1. Introduction
The demand for flexible electronic devices, such as flexible displays [
1], flexible radio frequency identification (RFID) tags [
2], wearable electronics and implantable devices [
3,
4], is rapidly growing. Flexible lithium-ion batteries (LIB) have gained considerable attention as a promising energy storage solution for such applications [
5,
6,
7,
8,
9,
10,
11,
12,
13]. Metal foils of Al or Cu are the conventional current collectors used in LIB, but are not suitable for flexible batteries, as loss of contact between the active material and the foil is very likely to occur during repeated bending [
14]. The high density of the metal foils also results in a low specific energy density for LIB. Chu et al. reported the use of a thin copper nanowire foil as a current collector to enhance the specific energy density for LIB [
15]. Films based on lightweight materials, such as carbon nanotubes (CNT) and graphene, have been widely investigated as current collectors for flexible LIB [
5,
7,
14,
16]. Yoon et al. reported that carbon nanotube films as negative electrodes for flexible LIB had high flexibility, good mechanical properties, and high electrical conductivity [
17]. However, these nanostructured current collectors and carbon-based materials are relatively expensive and require complex preparation processes. Carbon fibers (CF) have been investigated as an alternative current collector for LIB due to their high conductivity and good mechanical properties [
18,
19,
20]. Furthermore, polyacrylonitrile (PAN)-based CF can be used as active material for the negative electrode in LIB [
21,
22,
23], and Kjell et al. reported that CF can be used as both active material and current collector in LIB [
22]. Jabbour et al. showed that carbon/cellulose fibers composite sheets could be used as current collector-free negative electrodes [
19].
The conventional binder material for the electrodes in LIB is polyvinylidene fluoride (PVDF), which is relatively expensive and needs to dissolve in a toxic solvent, such as N-methyl-pyrrolidone (NMP). In addition, using PVDF as a binder limits the flexibility of the electrodes. Cellulose-based materials, including cellulose fibers, or cellulose nanofibrils (CNF) produced from wood in a sustainable way have gained considerable interest as a promising alternative binder for flexible paper electrodes due to excellent mechanical properties, low cost, and environmental friendliness [
9,
11,
24,
25].
In our previous work, we have shown that flexible electrodes can be fabricated using a low amount (4 wt%) of CNF [
24]. In the present work we have developed this concept further by incorporating lightweight CF current collectors and producing a full battery cell with a high degree of carbon based materials: CF current collectors; CF as active negative electrode material; and CNF as binder. The low amount of binder and the light weight of the CF increase the energy density of the LIB. Electrochemical properties and morphologies for both negative and positive electrodes were investigated from electrochemical charge-discharge characteristics, as well as scanning electron microscopy (SEM), X-ray diffraction (XRD), and Brunauer-Emmet-Teller (BET) measurements. Furthermore, this work demonstrates a facile fabrication process of lightweight and flexible electrodes with integrated current collectors, which is very beneficial from environmental and industrial perspectives.
2. Results and Discussion
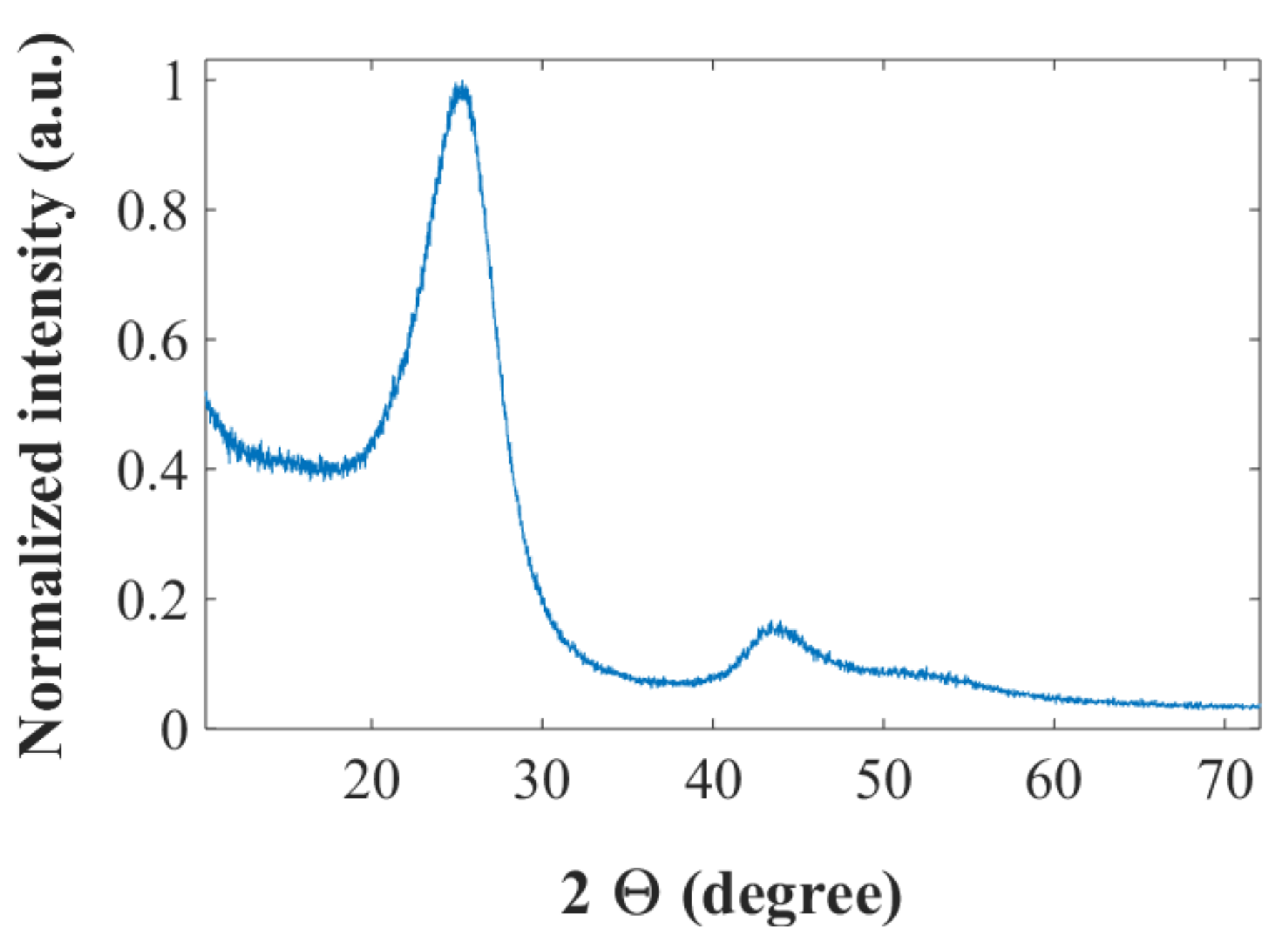
The XRD spectrum for the IMS65 CF, chopped with the 60 mesh (see Materials and Methods) by a Thomas Wiley mini-mill cutting mill, is shown in
Figure 1. The peak around 2θ = 26° corresponds to the (002) reflection for graphite, while the peaks around 2θ = 43° can be attributed to a superposition of the graphitic (100) reflection and the (101) reflection from the disordered CF [
26]. The interlayer spacing,
d002, and the crystallite sizes,
Lc, can be calculated with the Bragg and Scherrer formulae, respectively, to be
d002 = 3.50 Å and
Lc = 24.3 Å. For graphite typical values are
d002 = 3.35 Å and
Lc ≈ 400 Å. The relatively large interlayer spacing and the low values of
Lc for the CF indicate a largely disordered and amorphous structure with small crystal sizes, which is consistent with previous work [
23]. The BET surface area for the chopped CF is found to be 0.65 m
2 g
−1, slightly higher than the unchopped CF measured previously (0.42 m
2 g
−1 [
23]), which is due to the extra area from the chopped cross-sections. It is still very low, however, which is beneficial for a small solid-electrolyte interface (SEI)-layer and stable cycling performance. A summary of the crystallite parameters and the BET surface area is seen in
Table 1.
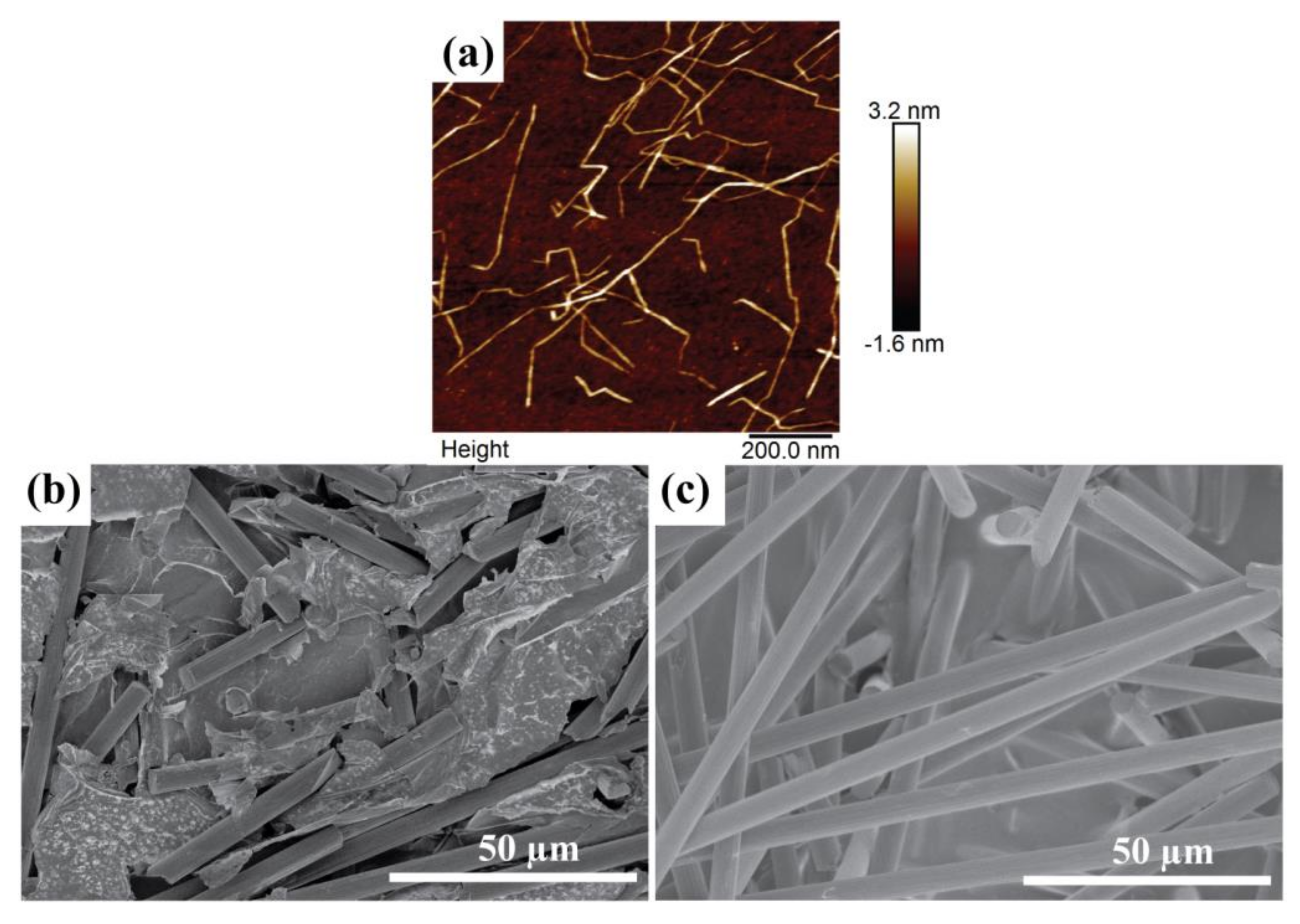
An atomic force microscope (AFM) height image of the CNF is presented in
Figure 2a. It is seen that every fibril is finely dispersed. The width and length of the fibrils are approximately 2–3.5 nm and 0.1–1 µm, respectively.
Figure 2b,c presents SEM images of the CF/CNF films, illustrating the different morphologies of the two sides of each layer. The bottom side (towards the filter) after the filtration process is smooth with densely packed CF bound by CNF sheets (
Figure 2b). The top side (
Figure 2c) is rough, with CF more loosely packed and sticking out from the CNF sheets—note though that the CF adhere strongly to the electrodes so CNF also works well as a binder for this part of the film. Likely the light weight of CF causes them to easily float over the film surface during the filtration.
In order to increase the conductivity of the CF/CNF electrodes, 2 wt% Super-P carbon (SPC) was added to their composition.
Figure 3 shows SEM images of surfaces and cross sections of the CF/SPC/CNF electrodes. The CF are homogenously dispersed by CNF sheets, and the SPC particles are relatively uniformly mixed into the CNF sheets, as seen in
Figure 3a. Some agglomeration is seen, which is normal for nano-sized particles. The thickness of the CF/SPC/CNF films is approximately 55 µm as shown in the cross sectional image in
Figure 3b. The inset in
Figure 3b shows that the diameter of a CF is ~5 µm, which is consistent with the manufacturer’s data. Each CF is wrapped by CNF sheets, which is in agreement with the surface SEM images, and the overall morphologies of the electrodes seem uniform.
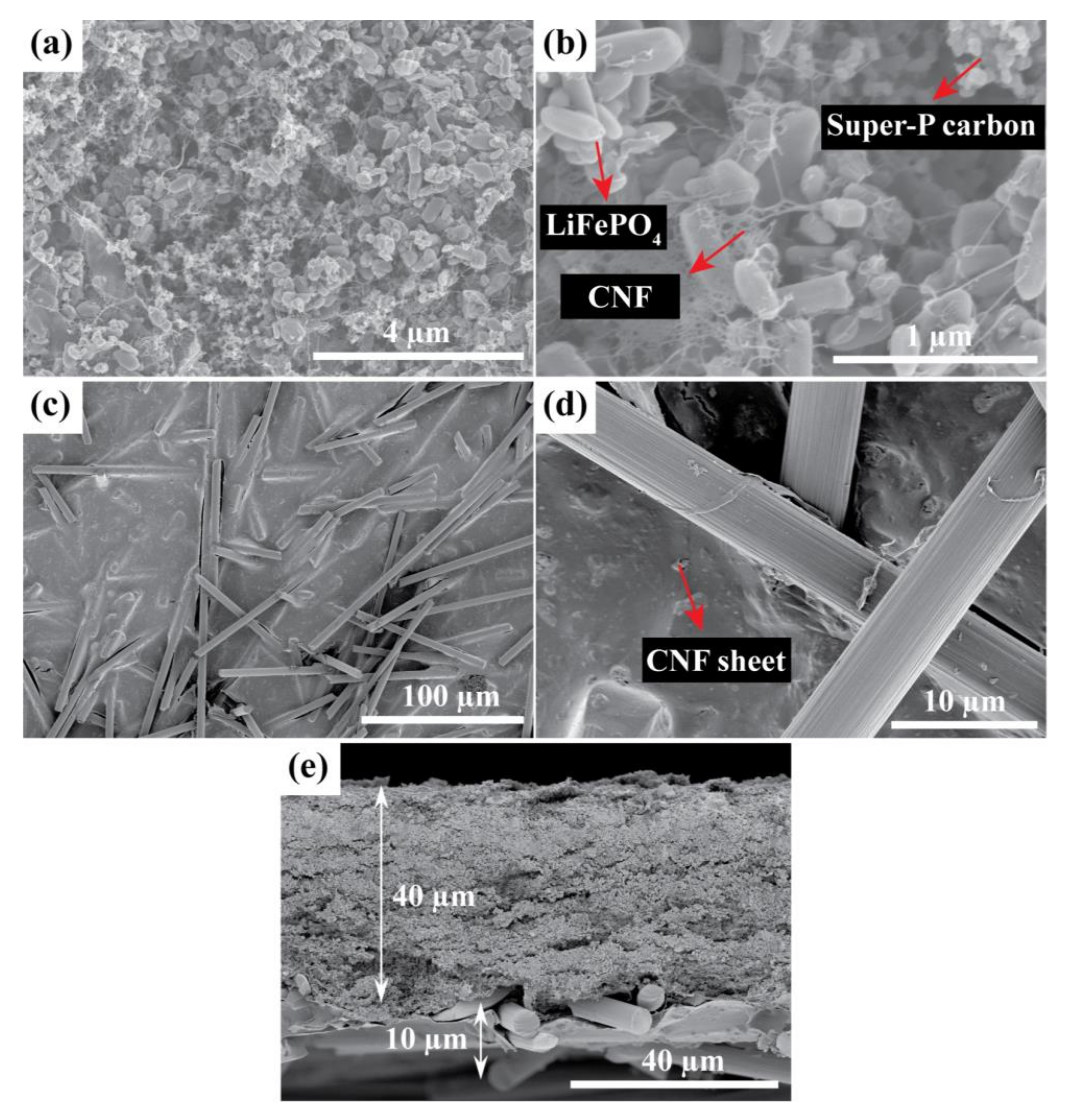
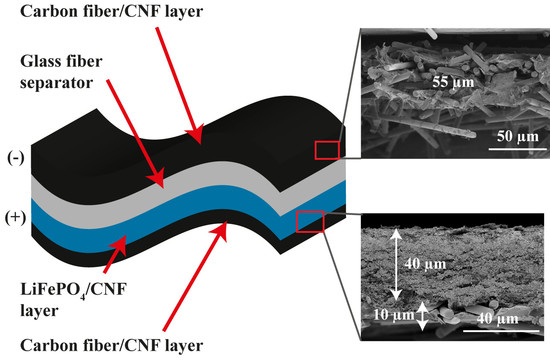
Figure 4 shows SEM images of the LFP/SPC/CNF electrode with integrated CF/SPC as the current collector (LFP-CF). The LFP and SPC nanoparticles are uniformly dispersed by the CNF web, as shown in
Figure 4a,b. The CF are relatively homogenously distributed and surrounded by CNF sheets, as seen in
Figure 4c,d. The cross-sectional SEM image in
Figure 4e shows that the thickness of the LFP and CF current collector layers are approximately 40 and 10 µm, respectively.
The mechanical properties and conductivities of the LFP-CF and CF/SPC/CNF electrodes are shown in
Table 2. Both exhibit similar strength and modulus since they both consist of ~4 wt% CNF as binder, which is the main contributor to the mechanical properties. Their ultimate tensile strengths are ~2 MPa. The Young’s modulus for the CF/SPC/CNF negative electrode is ~225 MPa, which is slightly higher than that of the LFP-CF positive electrode (~201 MPa). This could be ascribed to the higher concentration of CF, with advantageous mechanical properties, in the negative electrodes. The tensile strain at break for the CF/SPC/CNF is lower than that of the LFP-CF electrode, which is probably caused by the stiffness of the CF. The conductivity of the CF/SPC/CNF is 143 S m
−1; the high value is ascribed to the high CF content in the electrode (94 wt%). The conductivity of the LFP-CF electrode is 95 S m
−1. The lower value compared to the CF/SPC/CNF electrode is ascribed to the relatively lower amount of CF. Since the rate performance is good (see below), these are sufficient conductivities for usage without additional current collectors. The conductivities can be compared to our previous work, where an LFP/SPC/CNF electrode exhibited a conductivity of around 0.1–1 S m
−1, depending on the component ratios [
24]. Even with as much as 11 wt% SPC, the conductivity was no higher than 1.14 S m
−1. The much higher value clearly shows the effect of integrating a CF current collector film.
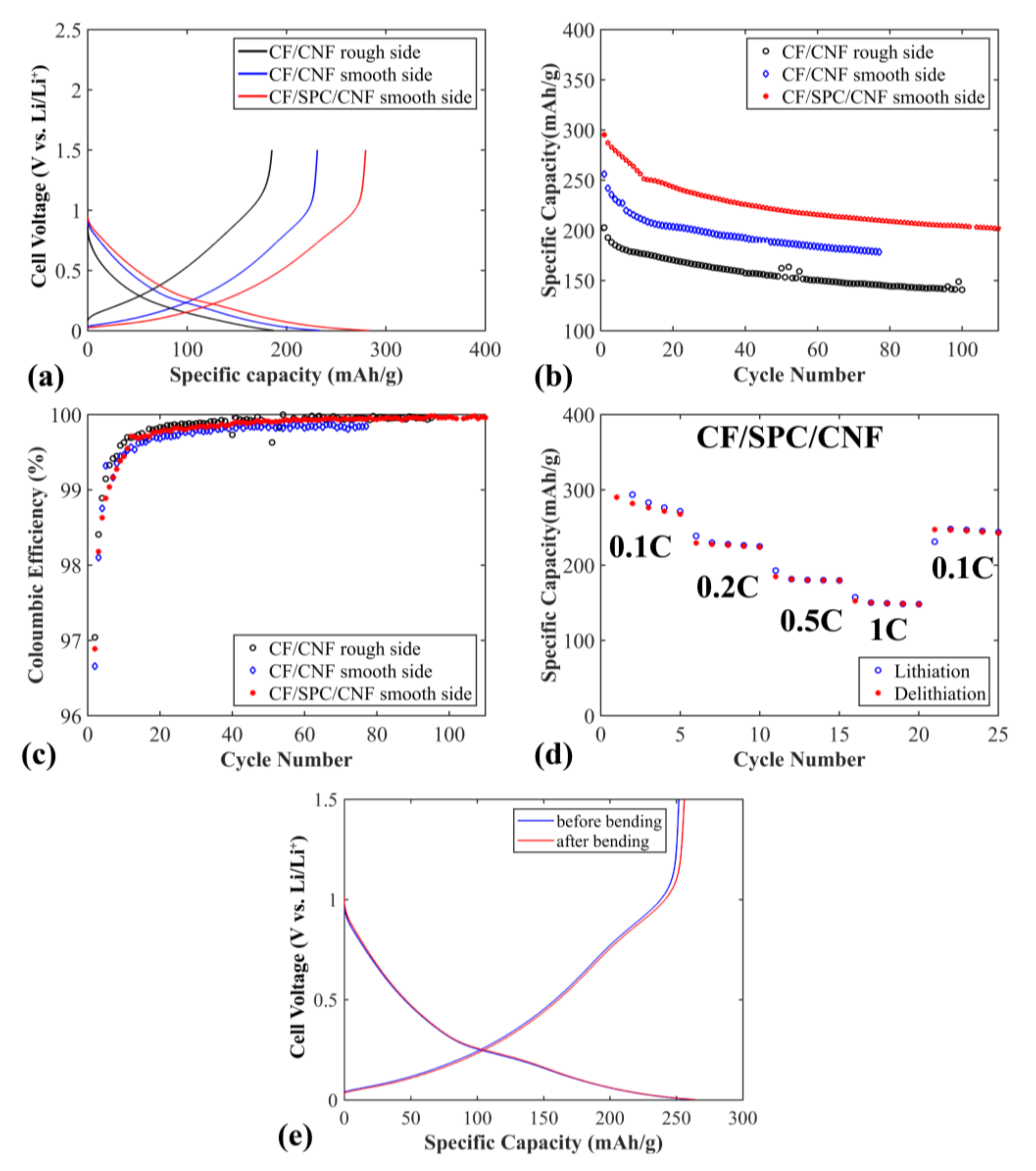
Figure 5 shows the electrochemical properties of the flexible CF/CNF and CF/SPC/CNF negative electrodes. The sloping voltage profile is typical for disordered carbon materials, where a distribution of Li
+ insertion sites generates a distribution of the chemical potentials [
27]. As shown in
Figure 5a, the electrode shows a higher specific capacity and lower polarization when the smooth side of a CF/CNF sheet is oriented towards the separator, compared to when the rough side of the CF/CNF films is pressed against the separator. This illustrates that the smooth side of the CF/CNF electrode, with densely-packed CF, seems to be beneficial for Li
+ transfer while the rough side is beneficial as the current collector side, as it contains less of the insulating CNF. When 2 wt% SPC is added to CF/CNF, increasing the conductivity between the CF, even better electrochemical properties are achieved, including higher specific capacity and lower polarization (see
Figure 5a,b). The initial specific capacity of the electrode is ~300 mAh g
−1, but drops rapidly to ~250 mAh g
−1 after 13 cycles, which is ascribed to the solid electrolyte interface (SEI) layer stabilization for CF electrodes [
23]. A slightly lower specific capacity is observed for these flexible electrodes compared to free standing, unchopped, electrodes of the same type of CF in previous work [
23] (300 mAh g
−1 versus 358 mAh g
−1). This is to be expected, since the fibers are chopped here which, in addition to the added CNF binder, lowers the conductivity of the electrodes (the conductivity of the non-chopped carbon fibers is 694 S cm
−1). The capacity retention is ~83% after 13 cycles, which is similar to previous studies [
23]. The electrode maintains a relatively stable specific capacity for the following cycles, with a specific capacity retention of ~80% after 110 cycles. The coulombic efficiency of the electrodes is shown in
Figure 5c. After 13 cycles, the coulombic efficiency of the CF/SPC/CNF electrode reaches 99.71% and increases to over 99.90% after 41 cycles. This illustrates that the CF/SPC/CNF electrode exhibits a good cycling stability with a low amount of side reactions.
Rate capabilities of the CF/SPC/CNF electrode are shown in
Figure 5d. The electrodes exhibit relatively stable capacities for all C-rates, except for a drop during the first five cycles at 0.1 C. This is caused by the SEI layer stabilization, as well as by Li being trapped in the disordered CF structure, which has been observed previously for PAN-based CF [
23]. The specific capacity decreases from ~267 to ~230 mAh g
−1 when the current rate increases from 0.1 to 0.2 C due to higher polarization at increased current density. At high currents, 0.5 C and 1 C, the specific capacities of the electrode are ~180 mAh g
−1 and ~150 mAh g
−1, respectively. The specific capacity at 1 C is higher than that of the CF paper electrode at 0.6 C (~75 mAh g
−1) mentioned in the work of Jabbour et al. [
19]. This is most probably due to the lower amount of CNF (4 wt%) that was used to prepare our electrode, compared to ~20 wt% cellulose fibers in Jabbour’s work [
19]. When the current rate changes back to 0.1 C, the electrode obtains a relatively stable capacity of ~242 mAh g
−1, with a capacity retention of 91% compared to the first 0.1 C rate capacity.
Figure 5e presents voltage profiles at 0.1 C for CF/SPC/CNF electrodes before and after 4000 times repeated bending. SEM pictures after bending and consecutive cycling of the same electrode is presented in
Figure S1a,b. No significant difference is observed, in either voltage profiles or morphology seen in the SEM, which indicates that the flexibility of the electrode is good, and that the electrochemical properties are unaffected by repeated bending.
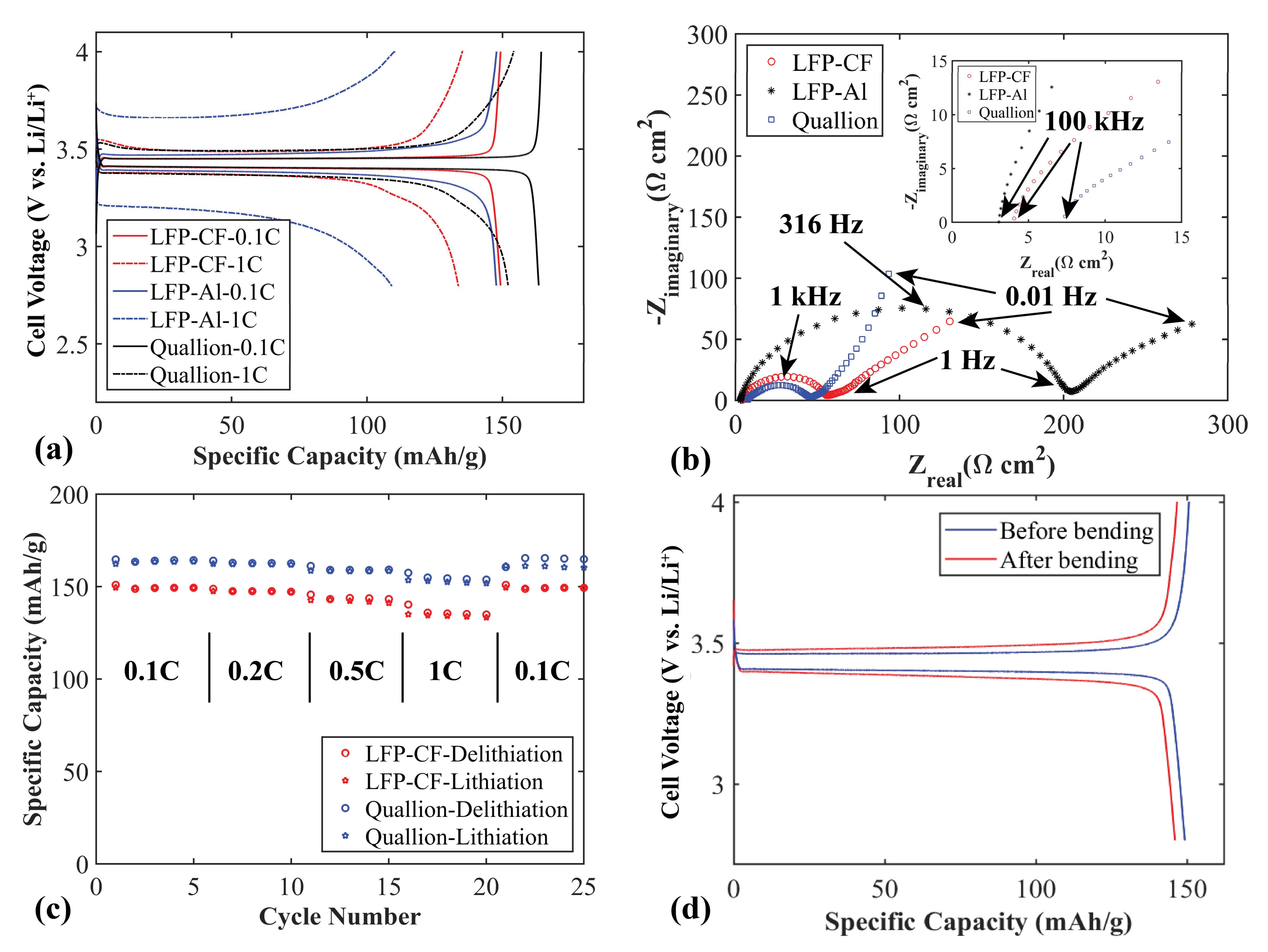
Figure 6a shows a comparison between the charge/discharge voltage profiles for flexible LFP electrodes using CF or Al as current collectors (LFP-CF and LFP-Al). At 0.1 C, the electrodes have similar specific capacities, but with a slightly larger charge and discharge overpotential for the electrode with Al as current collector. At 1 C the electrode with CF as current collector exhibits higher specific capacity and lower polarization (101 mV compared to 486 mV) than that of the electrode with an Al current collector. This indicates a significantly lower overall contact resistance when using CF as current collector compared to conventional Al current collectors, likely due to the close interface and good contact between the LFP and CF layers prepared by the filtration process.
To further evaluate the performance of the LFP-CF flexible electrode, a commercial Quallion LFP electrode was cycled. As seen in
Figure 6a, at 0.1 C both the Quallion electrode and the LFP-CF electrode reach their maximum specified capacities (170 mAh g
−1 and 150 mAh g
−1, respectively). The reason for the lower capacity of the LFP-CF compared to the Quallion electrode is that the LFP we use in this work has a specific capacity of ~150 mAh g
−1 according to the supplier (170 mAh g
−1 theoretical max for LFP). The LFP-CF and the Quallion electrodes show similar polarization behavior. In
Figure 6b, EIS Nyquist plots is presented for the three different electrodes. The higher polarization of the LFP-Al electrode is confirmed by a charge-transfer resistance (semicircle diameter) of ~200 Ω cm
2 compared to ~50 Ω cm
2 for the other electrodes. The LFP-CF electrode has a charge-transfer resistance similar to that of the Quallion electrode.
Figure 6c shows the rate capabilities of the LFP-CF and of the Quallion electrode. The specific capacities of the Quallion electrode are slightly higher overall than those of the LFP-CF electrode at different currents, for the same reason as explained above. The rate capabilities of the LFP-CF are very good, and similar to those of the Quallion electrode. The LFP-CF electrode obtains a specific capacity of ~150 mAh g
−1 at 0.1 C. After increasing current rates to 0.2 C and 0.5 C, it delivers a capacity of 147 and 142 mAh g
−1, respectively. Even at a high current rate (1 C) the electrode can keep a stable capacity of 133 mAh g
−1. After 20 cycles at different current rates, when the current is decreased to 0.1 C again, the electrode regains the full capacity as the first five cycles at 0.1 C (~100% retention).
Figure 6d shows voltage profiles at 0.1 C before and after 4000 times of repeated bending. SEM pictures after bending and cycling is seen in
Figure S1c,d. As with the CF/SPC/CNF electrode no large difference is observed in the voltage profiles, except a slightly lower capacity after bending which could be ascribed to local variations of active material distribution in the electrode. The SEM pictures revealed no visible difference in morphology after bending and cycling. It can be concluded that this electrode also has good flexibility, and that the electrochemical performance is maintained after being bent many times.
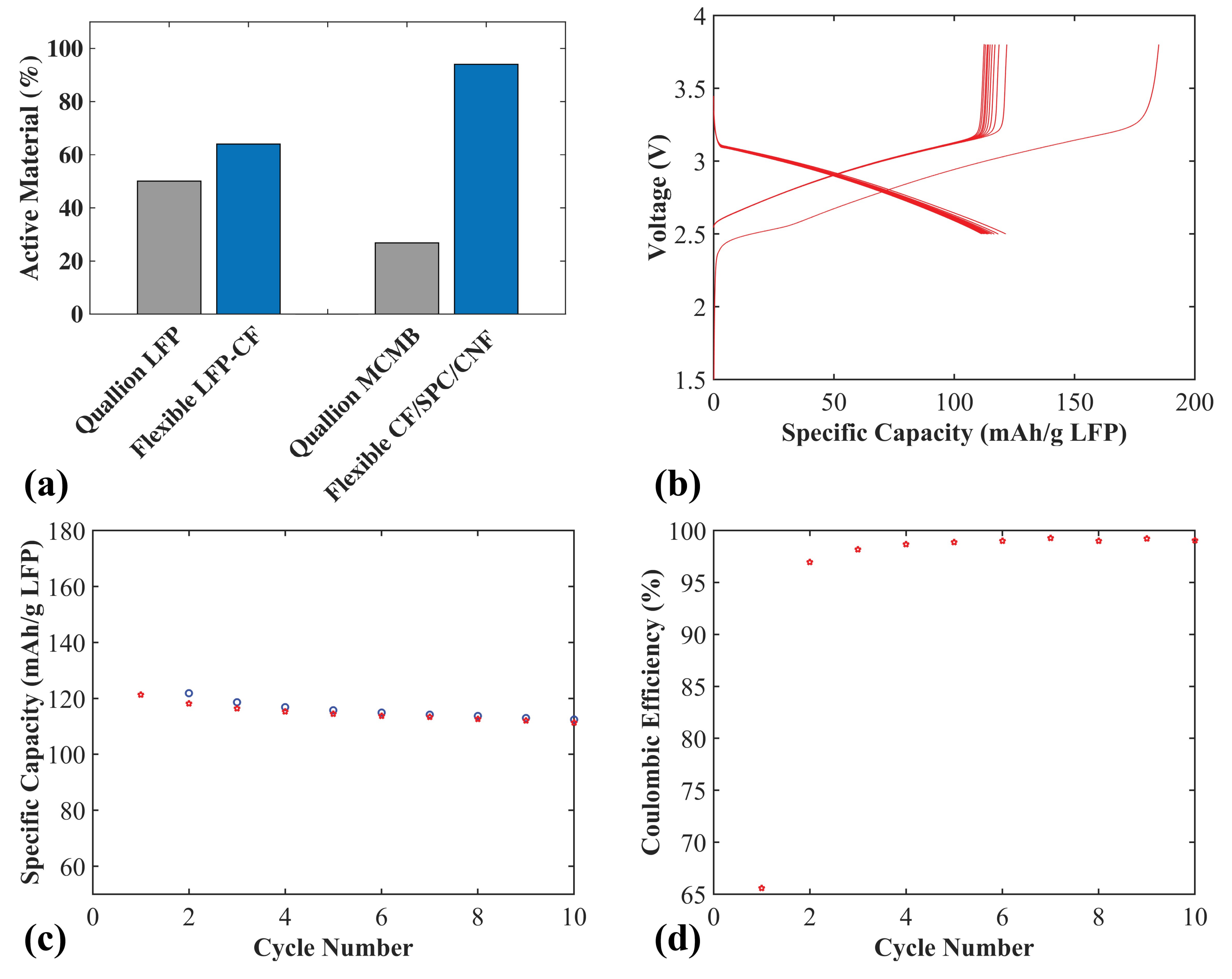
In order to evaluate the weight reduction for both electrodes when using CF as the active material and current collector, compared to conventional electrodes, the active weight percentage was calculated and compared to commercial Quallion electrodes (see
Figure 7a). The Quallion positive electrode is LFP based, with 84 wt% active material and a 20 µm Al current collector, with a full electrode thickness of 55 µm. The negative electrode is a graphite meso-carbon microbead (MCMB) electrode with 90 wt% active material and a 10 µm Cu current collector, with a full electrode thickness of 40 µm. When calculating the active material weight percentages for all electrodes in
Figure 7a, the weight of the current collectors were included. For the positive LFP-CF flexible electrode (thickness 50 µm), an increase of 28% active material, from 50.1% to 64%, was achieved. For the negative CF/SPC/CNF electrode (thickness 55 µm), a large increase of 250% (from 26.8% to 94%) was achieved. This is because, for the negative electrode, carbon fibers are used as both the active material and the current collector, the Cu current collector in the MCMB electrode makes up much of the total weight. This illustrates a large positive impact on the gravimetric energy density compared to conventional electrodes.
Figure 7b–d shows the electrochemical properties of a full flexible cell with a positive LFP-CF electrode and a negative CF/SPC/CNF electrode. The charge/discharge voltage profiles are shown in
Figure 7b. The specific capacity is based on the weight of the LFP. The irreversible discharge capacity of the cell for the first cycle is again likely caused by the SEI layer formation and trapped Li in the CF/SPC/CNF negative electrode, which is observed for the CF/SPC/CNF half-cell in
Figure 5.
Figure 7c,d show that the full cell obtains an initial specific capacity of ~121 mAh g
−1, a 92% capacity retention of the initial capacity after 10 cycles, and a coulombic efficiency of approximately 99%. The gravimetric energy density of the full cell for the initial cycle, based on the total mass of the CF current collectors and electrodes, is ~150 Wh kg
−1. The mass of the electrolyte, separator and the package was omitted since pouch cells were used, with an excess of electrolyte and a large 260 µm glass fiber separator in contrast to the lightweight separators normally used in commercial cells. Adding the weight of those components would lower the energy density slightly. However, the values obtained are still in the range of commercial LIB (100–200 Wh kg
−1 [
28]), which is a high value considering that commercial LIB are highly optimized, with typically double-coated current collectors. This illustrates that the flexible cell can be assembled with a positive flexible electrode using a lightweight CF current collector and a negative electrode based on CF flexible films without any additional current collector. This use of CF is a promising method to significantly raise the energy density and lower the cost of future batteries.