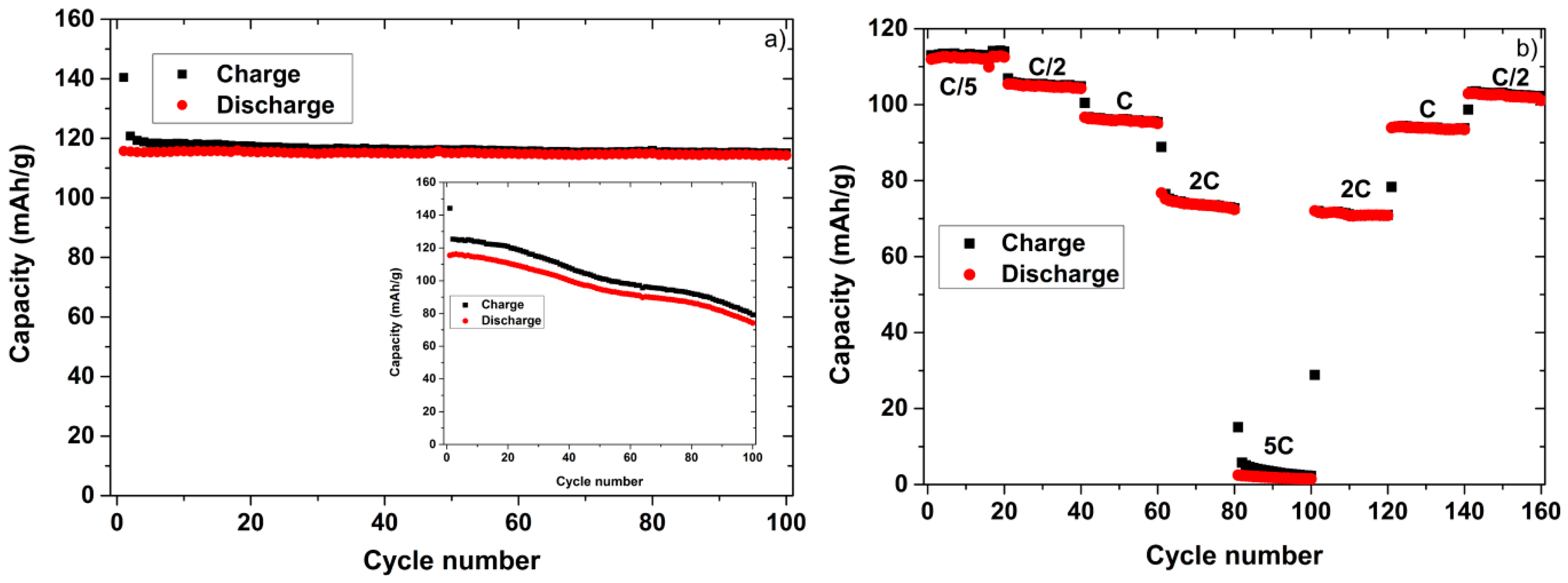
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kalhoff, J.; Eshetu, G.G.; Bresser, D.; Passerini, S. Safer Electrolytes for Lithium-Ion Batteries: State of the Art and Perspectives. ChemSuschem 2015, 8, 2154–2175. [Google Scholar] [CrossRef] [PubMed]
- Larcher, D.; Tarascon, J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Bloom, I.; Cole, B.W.; Sohn, J.J.; Jones, S.A.; Polzin, E.G.; Battaglia, V.S.; Henriksen, G.L.; Motloch, C.; Richardson, R.; Unkelhaeuser, T.; et al. An accelerated calendar and cycle life study of Li-ion cells. J. Power Sources 2001, 101, 238–247. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.; Veit, C.; Möller, K.-C.; Besenhard, J.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Tidblad, A.; Svens, A.P. Energirelaterad Fordonsforskning 2017. Available online: https://www.energimyndigheten.se/globalassets/forskning--innovation/konferenser/energirelaterad-fordonsforskning/program-energirelaterad-fordonsforskning-2017.pdf (accessed on 29 December 2017).
- Vervaeke, M.; Calabrese, G. Prospective design in the automotive sector and the trajectory of the Bluecar project: An electric car sharing system. Int. J. Veh. Des. 2015, 68, 245–264. [Google Scholar] [CrossRef]
- Smart, M.C.; Ratnakumar, B.V.; Gozdz, A.S.; Mani, S. The Effect of Electrolyte Additives upon the Lithium Kinetics of Li-Ion Cells Containing MCMB and LiNixCo1−xO2 Electrodes and Exposed to High Temperatures. ECS Trans. 2010, 25, 37–48. [Google Scholar] [CrossRef]
- Morishita, M.; Mukai, T.; Sakamoto, T.; Yanagida, M.; Sakai, T. Improvement of Cycling Stability at 80 °C for 4 V-Class Lithium-Ion Batteries and Safety Evaluation. J. Electrochem. Soc. 2013, 160, A1311–A1318. [Google Scholar] [CrossRef]
- Andersson, A.M.; Edström, K. Chemical composition and morphology of the elevated temperature SEI on Graphite. J. Electrochem. Soc. 2001, 148, A1100–A1109. [Google Scholar] [CrossRef]
- Markevich, E.; Pollak, E.; Salitra, G.; Aurbach, D. On the performance of graphitized meso carbon microbeads (MCMB)–meso carbon fibers (MCF) and synthetic graphite electrodes at elevated temperatures. J. Power Sources 2007, 174, 1263–1269. [Google Scholar] [CrossRef]
- Muto, S.; Sasano, Y.; Tatsumi, K.; Sasaki, T.; Horibuchi, K.; Takeuchi, Y.; Ukyo, Y. Capacity-fading mechanisms of LiNiO2-based lithium-ion batteries II. Diagnostic analysis by electron microscopy and spectroscopy. J. Electrochem. Soc. 2009, 156, A371–A377. [Google Scholar] [CrossRef]
- Sun, Y.-K. Structural degradation mechanism of oxysulfide spinel LiAl0.24Mn1.76O3.98S0.02 cathode materials on high temperature cycling. Electrochem. Commun. 2001, 3, 199–202. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Ohzuku, T. Electrochemical behaviors of LiCo1/3Ni1/3Mn1/3O2 in lithium batteries at elevated temperatures. J. Power Sources 2005, 146, 636–639. [Google Scholar] [CrossRef]
- Bodenes, L.; Dedryvère, R.; Martinez, H.; Fischer, F.; Tessier, C.; Pérès, J.-P. Lithium-ion batteries working at 85 °C: Aging phenomena and electrode/electrolyte interfaces studied by XPS. J. Electrochem. Soc. 2012, 159, A1739–A1746. [Google Scholar] [CrossRef]
- Bodenes, L.; Naturel, R.; Martinez, H.; Dedryvère, R.; Menetrier, M.; Croguennec, L.; Pérès, J.-P.; Tessier, C.; Fischer, F. Lithium secondary batteries working at very high temperature: Capacity fade and understanding of aging mechanisms. J. Power Sources 2013, 236, 265–275. [Google Scholar] [CrossRef]
- Mun, J.; Jung, Y.S.; Yim, T.; Lee, H.Y.; Kim, H.-J.; Kim, Y.G.; Oh, S.M. Electrochemical stability of bis (trifluoromethanesulfonyl) imide-based ionic liquids at elevated temperature as a solvent for a titanium oxide bronze electrode. J. Power Sources 2009, 194, 1068–1074. [Google Scholar] [CrossRef]
- Matsumoto, H.; Kubota, K. The High Temperature Operation of Lithium Secondary Batteries with Using Ionic Liquids. ECS Trans. 2014, 64, 425–432. [Google Scholar] [CrossRef]
- Plylahan, N.; Kerner, M.; Lim, D.-H.; Matic, A.; Johansson, P. Ionic liquid and hybrid ionic liquid/organic electrolytes for high temperature lithium-ion battery application. Electrochim. Acta 2016, 216, 24–34. [Google Scholar] [CrossRef]
- Hu, Q.; Osswald, S.; Daniel, R.; Zhu, Y.; Wesel, S.; Ortiz, L.; Sadoway, D.R. Graft copolymer-based lithium-ion battery for high-temperature operation. J. Power Sources 2011, 196, 5604–5610. [Google Scholar] [CrossRef] [Green Version]
- Wetjen, M.; Kim, G.-T.; Joost, M.; Winter, M.; Passerini, S. Temperature dependence of electrochemical properties of cross-linked poly(ethylene oxide)–lithium bis(trifluoromethanesulfonyl)imide–N-butyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide solid polymer electrolytes for lithium batteries. Electrochim. Acta 2013, 87, 779–787. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A Critical Review of Thermal Issues in Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, R1–R25. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Meakin, P.; Sun, J.; Amini, N.; Forsyth, M. Pyrrolidinium imides: A new family of molten salts and conductive plastic crystal phases. J. Phys. Chem. B 1999, 103, 4164–4170. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ohzuku, T. Electrochemistry of anatase titanium dioxide in lithium nonaqueous cells. J. Power Sources 1985, 14, 153–166. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X. Olivine LiFePO4: The remaining challenges for future energy storage. Energy Environ. Sci. 2015, 8, 1110–1138. [Google Scholar] [CrossRef]
- Delacourt, C.; Poizot, P.; Levasseur, S.; Masquelier, C. Size effects on carbon-free LiFePO4 powders the key to superior energy density. Electrochem. Solid State Lett. 2006, 9, A352–A355. [Google Scholar] [CrossRef]
- Wei, W.; Oltean, G.; Tai, C.-W.; Edström, K.; Björefors, F.; Nyholm, L. High energy and power density TiO2 nanotube electrodes for 3D Li-ion microbatteries. J. Mater. Chem. A 2013, 1, 8160–8169. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oltean, G.; Plylahan, N.; Ihrfors, C.; Wei, W.; Xu, C.; Edström, K.; Nyholm, L.; Johansson, P.; Gustafsson, T. Towards Li-Ion Batteries Operating at 80 °C: Ionic Liquid versus Conventional Liquid Electrolytes. Batteries 2018, 4, 2. https://doi.org/10.3390/batteries4010002
Oltean G, Plylahan N, Ihrfors C, Wei W, Xu C, Edström K, Nyholm L, Johansson P, Gustafsson T. Towards Li-Ion Batteries Operating at 80 °C: Ionic Liquid versus Conventional Liquid Electrolytes. Batteries. 2018; 4(1):2. https://doi.org/10.3390/batteries4010002
Chicago/Turabian StyleOltean, Gabriel, Nareerat Plylahan, Charlotte Ihrfors, Wei Wei, Chao Xu, Kristina Edström, Leif Nyholm, Patrik Johansson, and Torbjörn Gustafsson. 2018. "Towards Li-Ion Batteries Operating at 80 °C: Ionic Liquid versus Conventional Liquid Electrolytes" Batteries 4, no. 1: 2. https://doi.org/10.3390/batteries4010002




