On-Demand Micro-Power Generation from an Origami-Inspired Paper Biobattery Stack
Abstract
:1. Introduction
2. Experimental Sections
3. Results and Discussion
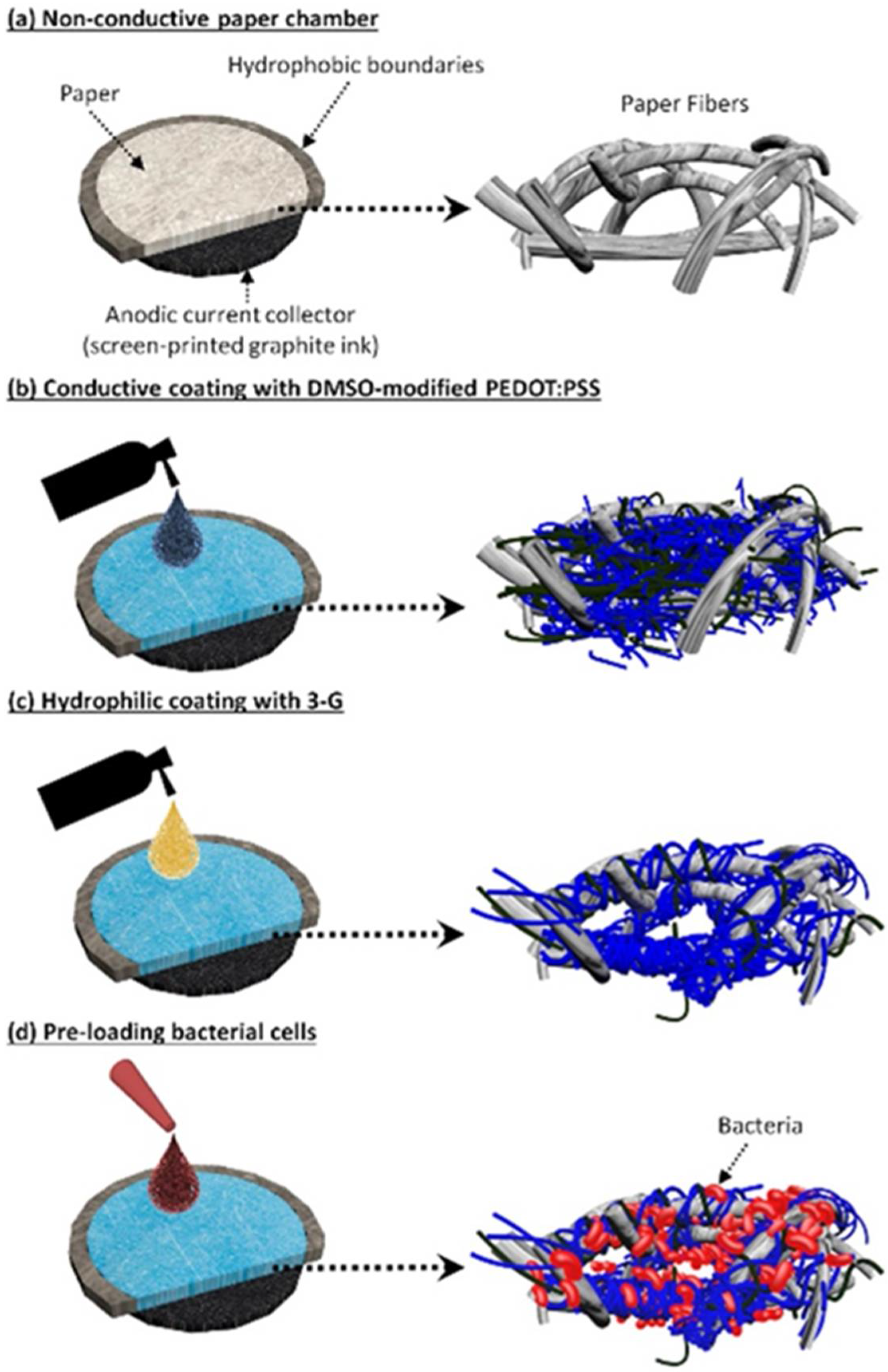
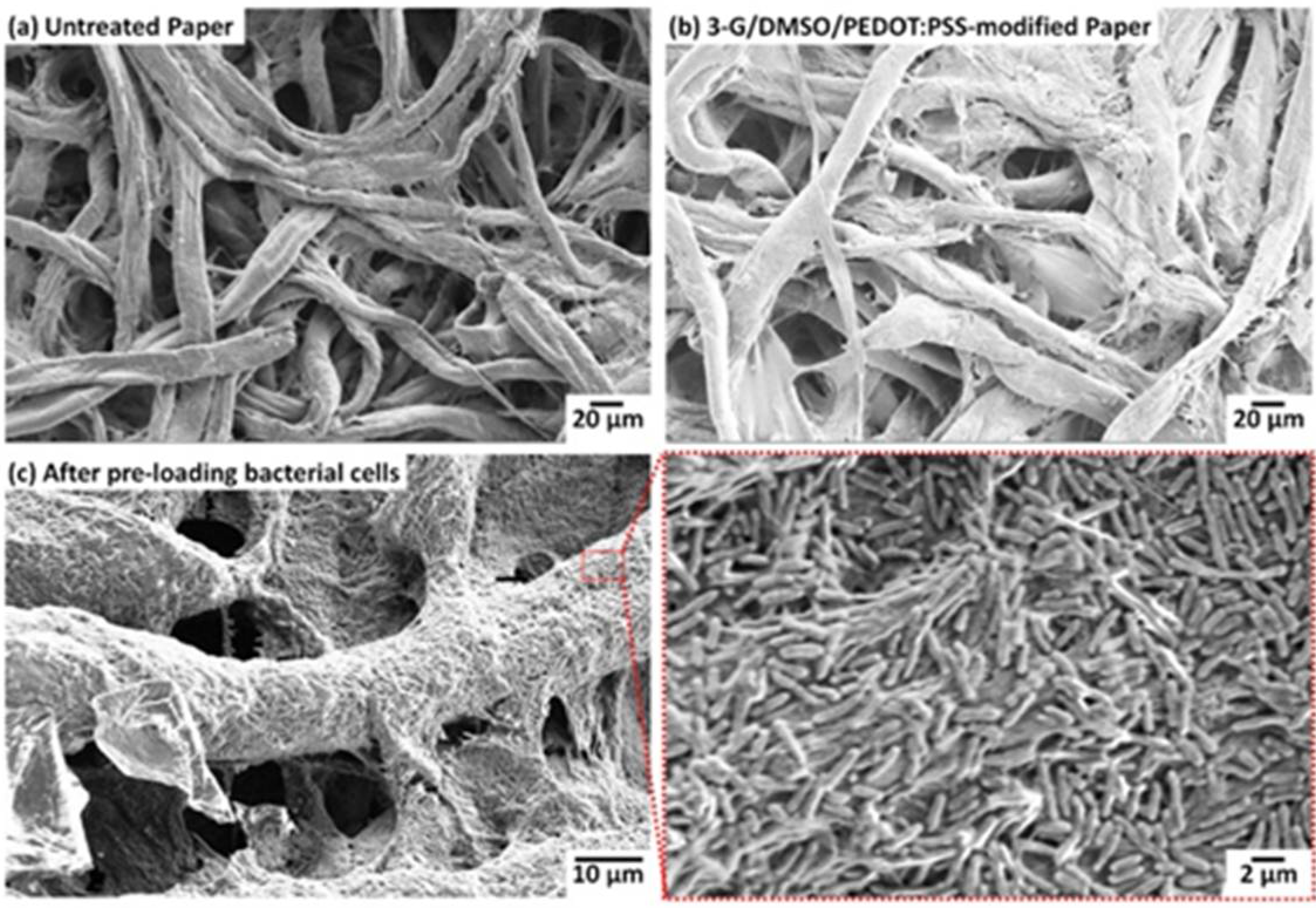
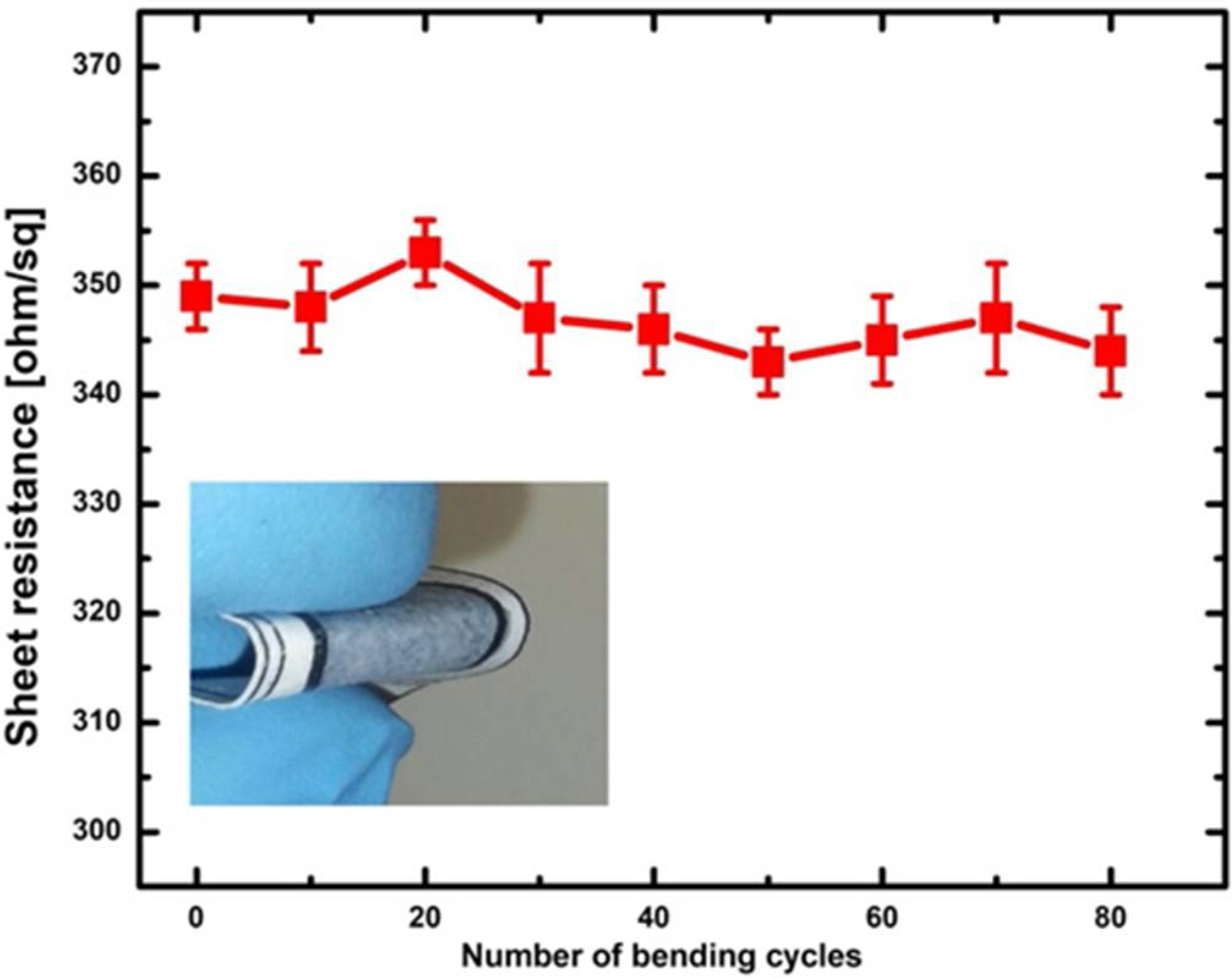
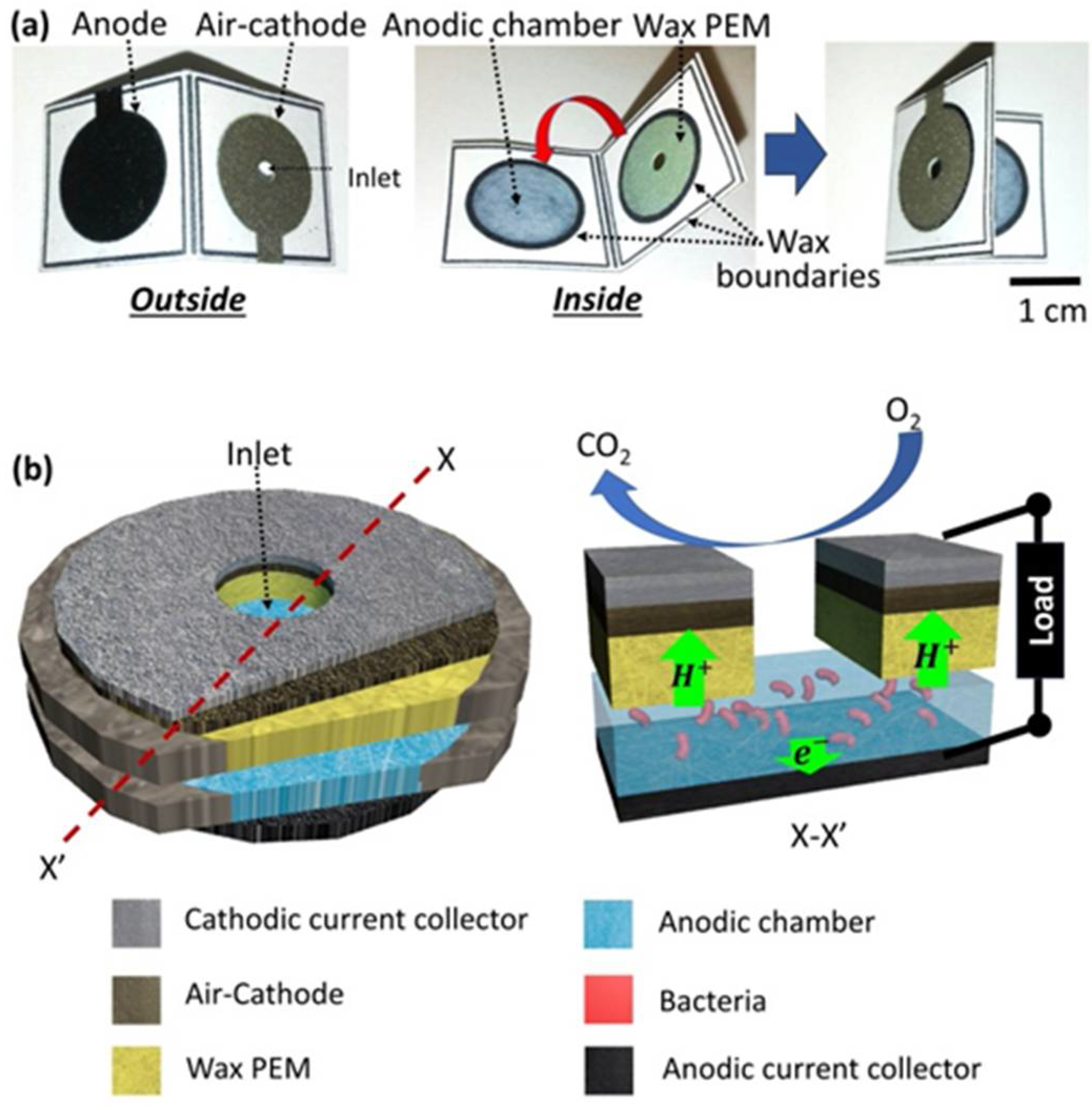
3.1. Conductive and Hydrophilic Anodic Chambers
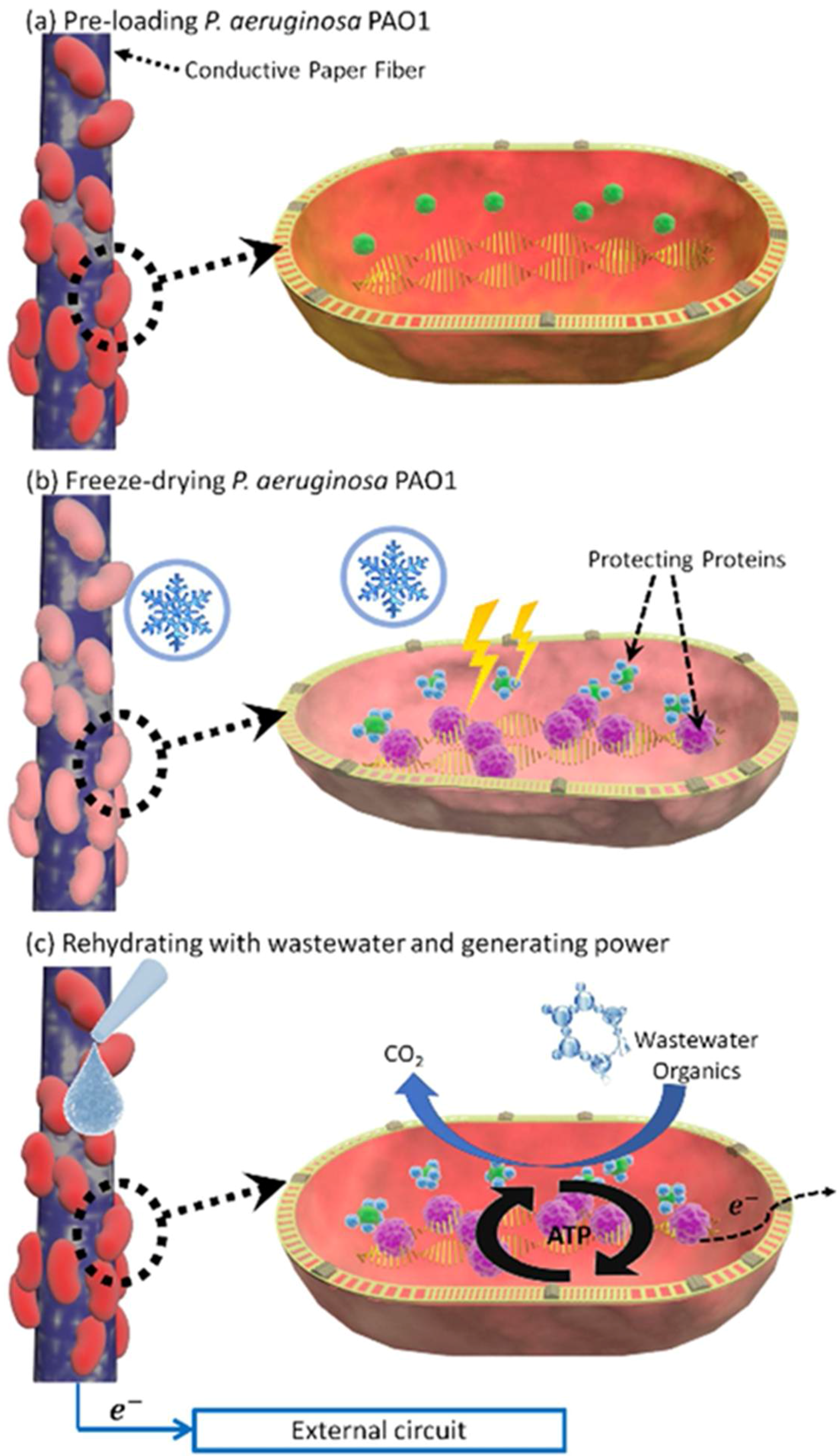
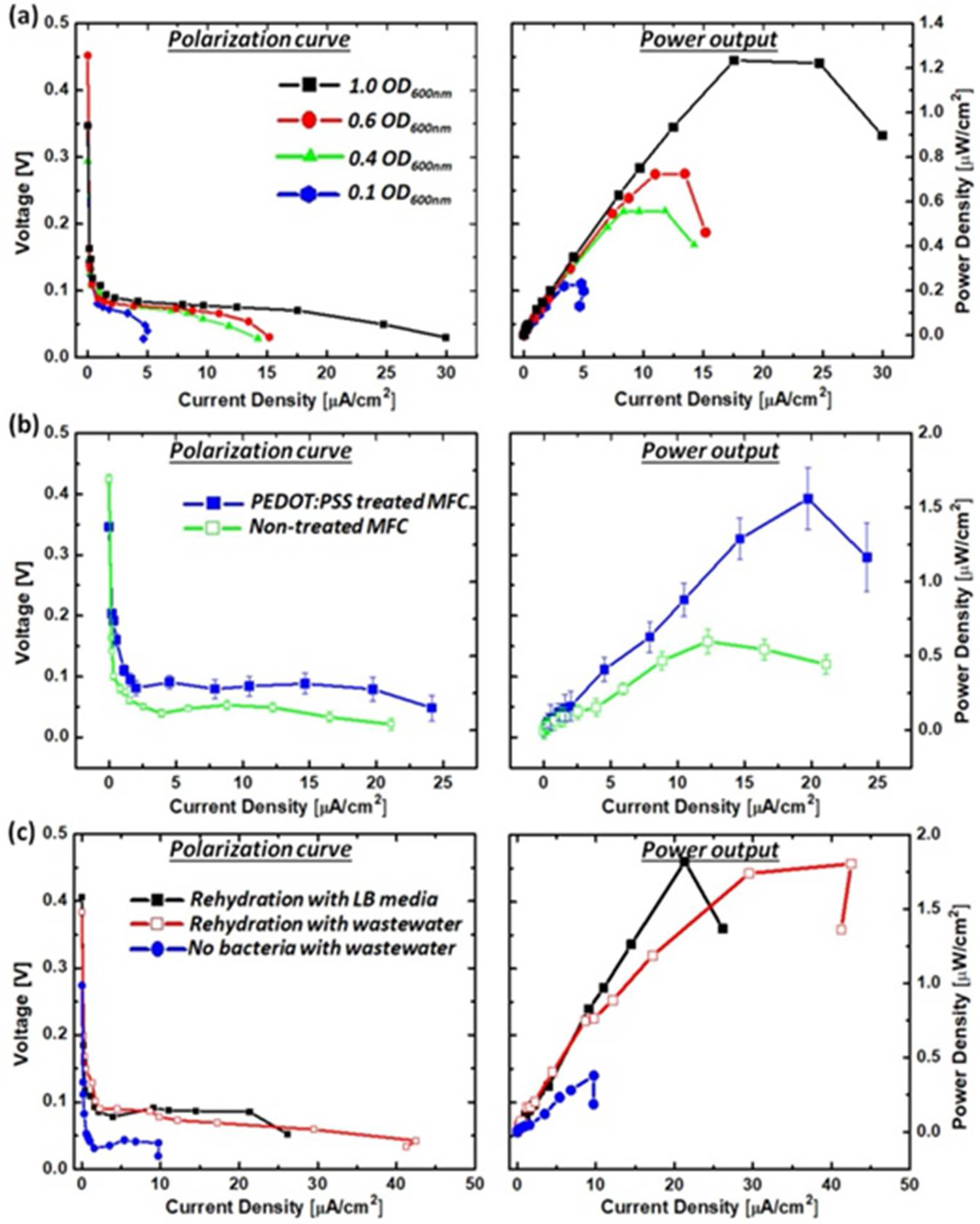
3.2. Freeze-Drying Bacterial Cells
3.3. Power Generation from a Single MFC Unit
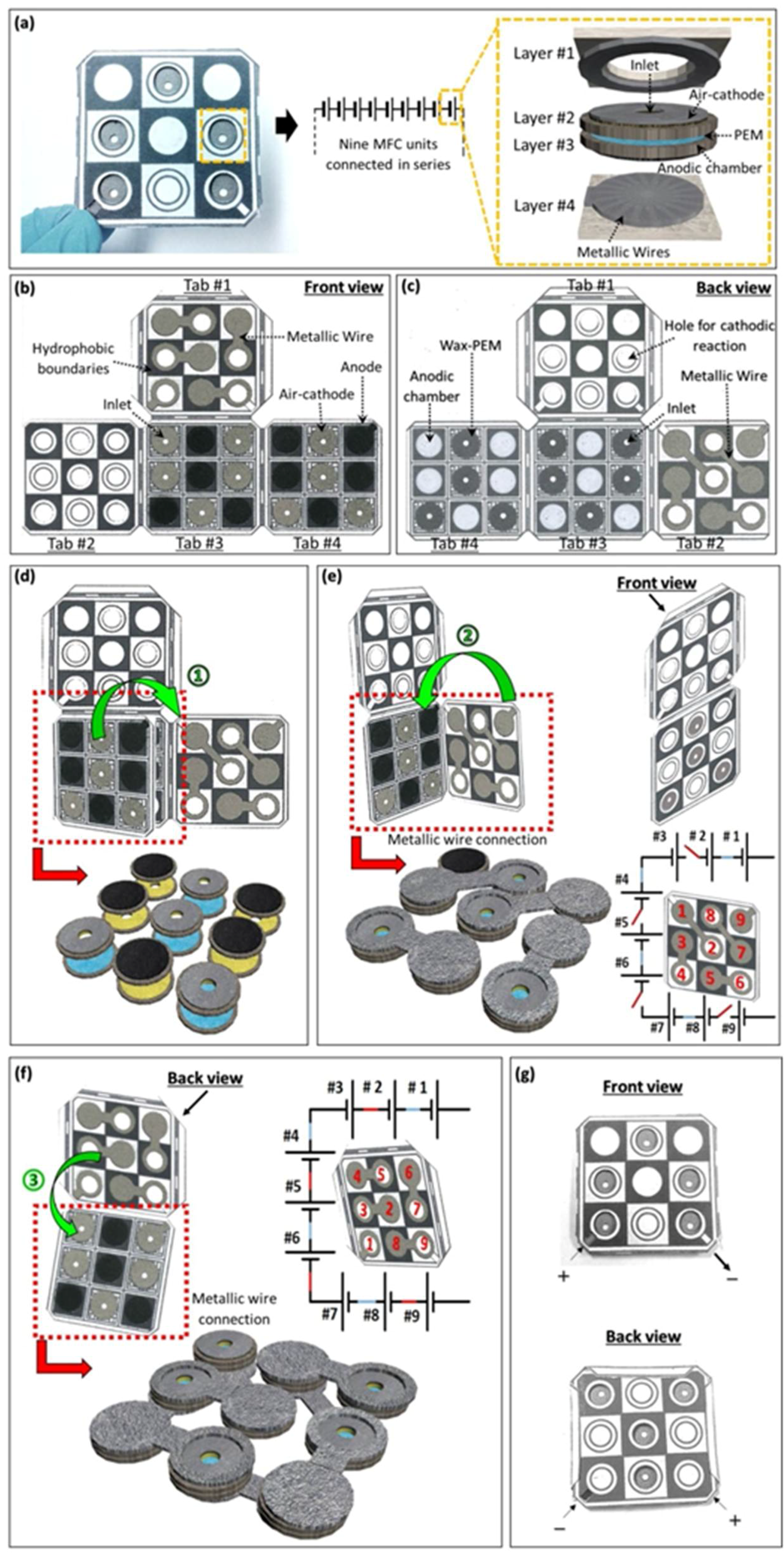
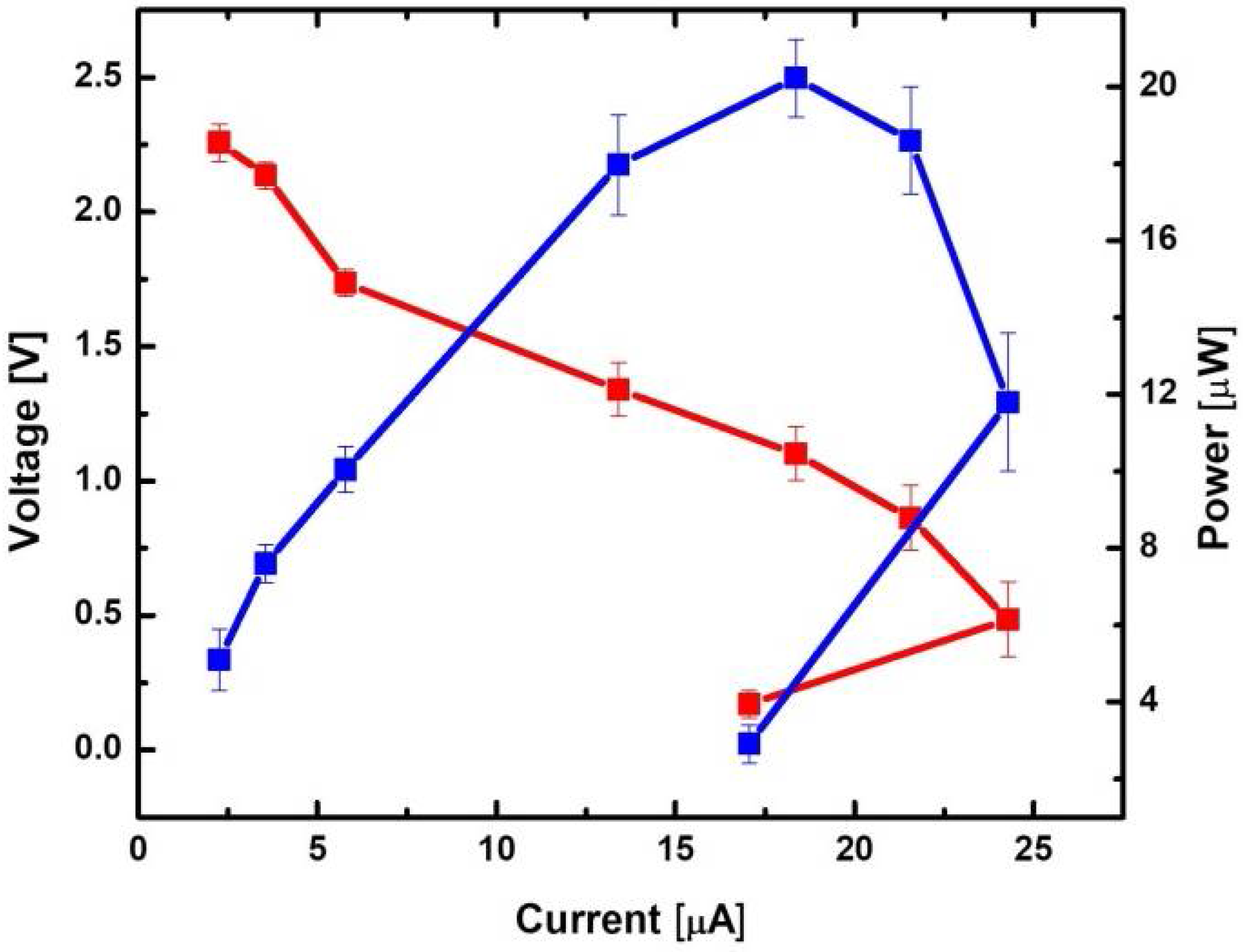
3.4. Power Generation from a Nine-Cell MFC Stack
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chaturvedi, V.; Verma, P. Microbial fuel cell: A green approach for the utilization of waste for the generation of bioelectricity. Bioresour. Bioprocess. 2016, 3, 38. [Google Scholar] [CrossRef]
- Li, S.; Cheng, C.; Thomas, A. Carbon-based microbial fuel cell electrodes: From conductive supports to active catalysts. Adv. Mater. 2017, 29, 1602547. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhai, L.-F.; Li, W.-W.; Yu, H.-Q. Harvest and utilization of chemical energy in wastes by microbial fuel cells. Chem. Soc. Rev. 2016, 45, 2847–2870. [Google Scholar] [CrossRef] [PubMed]
- Babauta, J.; Renslow, R.; Lewandowski, Z.; Beyenal, H. Electrochemically active biofilms: Facts and fiction. A review. Biofouling 2013, 28, 789–812. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E. Scaling up microbial fuel cells and other bioelectrochemical systems. Appl. Microbiol. Biotechnol. 2010, 85, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Shantaram, A.; Beyenal, H.; Raajan, R.; Veluchamy, A.; Lewandowski, Z. Wireless sensors powered by microbial fuel cells. Environ. Sci. Technol. 2005, 1, 5037–5042. [Google Scholar] [CrossRef]
- Choi, S. Microscale microbial fuel cells: Advances and challenges. Biosens. Bioelectron. 2015, 69, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Morse, D.E. Miniaturizing microbial fuel cells. Trends Biotechnol. 2011, 29, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bernarda, A.; Huang, C.; Lee, D.; Chang, J. Micro-sized microbial fuel cell: A mini-review. Bioresour. Technol. 2011, 102, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ali, M.A.; Xu, Z.; Halverson, L.J.; Dong, L. Integrated microfluidic flow-through microbial fuel cells. Sci. Rep. 2017, 7, 41208. [Google Scholar] [CrossRef] [PubMed]
- Siu, C.; Chiao, M. A microfabricated PDMS microbial fuel cell. J. Microelectromech. Syst. 2008, 17, 1329–1341. [Google Scholar] [CrossRef]
- Qian, F.; Baum, M.; Gu, Q.; Morse, D.E. A 1.5 µL microbial fuel cell for on-chip bioelectricity generation. Lab Chip 2009, 9, 3076–3081. [Google Scholar] [CrossRef] [PubMed]
- Fraiwan, A.; Lee, H.; Choi, S. A multi-Anode paper-based microbial fuel cell: A potential power source for disposable biosensors. IEEE Sens. J. 2014, 14, 3385–3390. [Google Scholar] [CrossRef]
- Thom, N.K.; Lewis, G.G.; DiTucci, M.J.; Phillips, S.T. Two general designs for fluidic batteries in paper-based microfluidic devices that provide predictable and tunable sources of power for on-chip assays. RSC Adv. 2013, 3, 6888–6895. [Google Scholar] [CrossRef]
- Phillips, S.T.; Lewis, G.G. The expanding role of paper in point-of-care diagnostics. Expert Rev. Mol. Diagn. 2014, 14, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Mabey, D.; Peeling, R.W.; Ustianowski, A.; Perkins, M.D. Diagnostics for the developing world. Nat. Rev. Microbiol. 2004, 2, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Veerubhotla, R.; Das, D.; Pradhan, D. A flexible and disposable battery powered by bacteria using eyeliner coated paper electrodes. Biosens. Bioelectron. 2017, 94, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ban, J.Y.; Oh, C.-H.; Park, H.-K.; Choi, S. A solvent-free microbial-activated air cathode battery paper platform made with pencil-traced graphite electrodes. Sci. Rep. 2016, 6, 28588. [Google Scholar] [CrossRef] [PubMed]
- Veerubhotla, R.; Bandopadhyay, A.; Das, D.; Chakraborty, S. Instant power generation from an air-breathing paper and pencil based bacterial bio-fuel cell. Lab Chip 2015, 15, 2580–2583. [Google Scholar] [CrossRef] [PubMed]
- Zuo, K.; Liu, H.; Zhang, Q.; Liang, P.; Huang, X.; Vecitis, C.D. A single-use paper-shaped microbial fuel cell for rapid aqueous biosensing. ChemSusChem 2015, 8, 2035–2040. [Google Scholar] [CrossRef] [PubMed]
- Winfield, J.; Chambers, L.D.; Rossiter, J.; Greenman, J.; Ieropoulos, I. Urine-activated origami microbial fuel cells to signal proof of life. J. Mater. Chem. A 2015, 3, 7058–7065. [Google Scholar] [CrossRef]
- Fraiwan, A.; Mukherjee, S.; Sundermier, S.; Lee, H.-S.; Choi, S. A paper-based Microbial Fuel Cell: Instant battery for disposable diagnostic devices. Biosens. Bioelectron. 2013, 49, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Fraiwan, A.; Choi, S. Bacteria-Powered Battery on Paper. Phys. Chem. Chem. Phys. 2014, 16, 26288–26293. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, S. An origami paper-based bacteria-powered battery. Nano Energy 2015, 15, 549–557. [Google Scholar] [CrossRef]
- Fraiwan, A.; Kwan, L.; Choi, S. A Disposable Power Source in Resource-limited Environments: A Paper-based Biobattery Generating Electricity from Wastewater. Biosens. Bioelectron. 2016, 85, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Choi, S. Stepping Towards Self-powered Papertronics: Integrating Biobatteries into a Single Sheet of Paper. Adv. Mater. Technol. 2017, 2, 1600194. [Google Scholar] [CrossRef]
- Hamedi, M.M.; Ainla, A.; Guder, F.; Christodouleas, D.C.; Fernandez-Abedul, M.; Whitesides, G.M. Integrating Electronics and Microfluidics on Paper. Adv. Mater. 2016, 28, 5054–5063. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, M.M.; Campbell, V.E.; Rothemund, P.; Guder, F.; Christodouleas, D.C.; Bloch, J.; Whitesides, G.M. Electrically Activated Paper Actuators. Adv. Funct. Mater. 2016, 26, 2446–2453. [Google Scholar] [CrossRef]
- Mohammadifar, M.; Choi, S.; Papertronics, A. On-demand and Disposable Biobattery: Saliva-activated Electricity Generation from Lyophilized Exoelectrogens pre-inoculated on Paper. Adv. Mater. Technol. 2007. in print. [Google Scholar] [CrossRef]
- Mohammadifar, M.; Zhang, K.; Choi, S. A saliva-powered paper biobattery for disposable biodevices. In Proceedings of the 2017 IEEE 30th International Conference on Micro Electro Mechanical Systems (MEMS), Las Vegas, NV, USA, 22–26 January 2017; pp. 121–124. [Google Scholar]
- Oh, S.-E.; Logan, B.E. Voltage reversal during microbial fuel cell stack operation. J. Power Source 2007, 167, 11–17. [Google Scholar] [CrossRef]
- Lee, C.; Lai, K.; Lin, C.; Li, C.; Ho, K.; Wu, C.; Lau, S.; He, H., Jr. A paper-based electrode using a graphene dot/PEDOT:PSS composite for flexible solar cells. Nano Energy 2017, 36, 260–267. [Google Scholar] [CrossRef]
- Huang, J.; Kekuda, D.; Chu, C.; Ho, K. Electrochemical characterization of the solvent-enhanced conductivity of poly(3,4-ethylenedioxythiophene) and its application in polymer solar cells. J. Mater. Chem. 2009, 19, 3704–3712. [Google Scholar] [CrossRef]
- Gao, Y.; Hassett, D.; Choi, S. Rapid Characterization of Bacterial Electrogenicity Using a Single-Sheet Paper-based Electrofluidic Array. Front. Bioeng. Biotechnol. 2017, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto-Shinohara, Y.; Sukenobe, J.; Imaizumi, T.; Nakahara, T. Survival of freeze-dried bacteria. J. Gen. Appl. Microbiol. 2008, 54, 9–24. [Google Scholar] [CrossRef]
- Wenfeng, S.; Gooneratne, R.; Glithero, N.; Weld, R.J.; Pasco, N. Appraising freeze-drying for storage of bacteria and their ready access in a rapid toxicity assessment assay. Appl. Microbiol. Biotechnol. 2013, 97, 10189–10198. [Google Scholar] [CrossRef] [PubMed]
- Doberenz, S.; Eckweiler, D.; Reichert, O.; Jensen, V.; Bunk, B.; Sproer, C.; Kordes, A.; Frangipani, E.; Luong, K.; Korlach, J.S.; et al. Identification of a Pseudomonas aeruginosa PAO1 DNA Methyltransferase, Its Targets, and Physiological Roles. mBio 2017, 8, e02312-16. [Google Scholar] [CrossRef] [PubMed]
- Whelan, D.R.; Hiscos, T.J.; Rood, J.I.; Bambery, K.R.; Mcnaughton, D.; Wood, B.R. Detection of an en masse and reversible B- to A-DNA conformational transition in prokaryotes in response to desiccation. J. R. Soc. Interface 2014, 11, 20140454. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.; Usall, J.; Teixido, N.; Garcia, N.; Vinas, I. Effect of protective agents, rehydration media and initial cell concentration on viability of Pantoea agglomerans strain CPA-2 subjected to freeze-drying. J. Appl. Microbiol. 2000, 89, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sharbrough, E.; Liu, H. Quantification of the Internal Resistance Distribution of Microbial Fuel Cells. Environ. Sci. Technol. 2008, 42, 8101–8107. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadifar, M.; Choi, S. On-Demand Micro-Power Generation from an Origami-Inspired Paper Biobattery Stack. Batteries 2018, 4, 14. https://doi.org/10.3390/batteries4020014
Mohammadifar M, Choi S. On-Demand Micro-Power Generation from an Origami-Inspired Paper Biobattery Stack. Batteries. 2018; 4(2):14. https://doi.org/10.3390/batteries4020014
Chicago/Turabian StyleMohammadifar, Maedeh, and Seokheun Choi. 2018. "On-Demand Micro-Power Generation from an Origami-Inspired Paper Biobattery Stack" Batteries 4, no. 2: 14. https://doi.org/10.3390/batteries4020014





