Challenges in Automotive Fuel Cells Recycling
Abstract
:1. Introduction
2. Theoretical Context
2.1. Proton Exchange Membrane Fuel Cells
2.2. Development of the Platinum Content
3. State-Of-The-Art in the Recycling Chain of Fuel Cell Vehicles
3.1. Collection
3.2. Dismantling
3.3. Disassembly and Pre-Processing
3.4. Material Recovery
4. Material Flow Analysis for Fuel Cell Vehicles
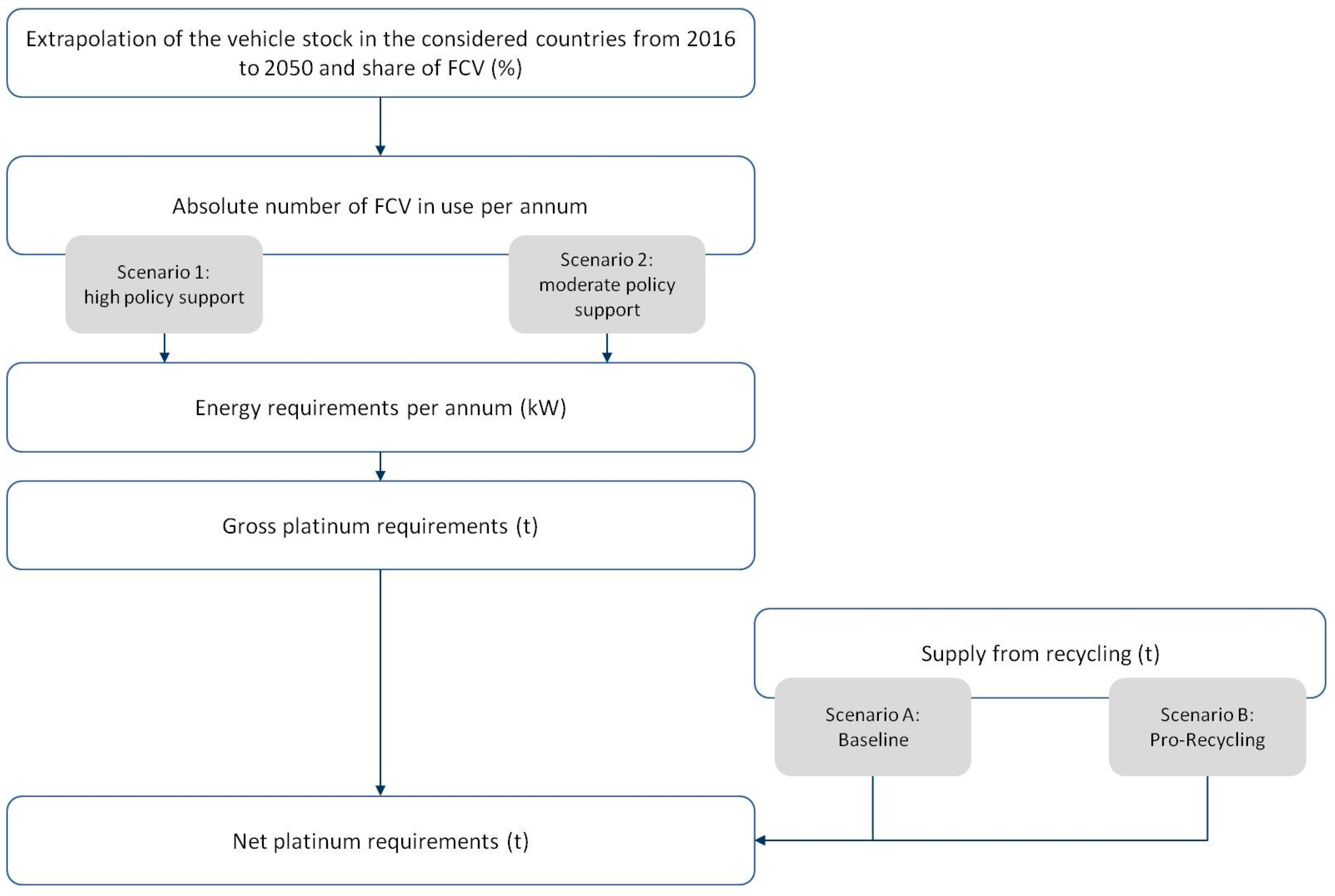
4.1. Research Design and Calculation Approach
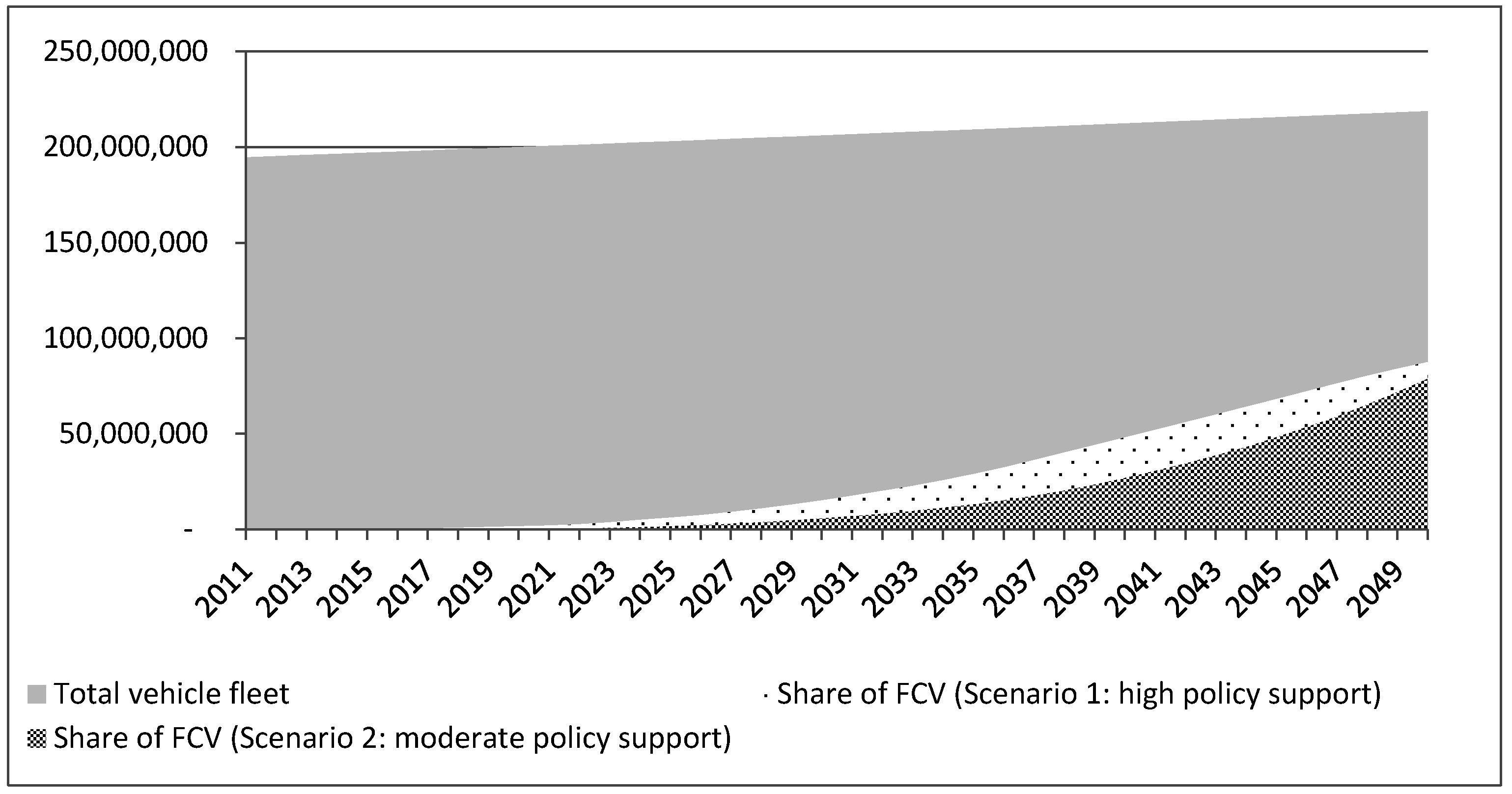
4.2. System Boundaries and Assumptions
“the hypothetic start of mass production has been shifted to 2016 and the number of first movers reduced to 4 which will ramp-up their plant utilization rate from 5% to 90% within a five year time frame (maximum production capacity of each of the four plants 100,000 units per year). After reaching full utilization of the production capacities of the first movers [...] after 5 years [...], it was assumed that followers are entering the market in a similar way and the first movers are doubling their production capacity”.[64] (p. 16)
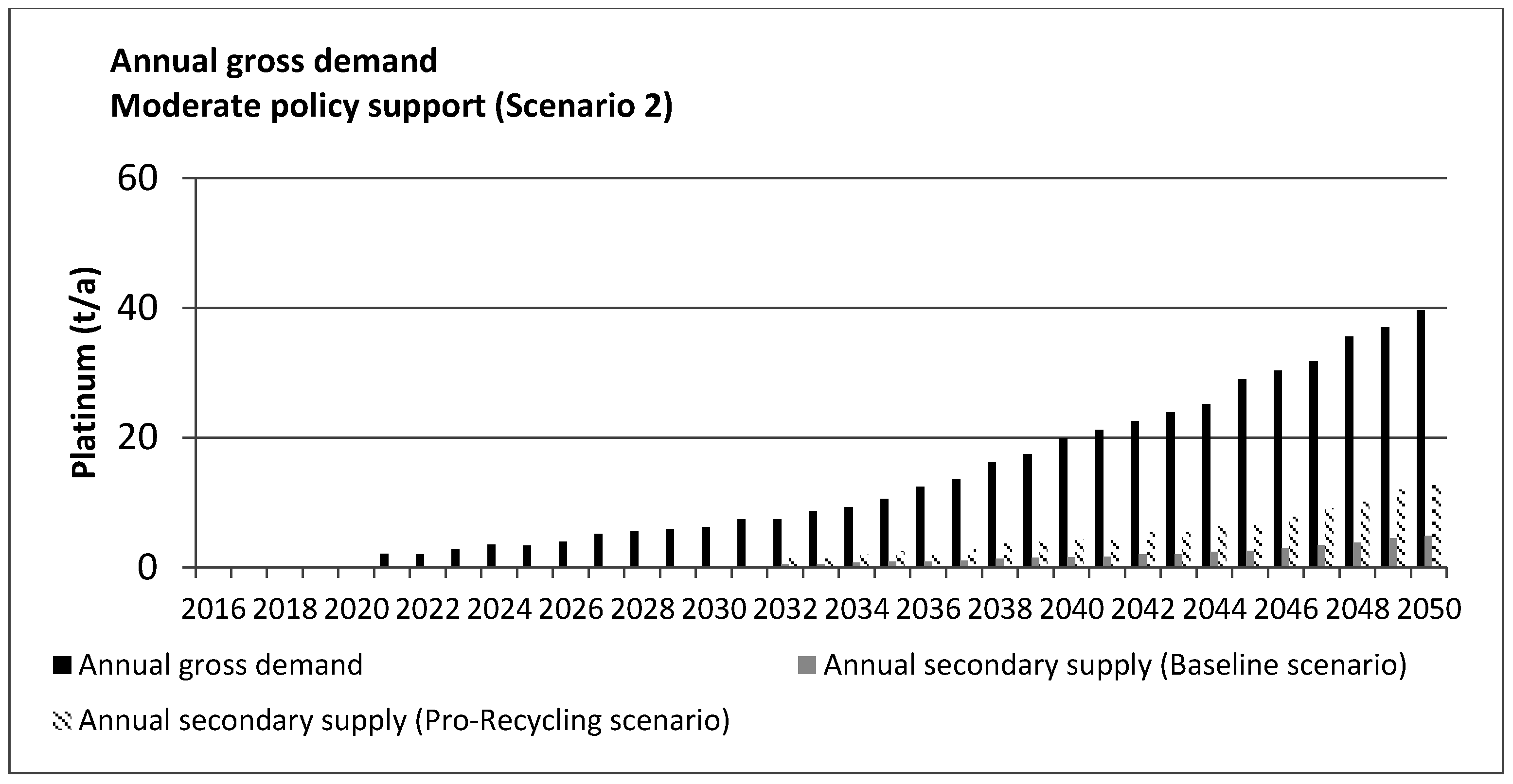
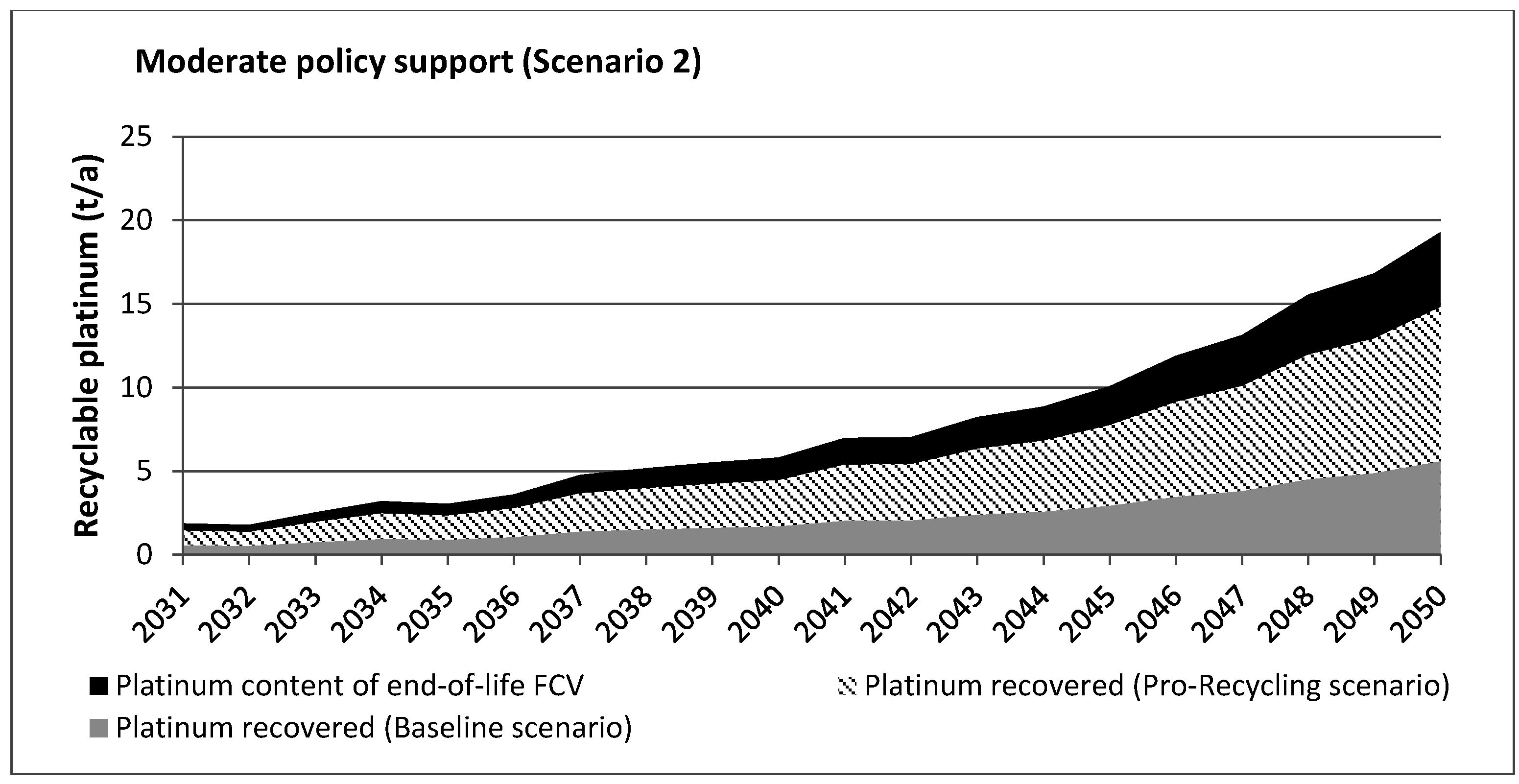
5. Results and Discussion
6. Conclusions and Implications for Further Research
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CO | Carbon Monoxide |
| CO2 | Carbon Dioxide |
| ELV | End-of-Life Vehicle |
| EU | European Union |
| FCV | Fuel Cell Vehicle |
| GDL | Gas Diffusion Layer |
| GHG | Greenhouse Gas |
| H | Hydrogen |
| HF | Hydrogen Fluoride |
| MEA | Membrane Electrode Assembly |
| MFA | Material Flow Analysis |
| PBI | Polybenzimidazole |
| PEM | Polymer Electrolyte Membrane |
| PEMFC | Polymer Electrolyte Membrane Fuel Cell |
| PGM | Platinum Group Metals |
| PTFE | Polytetrafluorethylene |
| US | United States of America |
| WIPO | World Intellectual Property Organization |
References
- European Commission. Communication from the Commission: A Roadmap for Moving to a Competitive Low Carbon Economy in 2050. COM (2011) 112 Final; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- European Commission. Reducing Emissions from Transport. Available online: http://ec.europa.eu/clima/policies/transport/index_en.htm (accessed on 27 October 2015).
- Mohrdieck, C.; Venturi, M.; Breitrück, K.; Schulze, H. Mobile Anwendungen. In Wasserstoff und Brennstoffzelle. Technologien und Marktperspektiven; Töpler, J., Lehmann, J., Eds.; Springer: Wiesbaden, Germany, 2014; pp. 59–111. [Google Scholar]
- Bundesministerium für Verkehr, Bau und Stadtentwicklung. Elektromobilität—Deutschland als Leitmarkt und Leitanbieter; Bundesministerium für Verkehr, Bau und Stadtentwicklung: Berlin, Germany, 2011. [Google Scholar]
- McKinsey & Company. A Portfolio of Power-Trains for Europe: A Fact-Based Analysis. The Role of Battery-Electric Vehicles, Plug-In Hybrids and Fuel Cell Electric Vehicles. Available online: http://www.fch.europa.eu/node/786 (accessed on 7 September 2016).
- Råde, I. Requirement and Availability of Scarce Metals for Fuel-Cell and Battery Electric Vehicles. Ph.D. Thesis, Chalmers University of Technology and Gothenburg University, Gothenburg, Sweden, 2001. [Google Scholar]
- Gordon, R.B.; Bertram, M.; Graedl, T.E. Metal stocks and sustainability. Proc. Natl. Acad. Sci. USA 2006, 103, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Elshkaki, A. Systems Analysis of Stock Buffering. Development of a Dynamic Substance Flow-Stock Model for the Identification and Estimation of Future Resources, Waste Streams and Emissions. Ph.D. Thesis, Leiden University, Leiden, The Netherlands, 6 September 2007. [Google Scholar]
- Angerer, G.; Marscheider-Weidemann, F.; Lüllmann, A.; Erdmann, L.; Scharp, M.; Handke, V.; Marwede, M. Rohstoffe für Zukunftstechnologien; Fraunhofer IRB Verlag: Stuttgart, Germany, 2009. [Google Scholar]
- Buchert, M.; Schüler, D.; Bleher, D. Critical Metals for Future Sustainable Technologies and Their Recycling Potential; Öko-Institut e.V. for United Nations Environment Program: Freiburg, Germany, 2009. [Google Scholar]
- Yang, C. An impending platinum crisis and its implications for the future of the automobile. Energy Policy 2009, 37, 1805–1808. [Google Scholar] [CrossRef]
- Buchert, M.; Jenseit, W.; Dittrich, S.; Hacker, F.; Schüler-Hainsch, E.; Ruhland, K.; Knöfel, S.; Goldmann, D.; Rasenack, K.; Treffer, F. Ressourceneffizienz und ressourcenpolitische Aspekte des Systems Elektromobilität; Öko-Institut e.V.: Darmstadt, Germany, 2011. [Google Scholar]
- Saurat, M.; Bringezu, S. Platinum Group Metal Flows on Europe, Part I. J. Ind. Ecol. 2008, 12, 754–767. [Google Scholar] [CrossRef]
- Mudd, G.M. Platinum group metals: A unique case study into the sustainability of mineral resources. In Proceedings of the 4th International Platinum Conference: Platinum in Transition ‘Boom or Bust’, Johannesburg, South Africa, 11–14 October 2010; The South African Institute of Mining and Metallurgy: Johannesburg, South Africa, 2010; pp. 113–120. [Google Scholar]
- Cairncross, E. Health and Environmental Impacts of Platinum Mining: Report from South Africa. 2014. Available online: http://www.thejournalist.org.za/wp-content/uploads/2014/09/Environmental-health-impacts-of-platinum-mining1.pdf (accessed on 29 August 2015).
- European Commission. Report on Critical Raw Materials for the EU; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Bernhart, W.; Riederle, S.; Yoon, M. Fuel Cells—A Realistic Alternative for Zero Emission? Roland Berger Strategy Consultants: Stuttgart, Germany, 2014. [Google Scholar]
- Carlson, E.J.; Anderson, A.; Clearly, B. Precious Metal Availability and Cost Analysis for PEMFC Commercialization. Hydrogen, Fuel Cells and Infrastructure Technologies FY 2003 Progress Report. Available online: https://www.eecbg.energy.gov/hydrogenandfuelcells/pdfs/iva4_carlson.pdf (accessed on 11 July 2015).
- Tiax LLC. Platinum Availability and Economics for PEMFC Commercialisation. Report to US DOE; Tiax LLC: Cambridge, MA, USA, 2003. [Google Scholar]
- Saurat, M.; Bringezu, S. Platinum Group Metal Flows on Europe, Part II. J. Ind. Ecol. 2009, 13, 406–421. [Google Scholar] [CrossRef]
- Hagelüken, C. Recycling the PGM—A European Perspective. Platin. Met. Rev. 2012, 56, 29–35. [Google Scholar] [CrossRef]
- Chan, C.C.; Bouscayrol, A.; Chen, K. Electric, Hybrid and Fuel-Cell Vehicles: Architectures and Modeling. IEEE Trans. Veh. Technol. 2010, 59, 589–598. [Google Scholar] [CrossRef]
- Kurzweil, P. Brennstoffzellentechnik. Grundlagen, Komponenten, Systeme, Anwendungen, 2nd ed.; Springer: Wiesbaden, Germany, 2013. [Google Scholar]
- Li, Q.; He, R.; Gao, J.A.; Jensen, J.O.; Bjerrum, N.J. The CO Poisoning Effect in PEMFCs Operational at Temperatures up to 200 °C. J. Electrochem. Soc. 2003, 150, A1599–A1605. [Google Scholar] [CrossRef] [Green Version]
- Simons, A.; Bauer, C. A life-cycle perspective on automotive fuel cells. Appl. Energy 2015, 157, 884–896. [Google Scholar] [CrossRef]
- Kaz, T. Herstellung und Charakterisierung von Membran-Elektroden-Einheiten für Niedertemperatur Brennstoffzellen. Ph.D. Thesis, University of Stuttgart, Stuttgart, Germany, 22 August 2008. [Google Scholar]
- Koraishy, B.; Meyers, J.M.; Wood, K.L. Manufacturing of Membrane Electrode Assemblies for Fuel Cells. 2009. Available online: http://www.sutd.edu.sg/cmsresource/idc/papers/2009-_Manufacturing_of_membrane_electrode_assemblies_for_fuel_cells.pdf (accessed on 4 September 2015).
- Spendelow, J.; Marcinkoski, J. Fuel Cell System Cost 2013. DOE Fuel Cell Technologies Office Record No. 14012. Available online: http://www.hydrogen.energy.gov/pdfs/14012_fuel_cell_system_cost_2013.pdf (accessed on 30 June 2015).
- Holton, O.T.; Stevenson, J.W. The Role of Platinum in Proton Exchange Membrane Fuel Cells. Platin. Met. Rev. 2013, 57, 259–271. [Google Scholar] [CrossRef]
- US Department of Energy. Hydrogen and Fuel Cells Program: Library. Available online: http://www.hydrogen.energy.gov/library.html (accessed on 30 June 2015).
- US Drive FCTT. Fuel Cell Technical Team Roadmap. Available online: http://energy.gov/eere/vehicles/downloads/us-drive-fuel-cell-technical-team-roadmap (accessed on 23 June 2015).
- Lehmann, J.; Luschtinetz, T. Wasserstoff und Brennstoffzellen. Unterwegs mit dem saubersten Kraftstoff; Springer: Wiesbaden, Germany, 2014. [Google Scholar]
- Sui, P.-C.; University of Victoria, Victoria, BC, Canada. Personal Communication, 2015.
- Heiskanen, J.; Kaila, J.; Vanhanen, H.; Pynnönen, H.; Silvennoinen, A. A look at the European Union’s End-of-Life Vehicle Directive—Challenges of treatment and disposal in Finland. In Proceedings of the 2nd International Conference on Final Sinks: Sinks—A Vital Element of Modern Waste Management, Espoo, Finland, 16–18 May 2013.
- Fergusson, M. End of Life Vehicles (ELV) Directive—An Assessment of the Current State of Implementation by Member States; Policy Department Economy and Science of the European Parliament: Brussels, Belgium, 2007. [Google Scholar]
- Wilts, H.; Bleischwitz, R. Combating Material Leakage: A Proposal for an International Metal Covenant. Surv. Perspect. Integr. Environ. Soc. 2011, 4, 1–9. [Google Scholar]
- Hagelüken, C.; Buchert, M.; Stahl, H. Stoffströme der Platingruppenmetalle. Systemanalyse und Maßnahmen für eine Nachhaltige Optimierung der Stoffströme der Platingruppenmetalle; GDMB Medienverlag: Clausthal-Zellerfeld, Germany, 2005. [Google Scholar]
- Axion Recycling. UK Closed-Loop Fuel Cell Component Recycling Is a Step Closer. Available online: http://www.axionpolymers.com/tag/fuel-cell/ (accessed on 30 August 2015).
- Schittl, G. Recyclingpotenzial von Kritischen Rohstoffen in Technologien zur Energieumwandlung. Master’s Thesis, University of Natural Resources and Life Sciences, Vienna, Austria, 7 March 2012. [Google Scholar]
- Cerri, I.; Lefebvre-Joud, F.; Holtappels, P.; Honegger, K.; Stubos, T.; Millet, P. Scientific Assessment in Support of the Materials Roadmap Enabling Low Carbon Energy Technologies: Hydrogen and Fuel Cells; Joint Research Centre, Institute for Energy and Transport: Petten, The Netherlands, 2012. [Google Scholar]
- Kromer, M.A.; Joseck, F.; Rhodes, T.; Guernsey, M.; Marcinkoski, J. Evaluation of a platinum leasing program for fuel cell vehicles. Int. J. Hydrogen Energy 2009, 34, 8276–8288. [Google Scholar] [CrossRef]
- Kwade, A.; Bärwaldt, G. LithoRec—Recycling von Lithium-Ionen-Batterien. Abschlussbericht des Verbundprojekts; Technische Universität Braunschweig: Brunswick, Germany, 2012. [Google Scholar]
- National Alternative Fuels Training Consortium. Safety Booklet Automotive Recycling. Available online: http://naftc.wvu.edu/cleancitieslearningprogram/firstrespondersafetytraining/towing-recovery (accessed on 2 July 2015).
- Schiemann, J.; Kerßenboom, A.; Prause, H.J.; Peil, S. Handbuch Verwertung von Brennstoffzellen und deren Peripherie-Systeme; Institut für Energie- und Umwelttechnik e.V.: Duisburg, Germany, 2007. [Google Scholar]
- Shore, L. Platinum Group Metal Recycling Technology Development. Final report to BASF Catalysts LLC and US DOE Office of Hydrogen, Fuel Cells, and Infrastructure Technologies Program. Available online: http://www.osti.gov/scitech/biblio/962699 (accessed on 29 July 2015).
- Wegner, R.; Fokkens, E.; Holdt, H.; Bukowsky, H.; Trautmann, M.; Nettesheim, S.; Jakubith, S.; Scholz, P.; Mollenhauer, T.; Theuring, S.; et al. React—Rückgewinnung und Wiedereinsatz von Edelmetallen aus Brennstoffzellen. In Recycling und Rohstoffe; Thomé-Kozmiensky, K.J., Goldmann, D., Eds.; Vivis TK-Verlag: Nietwerder, Germany, 2012; Volume 5, pp. 429–441. [Google Scholar]
- Handley, C.; Brandon, N.P.; van der Vort, R. Impact of the European Union vehicle waste directive on end-of-life options for polymer electrolyte fuel cells. J. Power Sources 2002, 106, 344–352. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Hydrogen Fluoride. Available online: http://www.epa.gov/ttnatw01/hlthef/hydrogen.html (accessed on 03 July 2015).
- Stolten, D. Hydrogen and Fuel Cells: Fundamentals, Technologies and Applications; John Wiley & Sons Ltd.: Bognor Regis, UK, 2010. [Google Scholar]
- Koehler, J.; Zuber, R.; Binder, M.; Baenisch, V.; Lopez, M. Process for Recycling Fuel Cell Components Containing Precious Metals. WIPO Patent No. WO/2006/024507, 9 March 2006. [Google Scholar]
- Jha, M.K.; Lee, J.; Kim, M.; Jeong, J.; Kim, B.-S.; Kumar, V. Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: A review. Hydrometallurgy 2013, 133, 23–32. [Google Scholar] [CrossRef]
- Duclos, L.; Svecova, L.; Laforest, V.; Mandil, G.; Thivel, P.-X. Process development and optimization for platinum recovery from PEM fuel cell catalyst. Hydrometallurgy 2016, 160, 79–89. [Google Scholar] [CrossRef]
- Xu, F.; Mu, S.; Pan, M. Recycling of membrane electrode assembly of PEMFC by acid processing. Int. J. Hydrogen Energy 2010, 35, 2976–2979. [Google Scholar] [CrossRef]
- Zhao, J.; He, X.; Tian, J.; Wan, C.; Jiang, C. Reclaim/recycle of Pt/C catalysts for PEMFC. Energy Convers. Manag. 2007, 48, 450–453. [Google Scholar] [CrossRef]
- Oki, T.; Katsumata, T.; Hashimoto, K.; Kobayashi, M. Recovery of Platinum Catalyst and Polymer Electrolyte from Used Small Fuel Cells by Particle Separation Technology. Mater. Trans. 2009, 50, 1864–1870. [Google Scholar] [CrossRef]
- Hagelüken, C.; Kayser, B.; Romero-Ojeda, J.; Kleinwächter, I. Process for the Concentration of Noble Metals from Fluorine-Containing Fuel Cell Components. WIPO Patent No. WO/2004/102711, 25 November 2004. [Google Scholar]
- Shore, L.; Matlin, R. Simplified Process for Leaching Precious Metals from Fuel Cell Membrane Electrode Assemblies. WIPO Patent No. WO/2009/029463, 5 March 2009. [Google Scholar]
- Shore, L.; Matlin, R.; Heinz, R. Method and Apparatus for Recovering Catalytic Elements from Fuel Cell Membrane Electrode Assemblies. WIPO Patent No. WO/2009/149241, 10 December 2009. [Google Scholar]
- Shore, L.; Matlin, R.; Heinz, R. Method for Recovering Catalytic Elements from Fuel Cell Membrane Electrode Assemblies. WIPO Patent No. WO/2010/132156, 18 November 2010. [Google Scholar]
- Shore, L.; Robertson, A.B.; Shulman, H.S.; Fall, M.L. Process for Recycling Components of a PEM Fuel Cell Membrane Electrode Assembly. WIPO Patent No. WO/2006/115684, 2 November 2006. [Google Scholar]
- Shore, L. Process for Recycling Components of a PEM Fuel Cell Membrane Electrode Assembly. WIPO Patent No. WO/2007/149904, 27 December 2007. [Google Scholar]
- Romero, J.; Meyer, H.; Voss, S. Device and Method for the Thermal Treatment of products Containing Fluorine and Precious Metals. European Patent No. EP2700726A1, 26 February 2014. [Google Scholar]
- Paepke, M. Method for Recycling Membrane/Electrode Units of a Fuel Cell. WIPO Patent No. WO/2015/010793, 29 January 2015. [Google Scholar]
- European Commission. HyWays—The European Hydrogen Roadmap. Project Report EUR 23123; Office for official publications of the European Communities: Luxembourg, Luxembourg, 2008. [Google Scholar]
- Bundesministerium für Verkehr, Bau und Stadtentwicklung. Szenarien der Mobilitätsentwicklung unter Berücksichtigung von Siedlungsstrukturen bis 2050; Bundesministerium für Verkehr, Bau und Stadtentwicklung, Traffic and Mobility Planning GmbH, Deutsches Institut für Urbanistik, Institut für Wirtschaftsforschung Halle: Magdeburg, Germany, 2006. [Google Scholar]
- Shell Germany. Shell PKW Szenarien bis 2040. Available online: http://www.shell.de/aboutshell/media-centre/annual-reports-and-publications/shell-pkwszenarien.html#vanity-aHR0cDovL3d3dy5zaGVsbC5kZS9wa3dzdHVkaWU (accessed on 9 October 2015).
- World Energy Council. Global Transport Scenarios 2050. Available online: https://www.worldenergy.org/publications/2011/global-transport-scenarios-2050/ (accessed on 9 October 2015).
- Adamson, K. The Fuel Cell and Hydrogen Annual Review. 2015. Available online: http://www.4thenergywave.co.uk/annual-review/ (accessed on 9 October 2015).
- Ultrasonic Systems Inc. Fuel Cell. Available online: http://www.ultraspray.com/markets/fuel-cell (accessed on 6 October 2015).
- Sonaer Ultrasonics. Fuel Cell Coatings. Available online: http://sonozap.com/Fuel_Cell_Coating.html (accessed on 6 October 2015).
- Sonotek. Ultrasonic Nozzle Overview. Available online: http://www.sono-tek.com/ultrasonic-nozzle-overview/ (accessed on 6 October 2015).
- Ehrenberger, S.; Deutsches Zentrum für Luft- und Raumfahrt, Brunswick, Germany. Personal Communication, 2015.
- Wagner, P.; Next Energy, Oldenburg, Germany. Personal Communication, 2015.
- Betrieblicher Umweltschutz Baden-Württemberg. Nasslackieren (Spritzlackieren). Available online: http://www.bubw.de/?lvl=465 (accessed on 9 October 2015).
- Dyck, A.; Next Energy, Oldenburg, Germany. Personal Communication, 2015.
- USGS. Mineral Commodities Summary 2011. Available online: http://minerals.usgs.gov/minerals/pubs/mcs/ (accessed on 8 July 2015).
- World Platinum Investment Council. Platinum Quarterly: Q4 2014. Available online: http://www.platinuminvestment.com/files/WPIC_Platinum_Quarterly_Q4_2014.pdf (accessed on 11 July 2015).
- Johnson Matthey. Price Charts. Available online: http://www.platinum.matthey.com/prices/price-charts (accessed on 11 June 2015).

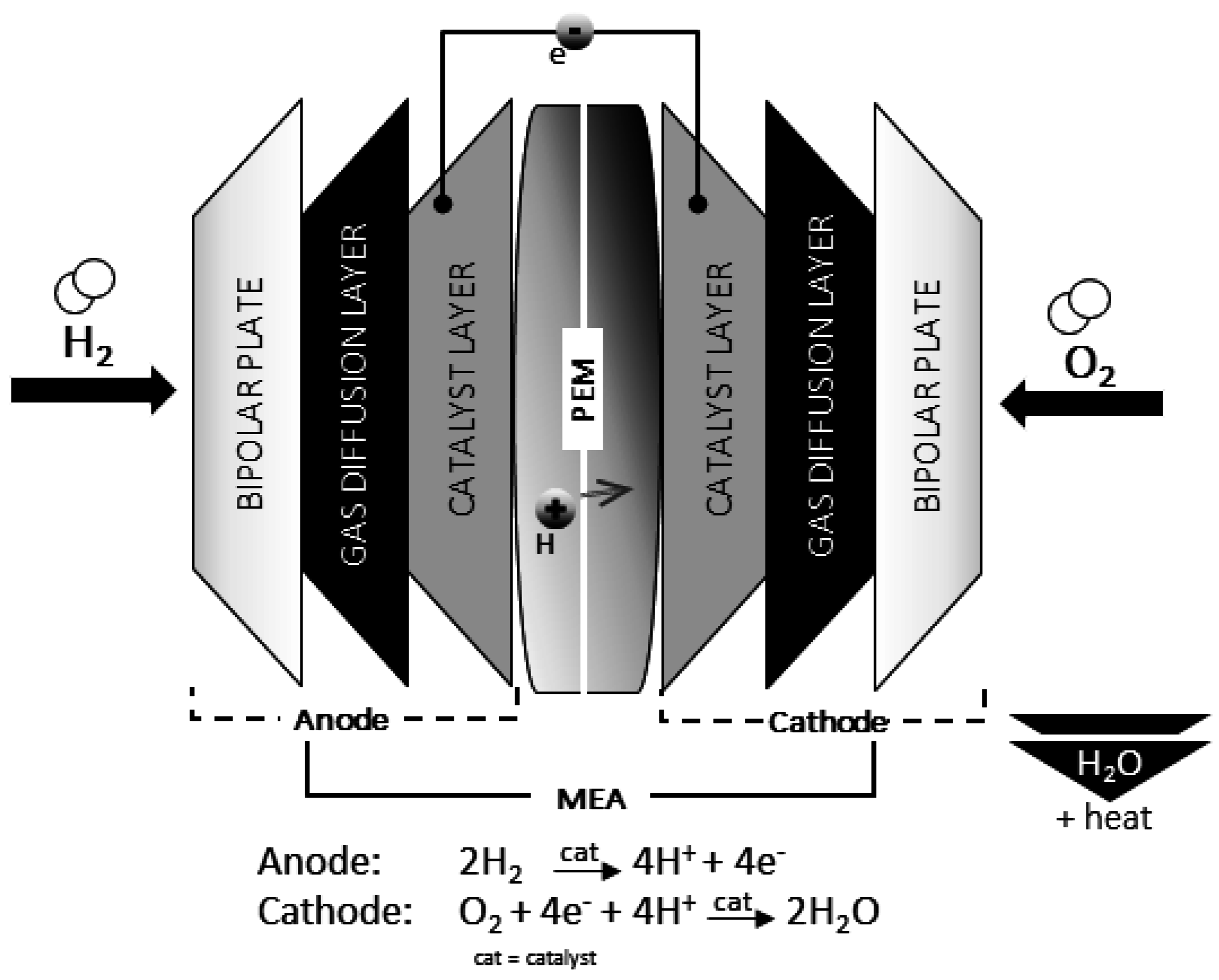


| Characteristics | Unit | Status 2013 | Target 2020 |
|---|---|---|---|
| Total platinum content | g/kWrated | 0.14 | 0.125 |
| Total platinum loading | mg/cm2 electrode area | 0.15 | 0.125 |
| Disassembly Step | Action Taken | Fraction Produced |
|---|---|---|
| Step 1 | Removal of tie rods and casing | Connecting elements: high-grade steel |
| Step 2 | Removal of end plates | Connecting elements: high-grade steel; |
| Insulation: plastics; | ||
| End plates: high-grade steel or aluminium alloy | ||
| Step 3 | Withdrawal of the stack’s individual layers, each consisting of bipolar plates, seal, gas diffusion layer and membrane electrode assembly | Seal: plastics |
| Bipolar plates: graphite, polymeric binders, additives, separating agents | ||
| Gas Diffusion Layer (GDL): non-woven fabric, carbon paper | ||
| Membrane Electrode Assembly (MEA): polymeric membrane coated with graphite and platinum |
| Patent Number | Inventor/s | Proprietor | Materials Recovered (and Rate in %) | Procedure | Source and Technology |
|---|---|---|---|---|---|
| WO/2004/102711 | Hagelüken, Kayser, Romero-Odeja, Kleinwächter | Umicore AG & Co. KG | PGM (95%) | Binding of HF through inorganic additive | [56], thermal pretreatment followed by hydro-metallurgy |
| WO/2006/024507 | Koehler, Zuber, Binder, Baenisch, Lopez | Umicore AG & Co KG | PGM and/or fluorine-containing constituents (no rate provided) | Separation of membrane and catalyst layer by treatment with medium in supercritical state | [50], thermal pretreatment (supercritical status) followed by hydro-metallurgy |
| WO/2006/115684 | Shore, Robertson, Shulman, Fall, Matlin, Heinz | BASF Catalysts LLC | PGM and/or ionomer (up to 99%) | Leaching using aqua regia | [57,58,59,60,61], hydrometallurgy |
| WO/2007/149904 | |||||
| WO/2009/029463 | |||||
| WO/2009/149241 | |||||
| WO/2010/132156 | |||||
| EP2700726A1 | Romero, Meyer, Voss | Heraeus Precious Metals GmbH | PGM (no rate provided) | HF-resistant furnace | [62],thermal treatment only |
| WO/2015/010793 | Paepke | Daimler AG | PGM and/or ionomer (>98%) | Separation of membrane and catalyst layer through ultrasound and solvent addition | [63], pretreatment by ultrasonic bath followed by pyrometallurgy |
| Parameter | System Boundaries/Assumptions | Sources |
|---|---|---|
| Geographic boundary | Finland, France, Germany, Italy, the Netherlands, Norway, Poland, Spain, the United Kingdom | [64] |
| Time scale | 2016 to 2050 | Authors’ choice based on data availability |
| Market penetration scenarios | High policy support, modest learning rate (Scenario 1); modest policy support, modest learning rate (Scenario 2) | [64] |
| Average annual growth rate of vehicle fleet | 0.03% | Mean value of [5,65,66,67] |
| Recycling scenarios | Baseline (Scenario A), Pro-Recycling (Scenario B) | Authors’ choice |
| Type of vehicle | Light-duty passenger vehicle | Authors’ choice based on vehicle segmentation and GHG emissions |
| Average vehicle lifetime | 10 years | [25] |
| Average vehicle power | 80 kW | [30] |
| Average vehicle weight | 1447 kg | [25] |
| Average weight of fuel cell stack | 40.8 kg | [25] |
| Platinum load per kW | Continuously declining from 2020: 0.125 g, 2030: 0.07 g, 2050: 0.07 g | [31,68] |
| Loss rate of catalyst ink in manufacturing | Continuously declining from 2020: 15%; 2030: 5.2%; 2050: 1.1% | Authors’ estimation based on [69,70,71,72,73,74] and average annual improvement rate of ~0.8% |
| Use phase loss | 0.67% | [41,75] |
| Parameter | Baseline Scenario (A) | Pro-Recycling Scenario (B) |
|---|---|---|
| Share of End-of-Life Vehicles collected by recycling facilities | 33% | 85% |
| Share of fuel cell stacks recovered in dismantling step | 96% | 99% |
| Share of platinum-containing components recovered in pre-processing step | 96% | 98% |
| Share of platinum recovered in material recovery step | 95% | 98% |
| Total recycling chain efficiency | 28.9% | 80.8% |
| Task | Scenario 1 (High Policy Support) | Scenario 2 (Moderate Policy Support) |
|---|---|---|
| Cumulative platinum demand for Fuel Cell Vehicle (FCV) production | 537.06 t | 459.24 t |
| Cumulative platinum content of end-of-life FCV | 293.51 t | 155.23 t |
| Cumulative amount of platinum recovered (Scenario A: Baseline) | 84.8 t (208.71 t lost) | 44.85 t (110.38 t lost) |
| Cumulative amount of platinum recovered (Scenario B: Pro-Recycling) | 230.02 t (63.49 t lost) | 119.17 t (36.06 t lost) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wittstock, R.; Pehlken, A.; Wark, M. Challenges in Automotive Fuel Cells Recycling. Recycling 2016, 1, 343-364. https://doi.org/10.3390/recycling1030343
Wittstock R, Pehlken A, Wark M. Challenges in Automotive Fuel Cells Recycling. Recycling. 2016; 1(3):343-364. https://doi.org/10.3390/recycling1030343
Chicago/Turabian StyleWittstock, Rikka, Alexandra Pehlken, and Michael Wark. 2016. "Challenges in Automotive Fuel Cells Recycling" Recycling 1, no. 3: 343-364. https://doi.org/10.3390/recycling1030343







