Fast Thermal Runaway Detection for Lithium-Ion Cells in Large Scale Traction Batteries
Abstract
:1. Introduction
2. Thermal Runaway Impact
3. Tested Sensors Set
4. Experiment
5. Measurements
6. Discussion
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BEV | Battery electric vehicle |
| PHEV | Plug in hybrid electrical vehicle |
| HEV | Hybrid electric vehicle |
| GTR EVS | Global technical regulation on electrical vehicle safety |
| SnO | Tin dioxide |
| CH | Methane |
| CH | Propane |
| CO | Carbon monoxide |
| IR | Infra red |
| LED | Light emitting diode |
| PCB | Printed circuit board |
| NMC | LiNiMnCoO |
| SOC | State of charge |
References
- Latif, A.A. Volkswagen brand: The fall of an auto empire. J. Glob. Bus. Adv. 2017, 10, 281–304. [Google Scholar] [CrossRef]
- Bergmann, J. When compliance fails. Compliance Elliance J. 2016, 2, 85–94. [Google Scholar]
- Scrosati, B.; Hassoun, J.; Sun, Y.K. Lithium-ion batteries. A look into the future. Energy Environ. Sci. 2011, 4, 3287–3295. [Google Scholar] [CrossRef]
- Kern, R.; Bindel, R.; Uhlenbrock, R. Durchgängiges Sicherheitskonzept für die Prüfung von Lithium-Ionen- Batteriesysteme. ATZ Elektron. 2009, 4, 22–29. [Google Scholar] [CrossRef]
- Barnett, B.; Ofer, D.; Sriramulu, S.; Stringfellow, R. Lithium-ion batteries, safety. In Batteries for Sustainability; Springer: New York, NY, USA, 2013; pp. 285–318. [Google Scholar]
- Brousse, T.; Taberna, P.L.; Crosnier, O.; Dugas, R.; Guillemet, P.; Scudeller, Y.; Zhou, Y.; Favier, F.; Bélanger, D.; Simon, P. Long-term cycling behavior of asymmetric activated carbon/MnO2 aqueous electrochemical supercapacitor. J. Power Sources 2007, 173, 633–641. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Draft Global Technical Regulation on Electric Vehicle Safety, 2017. ECE/TRANS/WP.29/GRSP/2017/2. Available online: https://www.unece.org/fileadmin/DAM/trans/doc/2017/wp29grsp/GRSP-61-07.pdf (accessed on 23 August 2017).
- Feng, X.; Weng, C.; Ouyang, M.; Sun, J. Online internal short circuit detection for a large format lithium ion battery. Appl. Energy 2016, 161, 168–180. [Google Scholar] [CrossRef]
- Roscher, M.A.; Kuhn, R.M.; Döring, H. Error detection for PHEV, BEV and stationary battery systems. Control Eng. Prac. 2013, 21, 1481–1487. [Google Scholar] [CrossRef]
- Spotnitz, R.; Franklin, J. Abuse behavior of high-power, lithium-ion cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Harris, S.; Timmons, A.; Pitz, W. A combustion chemistry analysis of carbonate solvents used in Li-ion batteries. J. Power sources 2009, 193, 855–858. [Google Scholar] [CrossRef]
- MacNeil, D.; Dahn, J. Test of reaction kinetics using both differential scanning and accelerating rate calorimetries as applied to the reaction of LixCoO2 in non-aqueous electrolyte. J. Phys. Chem. A 2001, 105, 4430–4439. [Google Scholar] [CrossRef]
- Maleki, H.; AlHallaj, S.; Selman, J.; Dinwiddie, R.; Wang, H. Thermal properties of lithium-ion battery and components. J. Elecrochem. Soc. 1999, 146, 947–954. [Google Scholar] [CrossRef]
- Yang, H.; Bang, H.; Amine, K.; Prakash, J. Investigations of the exothermic reactions of natural graphite anode for Li-ion batteries during thermal runaway. J. Elecrochem. Soc. 2005, 152, A73–A79. [Google Scholar] [CrossRef]
- Arai, H.; Tsuda, M.; Saito, K.; Hayashi, M.; Sakurai, Y. Thermal reactions between delithiated lithium nickelate and electrolyte solutions. J. Elecrochem. Soc. 2002, 149, A401–A406. [Google Scholar] [CrossRef]
- Feng, X.; Fang, M.; He, X.; Ouyang, M.; Lu, L.; Wang, H.; Zhang, M. Thermal runaway features of large format prismatci lithium ion battery using extended volume accelerating rate calorimetry. J. Power Sources 2014, 255, 294–301. [Google Scholar] [CrossRef]
- Feng, X.; He, X.; Ouyang, M.; Lu, L.; Wu, P.; Kulp, C.; Prasser, S. Thermal runaway propagation model for designing a safer battery pack with 25 Ah LiNixCoyMnzO2 large format lithium ion battery. Appl. Energy 2015, 154, 74–91. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Ramesh, R.; Kumar, T.P. Safety mechanisms in lithium-ion batteries. J. Power Sources 2006, 155, 401–414. [Google Scholar] [CrossRef]
- Tobishima, S.; Yamaki, J. A consideration of lithium cell safety. J. Power Sources 1999, 81–82, 882–886. [Google Scholar] [CrossRef]
- Ponchaut, N.; Marr, K.; Colella, F.; Somadepalli, V.; Horn, Q. Thermal Runaway and Safety of Large Lithium-Ion Battery Systems. In Proceedings of the The Battcon 2015, Orlando, FL, USA, 12–14 May 2015; pp. 17.1–17.10. [Google Scholar]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issue for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Ohsaki, T.; Kishi, T.; Kuboki, T.; Takami, N.; Shimura, N.; Sato, Y.; Sekino, M.; Satoh, A. Overcharge reaction of lithium-ion batteries. J. Power Sources 2005, 146, 97–100. [Google Scholar] [CrossRef]
- Doughty, D.; Roth, E.; Crafts, C.; Nagasubramanian, G.; Henriksen, G.; Amine, K. Effects of additives on thermal stability of Li ion cells. J. Power Sources 2005, 146, 116–120. [Google Scholar] [CrossRef]
- Abraham, D.; Roth, E.; Kostecki, R.; McCarthy, K.; MacLaren, S.; Doughty, D. Diagnostic examination of thermally abused high-power lithium-ion cells. J. Power Sources 2006, 161, 648–657. [Google Scholar] [CrossRef]
- Majimaa, M.; Tadab, T.; Ujiieb, S.; Yagasakib, E.; Inazawaa, S.; Miyazakia, K. Design and characteristics of large-scale lithium ion battery. J. Power Sources 1999, 81–82, 877–881. [Google Scholar] [CrossRef]
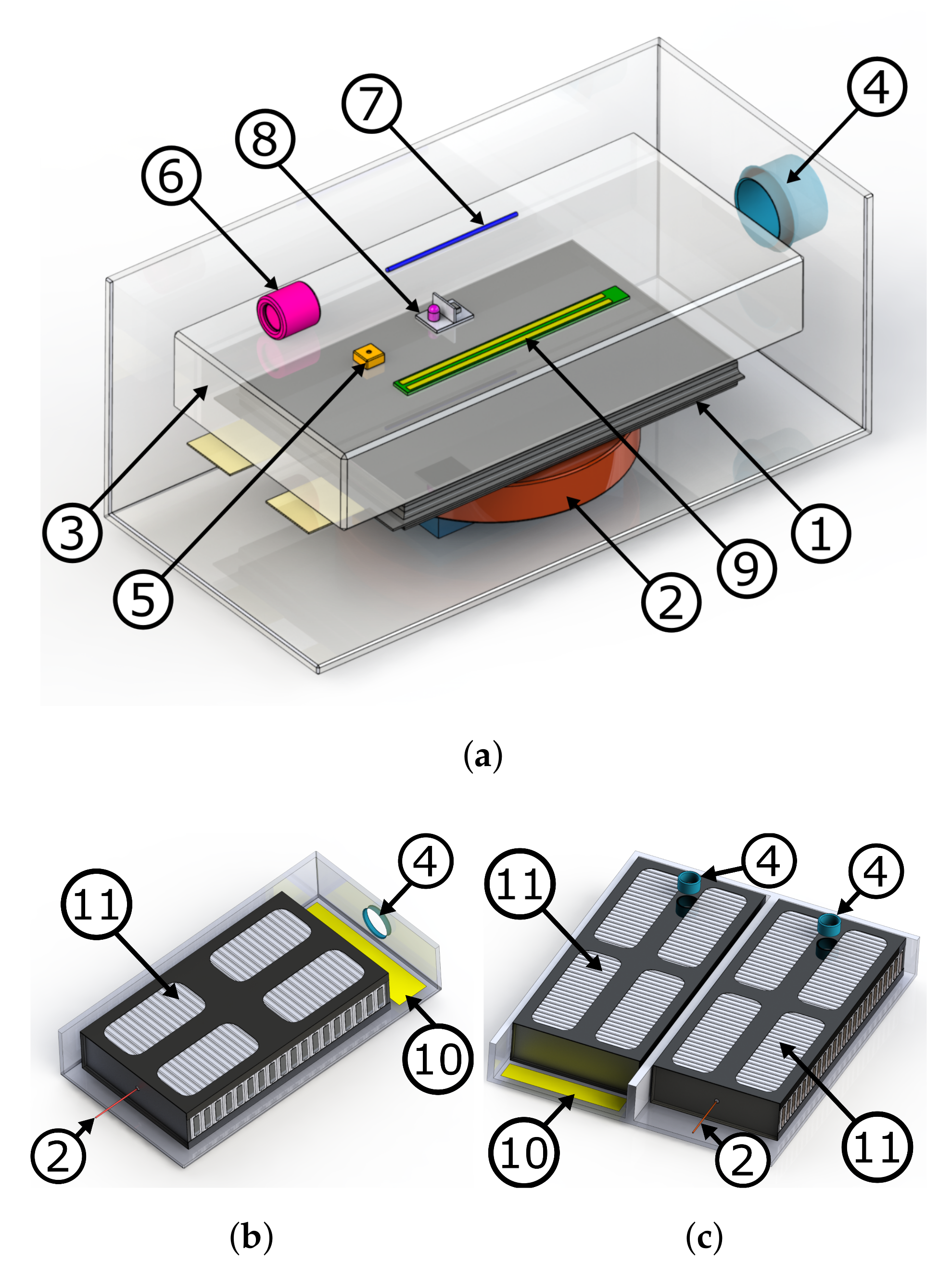
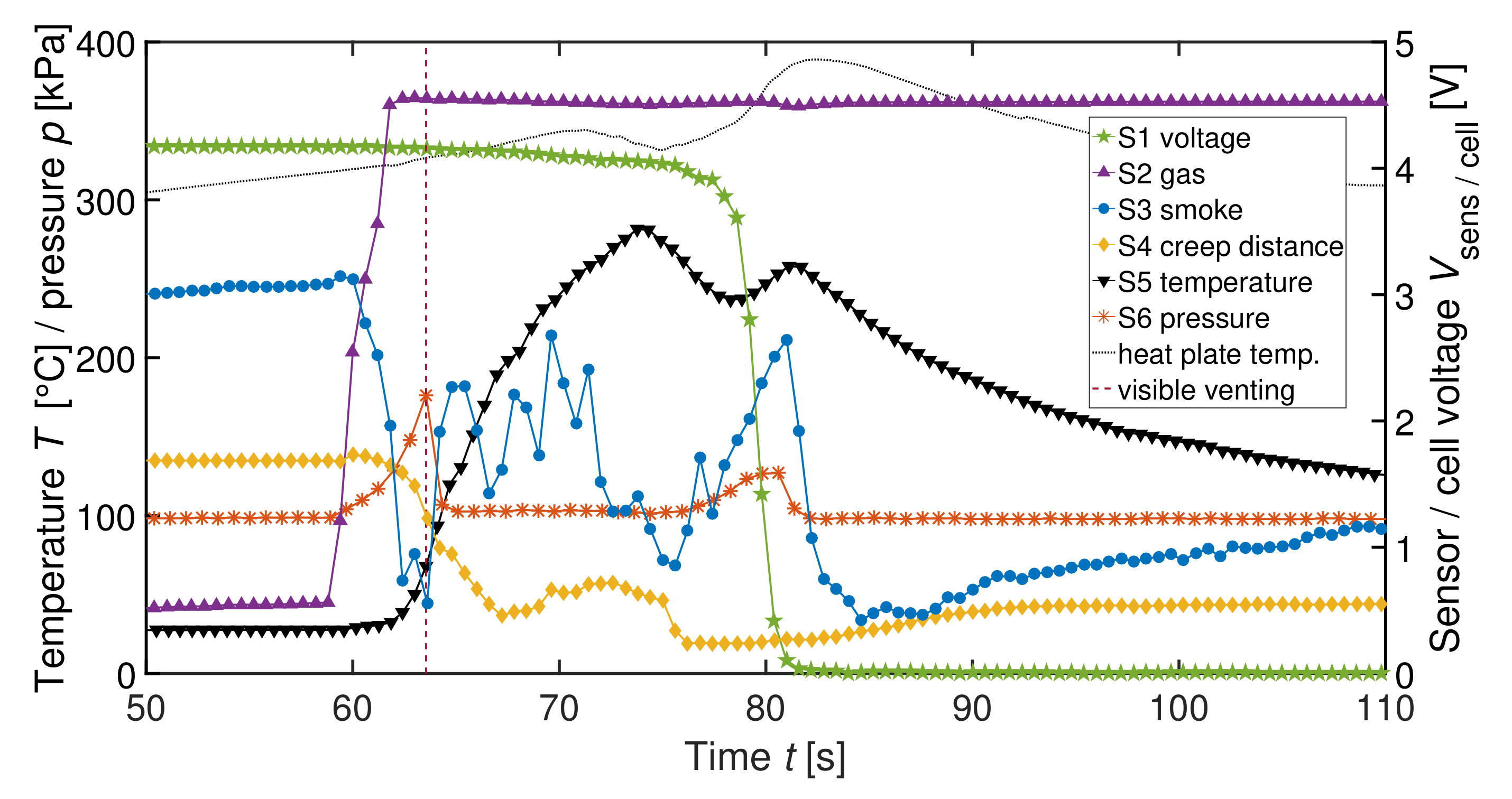
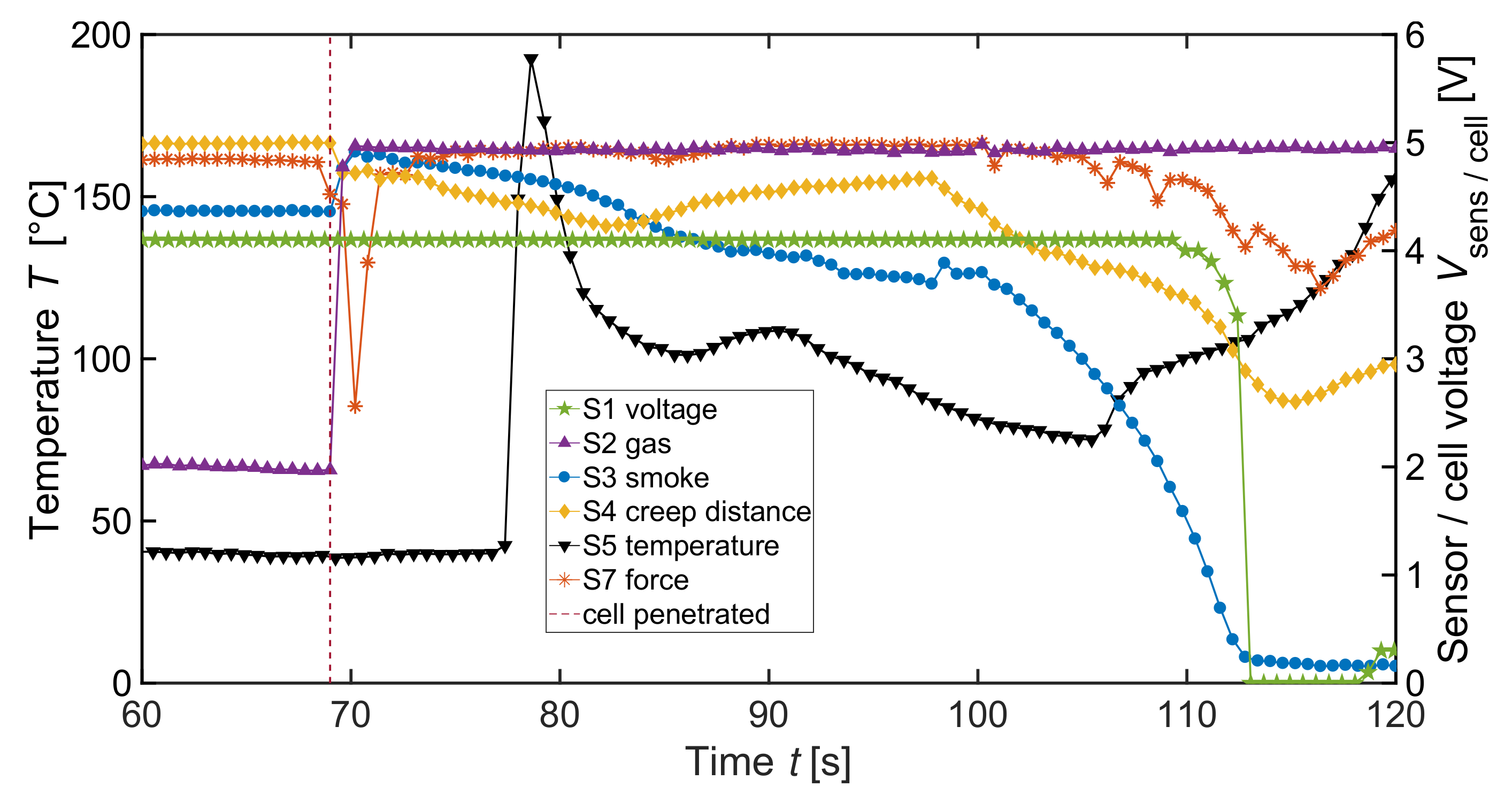
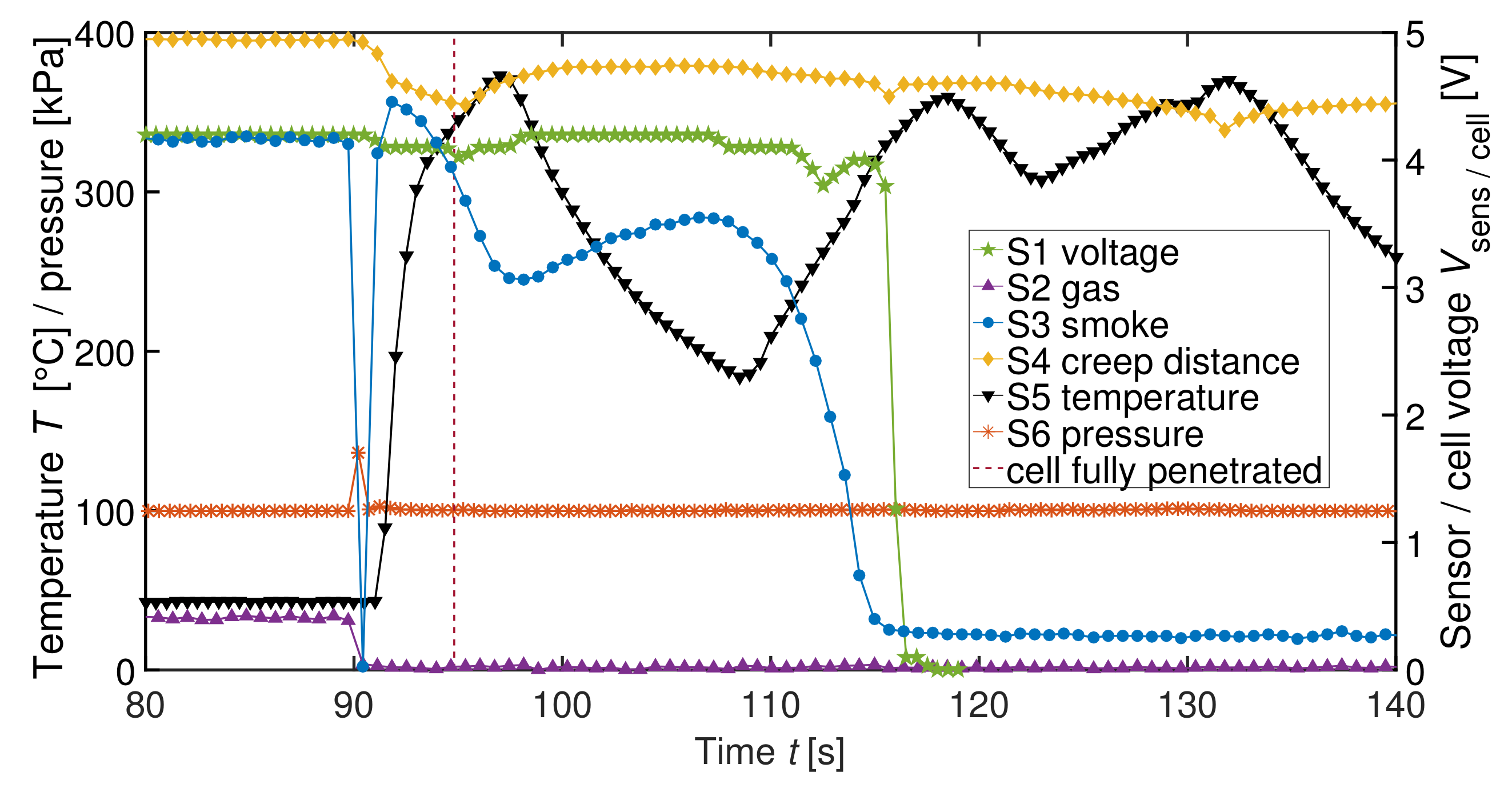
| Label | Sensor | Measured Physical Dimension |
|---|---|---|
| S1 | voltage sensor | voltage of first logical cell |
| S2 | gas detector | presence of chemicals |
| S3 | smoke detector | smoke particle density |
| S4 | creep distance sensor | electrical resistivity of surface |
| S5 | temperature sensor | gas temperature |
| S6 | pressure sensor | pressure |
| S7 | force sensor | force between cells |
| Test | Cell Volume | Cell Energy Density | Connection | Module Count | Trigger | Sensors |
|---|---|---|---|---|---|---|
| V1 | 210 cm | 350 Wh L | 1p | 1 | Heating | S1-S6 |
| V2 | 370 cm | 700 Wh L | 2p | 1 | Nail penetration | S1–S5, S7 |
| V3 | 390 cm | 540 Wh L | 2p | 2 | Nail penetration | S1-S6 |
| Sensor | Detection Speed | Signal Clarity | Sensor Feasibility |
|---|---|---|---|
| S1 voltage | - | + | + |
| S2 gas | + | + | - |
| S3 smoke | - | 0 | 0 |
| S4 creep distance | - | - | + |
| S5 temperature | 0 | 0 | 0 |
| S6 pressure | + | - | + |
| S7 force | + | - | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, S.; Birke, K.P.; Kuhn, R. Fast Thermal Runaway Detection for Lithium-Ion Cells in Large Scale Traction Batteries. Batteries 2018, 4, 16. https://doi.org/10.3390/batteries4020016
Koch S, Birke KP, Kuhn R. Fast Thermal Runaway Detection for Lithium-Ion Cells in Large Scale Traction Batteries. Batteries. 2018; 4(2):16. https://doi.org/10.3390/batteries4020016
Chicago/Turabian StyleKoch, Sascha, Kai Peter Birke, and Robert Kuhn. 2018. "Fast Thermal Runaway Detection for Lithium-Ion Cells in Large Scale Traction Batteries" Batteries 4, no. 2: 16. https://doi.org/10.3390/batteries4020016





