Immobilization of Titanium(IV) Oxide onto 3D Spongin Scaffolds of Marine Sponge Origin According to Extreme Biomimetics Principles for Removal of C.I. Basic Blue 9
Abstract
:1. Introduction
2. Materials and Methods
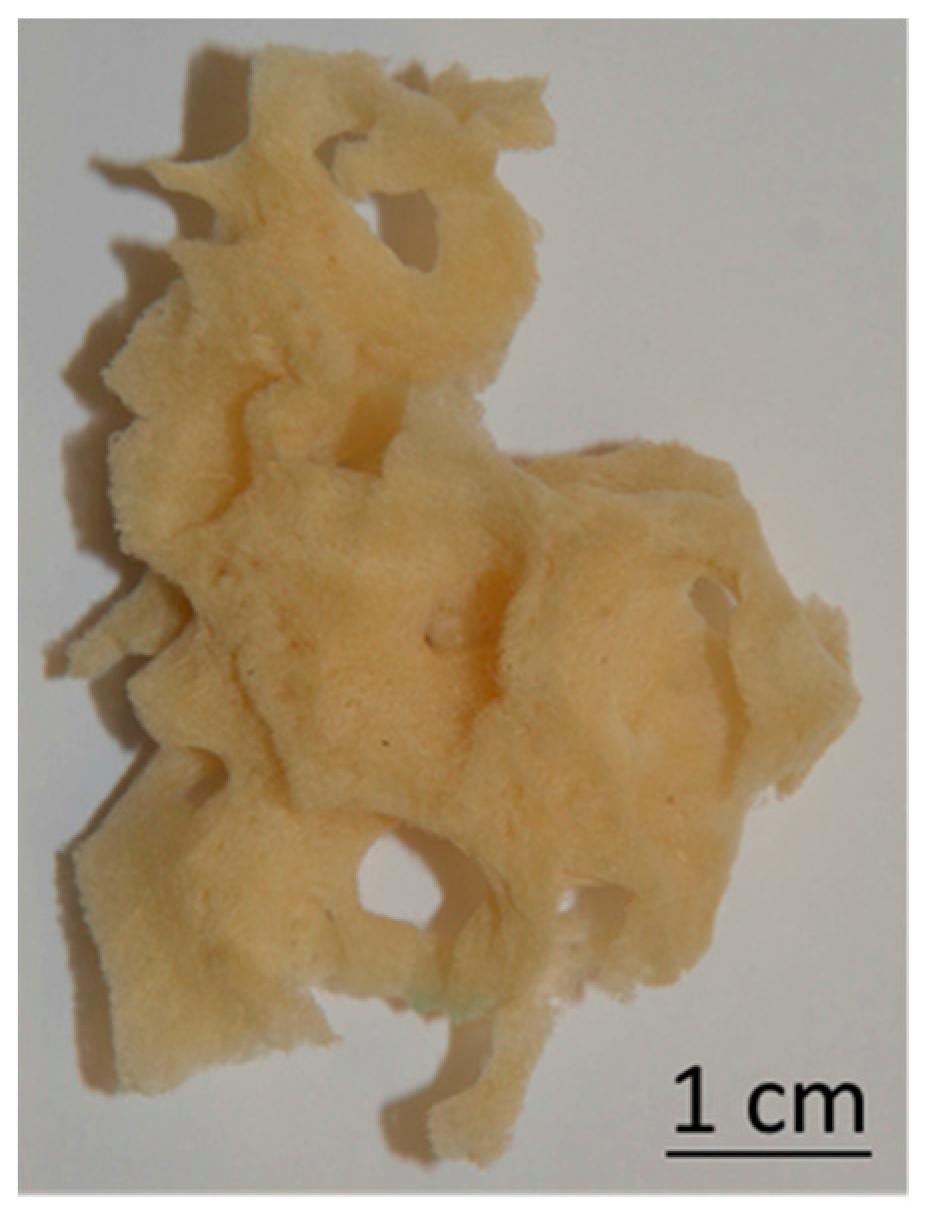
2.1. Isolation of Spongin Scaffolds
2.2. Hydrothermal Synthesis of TiO2 Immobilized onto Spongin Scaffold
2.3. Characterization Techniques
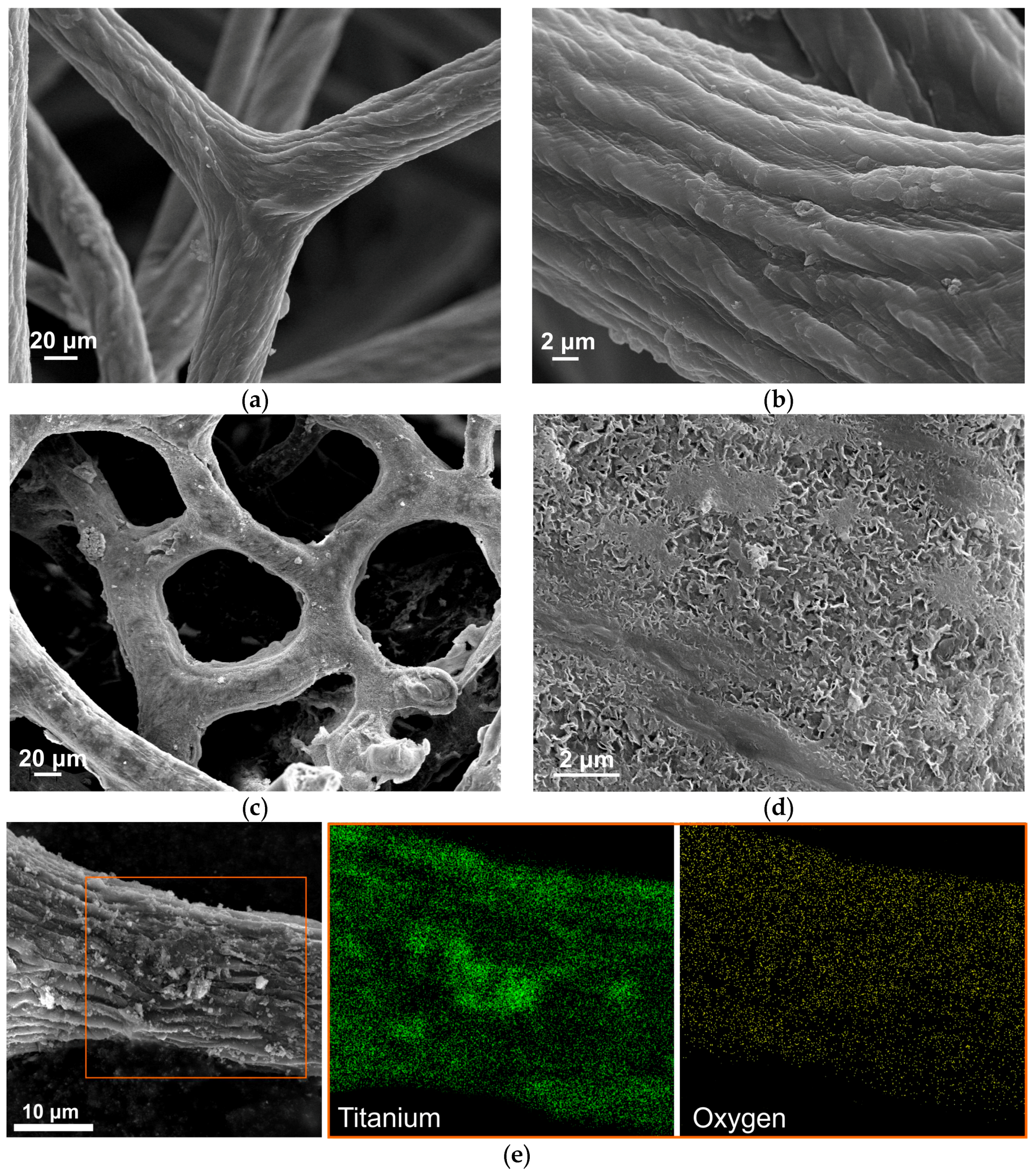
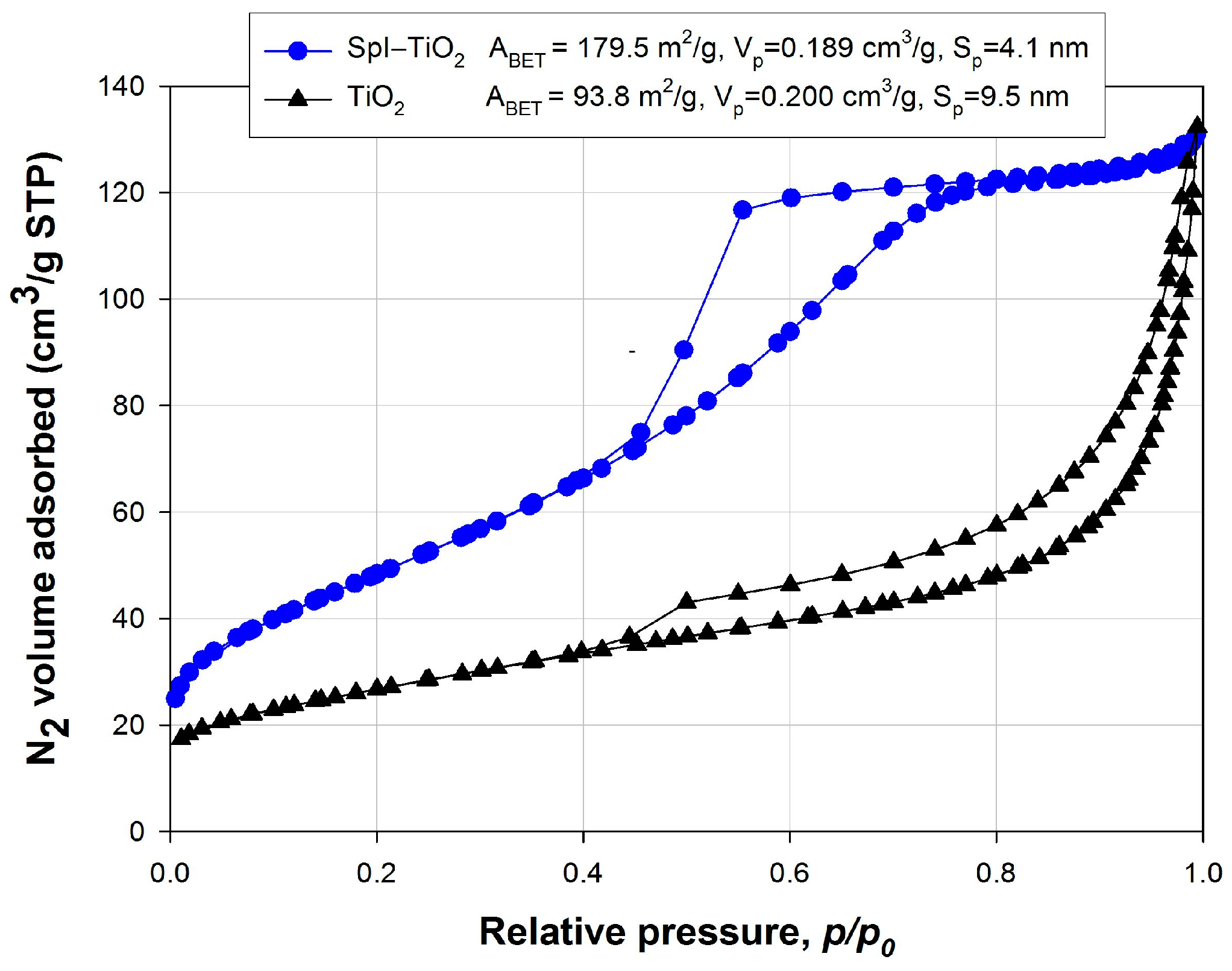
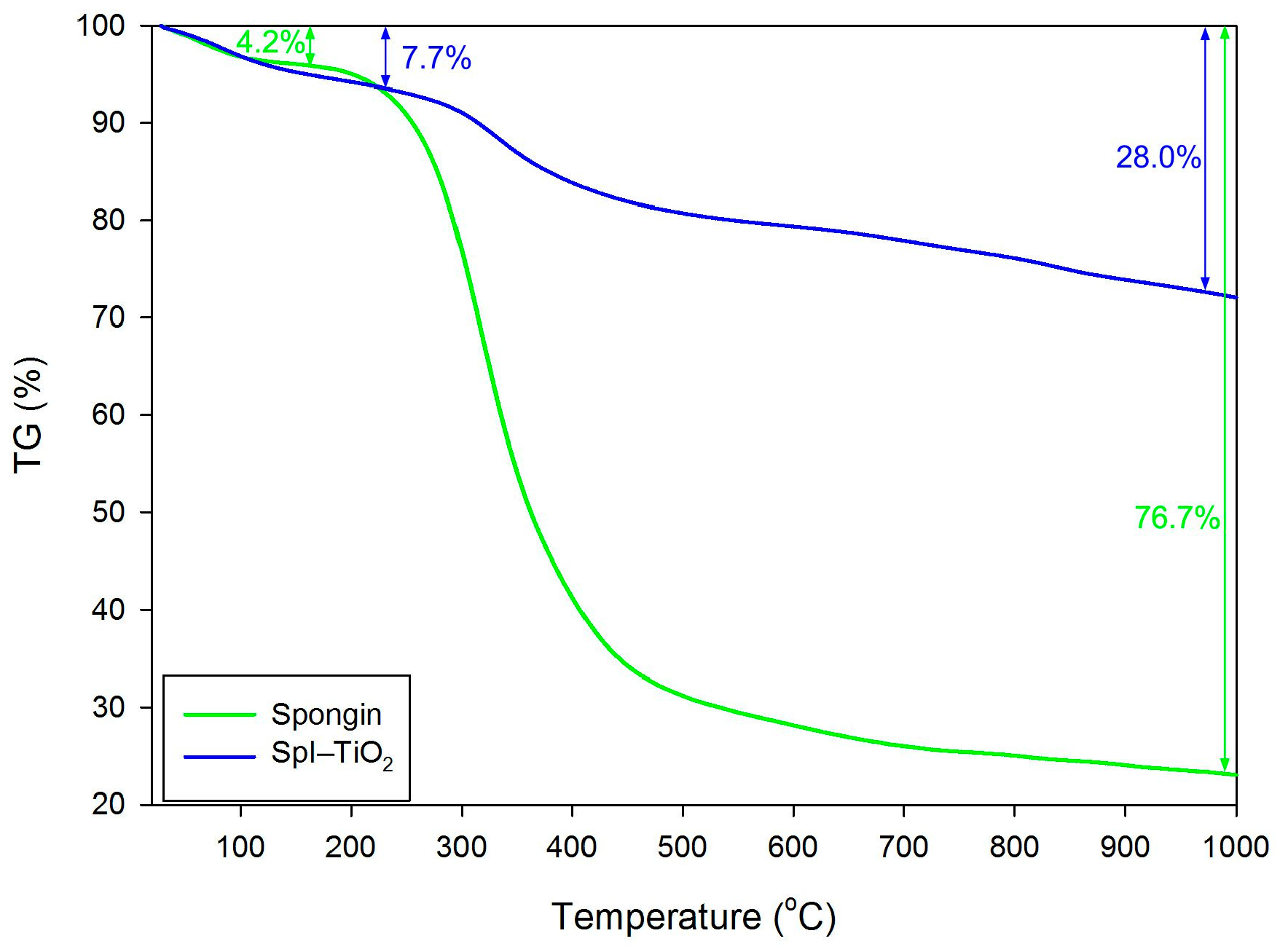
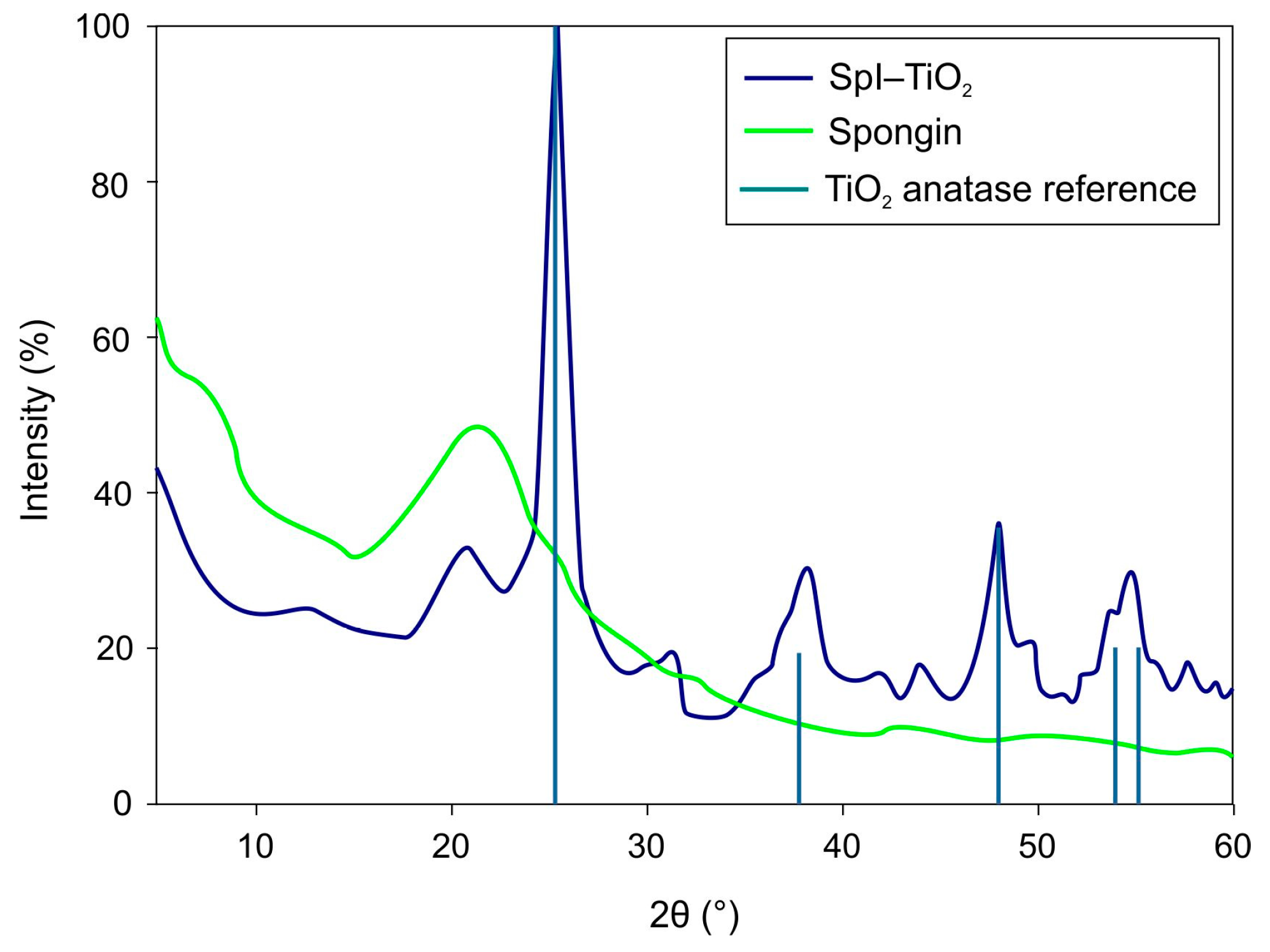
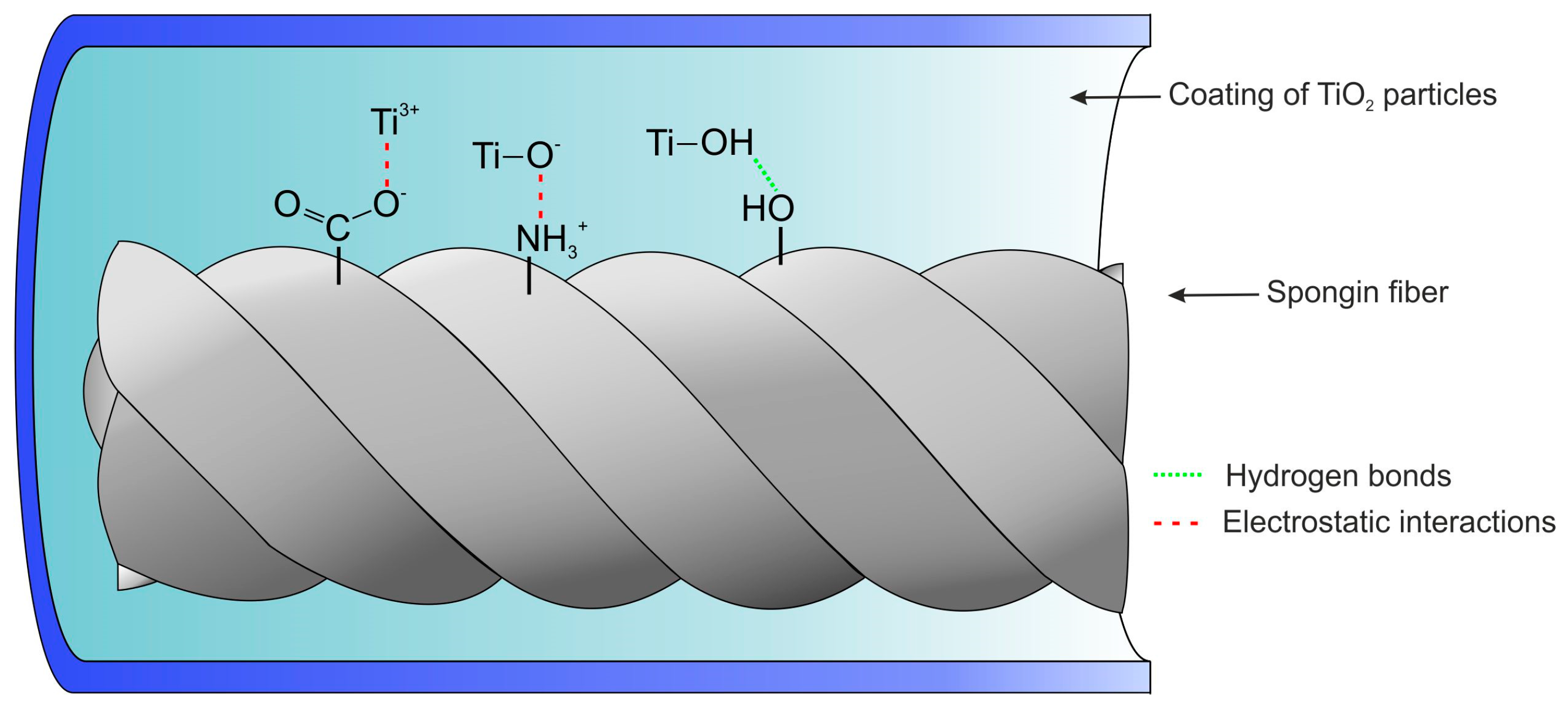
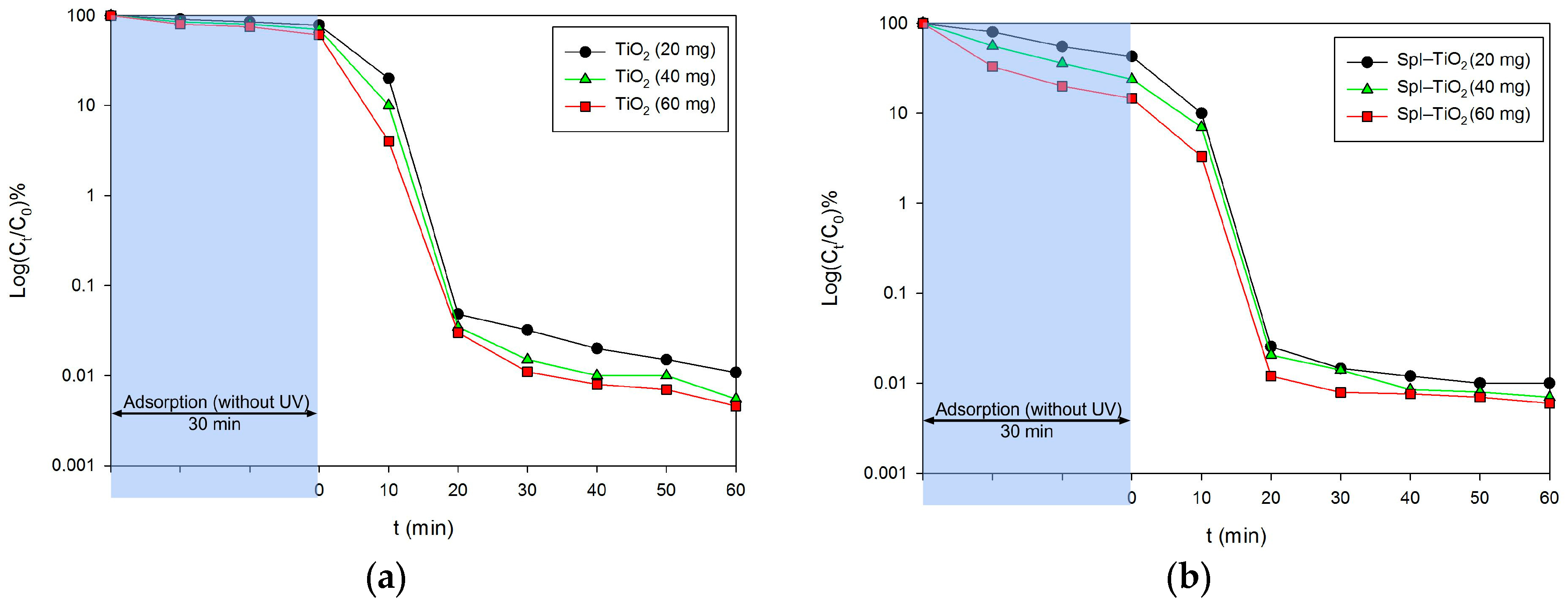
3. Results and Discussion
4. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xu, A.-W.; Ma, Y.; Cölfen, H. Biomimetic mineralization. J. Mater. Chem. 2007, 17, 415–449. [Google Scholar] [CrossRef]
- Ehrlich, H. Chitin and collagen as universal and alternative templates in biomineralization. Int. Geol. Rev. 2010, 52, 661–699. [Google Scholar] [CrossRef]
- Sanchez, C.; Arribart, H.; Guille, M.M.G. Biomimetism and bioinspiration as tools for the design of innovative materials and systems. Nat. Mater. 2005, 4, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, F.; Sommerdijk, N. Biomineralization as an inspiration for materials chemistry. Angew. Chem. Int. Ed. Engl. 2012, 51, 6582–6596. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Simon, P.; Motylenko, M.; Wysokowski, M.; Bazhenov, V.V.; Galli, R.; Stelling, A.L.; Stawski, D.; Ilan, M.; Stöcker, H.; et al. Extreme Biomimetics: Formation of zirconium dioxide nanophase using chitinous scaffolds under hydrothermal conditions. J. Mater. Chem. B 2013, 1, 5092–5099. [Google Scholar] [CrossRef]
- Szatkowski, T.; Jesionowski, T. Hydrothermal synthesis of spongin-based materials. In Extreme Biomimetics; Ehrlich, H., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 251–274. [Google Scholar]
- Wysokowski, M.; Petrenko, I.; Stelling, A.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan chitin as a versatile template for extreme biomimetics. Polymers 2015, 7, 235–265. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Beyer, J.; Makarova, A.; Stöcker, H.; Walter, J.; Galli, R.; Kaiser, S.; Vyalikh, D.; Bazhenov, V.V.; et al. Extreme biomimetic approach for development of novel chitin-GeO2 nanocomposites with photoluminescent properties. Nano Res. 2015, 8, 2288. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Walter, J.; Lota, G.; Wojciechowski, J.; Stöcker, H.; Galli, R.; Stelling, A.L.; Himcinschi, C.; Niederschlag, E.; et al. Synthesis of nanostructured chitin–hematite composites under extreme biomimetic conditions. RSC Adv. 2014, 4, 61743–61752. [Google Scholar] [CrossRef]
- Ehrlich, H.; Bazhenov, V.; Meschke, S.; Bürger, M.; Ehrlich, A.; Petovic, S.; Durovic, M. Marine invertebrates of Boka Kotorska Bay unique sources for bioinspired materials science. In The Handbook of Environmental Chemistry; Joksimovic, A., et al., Eds.; Springer: Berlin, Heidelberg, Germany, 2016; pp. 1–22. [Google Scholar]
- Ehrlich, H.; Maldonado, M.; Spindler, K.; Eckert, C.; Hanke, T.; Born, R.; Goebel, C.; Simon, P.; Heinemann, S.; Worch, H. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (Demospongia: Porifera). J. Exp. Zool. 2007, 356, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; Schupp, P.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Biol. Macromol. 2010, 47, 132–40. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Steck, E.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part II: Biomimetic potential and applications. Int. J. Biol. Macromol. 2010, 47, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Rigby, J.K.; Botting, J.P.; Tsurkan, M.V.; Werner, C.; Schwille, P.; Petrášek, Z.; Pisera, A.; Simon, P.; Sivkov, V.N.; et al. Discovery of 505-million-year old chitin in the basal demosponge Vauxia gracilenta. Sci. Rep. 2013, 3, 3497. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Motylenko, M.; Bazhenov, V.V.; Stawski, D.; Petrenko, I.; Ehrlich, A.; Behm, T.; Kljajic, Z.; Stelling, A.L.; Jesionowski, T.; et al. Poriferan chitin as a template for hydrothermal zirconia deposition. Front. Mater. Sci. 2013, 7, 248–260. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Stöcker, H.; Bazhenov, V.V.; Langer, E.; Dobrowolska, A.; Czaczyk, K.; Galli, R.; Stelling, A.L.; Behm, T.; et al. An extreme biomimetic approach: hydrothermal synthesis of β-chitin/ZnO nanostructured composites. J. Mater. Chem. B 2013, 1, 6469–76. [Google Scholar] [CrossRef]
- Wysokowski, M.; Petrenko, I.; Motylenko, M.; Langer, E.; Bazhenov, V.V.; Galli, R.; Stelling, A.L.; Kljajić, Z.; Szatkowski, T.; Kutsova, V.Z.; et al. Renewable chitin from marine sponge as a thermostable biological template for hydrothermal synthesis of hematite nanospheres using principles of extreme biomimetics. Bioinspired Mater. 2015, 1, 12–22. [Google Scholar] [CrossRef]
- Junqua, S.; Robert, L.; Vacelet, J.; Garrone, R.; De Ceccatty, M.P.; Vacelet, J. Biochemical and morphological studies on collagens of horny sponges. Ircinia filaments compared to spongines. Connect. Tissue Res. 1974, 2, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Green, D.; Walsh, D.; Mann, S. The potential of biomimesis in bone tissue engineering lessons from the design and synthesis of inverterbrate skeletons. Bone 2002, 30, 810–815. [Google Scholar] [CrossRef]
- Green, D.; Howard, D.; Yang, X.; Kelly, M.; Oreffo, R.O.C. Natural marine sponge fiber skeleton: A biomimetic scaffold. Tissue Eng. 2003, 9, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Green, D.W. Tissue bionics: examples in biomimetic tissue engineering. Biomed. Mater. 2008, 3, 34010. [Google Scholar] [CrossRef] [PubMed]
- Green, D.W.; Lai, W.F.; Jung, H.S. Evolving marine biomimetics for regenerative dentistry. Mar. Drugs 2014, 12, 2877–2912. [Google Scholar] [CrossRef] [PubMed]
- Byrappa, K.; Adschiri, T. Hydrothermal technology for nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef]
- Szatkowski, T.; Wysokowski, M.; Lota, G.; Pęziak, D.; Bazhenov, V.V.; Nowaczyk, G.; Walter, J.; Molodtsov, S.L.; Stöcker, H.; Himcinschi, C.; et al. Novel nanostructured hematite–spongin composite developed using an extreme biomimetic approach. RSC Adv. 2015, 5, 79031–79040. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Z.; Zhang, X. A One-step process for the modification of wool fibers with titanate tetrabutyl by low temperature hydrothermal method. Res. J. Text. Appar. 2014, 18, 23–31. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Z.; Zhang, X.; Mao, N. Photocatalytic effects of wool fibers modified with solely TiO2 nanoparticles and N-doped TiO2 nanoparticles by using hydrothermal method. Chem. Eng. J. 2014, 254, 106–114. [Google Scholar] [CrossRef]
- Cai, L.; Liao, X.; Shi, B. Using collagen fiber as a template to synthesize TiO2 and Fex/TiO2 nanofibers and their catalytic behaviors on the visible light-assisted degradation of Orange II. Ind. Eng. Chem. Res. 2010, 49, 3194–3199. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, D.D.; Guo, P.; Leckie, J.O. One-step fabrication and high photocatalytic activity of porous TiO2 hollow aggregates by using a low-temperature hydrothermal method without templates. Chem. - A Eur. J. 2006, 13, 1851–1855. [Google Scholar] [CrossRef] [PubMed]
- Samarghandi, M.R.; Nouri, J.; Mesdaghinia, A.R.; Mahvi, A.H.; Nasseri, S.; Vaezi, F. Efficiency removal of phenol, lead and cadmium by means of UV/TiO2/H2O2 processes. Int. J. Environ. Sci. Technol. 2007, 4, 19–25. [Google Scholar] [CrossRef]
- Meng, X.; Qian, Z.; Wang, H.; Gao, X.; Zhang, S.; Yang, M. Sol–gel immobilization of SiO2/TiO2 on hydrophobic clay and its removal of methyl orange from water. J. Sol-Gel Sci. Technol. 2008, 46, 195–200. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, C.-Y.; Mazyck, D.W. Microwave-assisted preparation of TiO2/activated carbon composite photocatalyst for removal of methanol in humid air streams. Ind. Eng. Chem. Res. 2006, 45, 5110–5116. [Google Scholar] [CrossRef]
- Kibanova, D.; Cervini-Silva, J.; Destaillats, H. Efficiency of clay−TiO2 nanocomposites on the photocatalytic elimination of a model hydrophobic air pollutant. Environ. Sci. Technol. 2009, 43, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Ao, C.H.; Lee, S.C. Indoor air purification by photocatalyst TiO2 immobilized on an activated carbon filter installed in an air cleaner. Chem. Eng. Sci. 2005, 60, 103–109. [Google Scholar] [CrossRef]
- Sotelo-Vazquez, C.; Noor, N.; Kafizas, A.; Quesada-Cabrera, R.; Scanlon, D.O.; Taylor, A.; Durrant, J.R.; Parkin, I.P. Multifunctional P-doped TiO2 films: A new approach to self-cleaning, transparent conducting oxide materials. Chem. Mater. 2015, 27, 3234–3242. [Google Scholar] [CrossRef]
- Guan, K. Relationship between photocatalytic activity, hydrophilicity and self-cleaning effect of TiO2/SiO2 films. Surf. Coatings Technol. 2005, 191, 155–160. [Google Scholar] [CrossRef]
- Latthe, S.S.; Liu, S.; Terashima, C.; Nakata, K.; Fujishima, A. Transparent, adherent, and photocatalytic SiO2-TiO2 coatings on polycarbonate for self-cleaning applications. Coatings 2014, 4, 497–507. [Google Scholar] [CrossRef]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; dos Santos, V.A.P.M.; Fernández-García, M.; et al. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.H.; Salam, N.R.A.; Zainal, N.; Basha, R.K.; Talib, R.A. Antimicrobial activity of TiO2 nanoparticle-coated film for potential food packaging applications. Int. J. Photoenergy 2014, 2014. Article ID 945930, 6 pages. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, H. In situ synthesis and hydrothermal crystallization of nanoanatase TiO2–SiO2 coating on aramid fabric (HTiSiAF) for UV protection. Microsc. Res. Tech. 2015, 78, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Seentrakoon, B.; Junhasavasdikul, B.; Chavasiri, W. Enhanced UV-protection and antibacterial properties of natural rubber/rutile-TiO2 nanocomposites. Polym. Degrad. Stab. 2013, 98, 566–578. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Shaheen, T.I.; Zaghloul, S.; El-Rafie, M.H.; Hebeish, A. Antibacterial activities and UV protection of the in situ synthesized titanium oxide nanoparticles on cotton fabrics. Ind. Eng. Chem. Res. 2016, 55, 2661–2668. [Google Scholar] [CrossRef]
- Zhang, S.; Plessow, P.N.; Willis, J.J.; Dai, S.; Xu, M.; Graham, G.W.; Cargnello, M.; Abild-Pedersen, F.; Pan, X. Dynamical observation and detailed description of catalysts under strong metal-support interaction. Nano Lett. 2016, 16, 4528–4534. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.T.; Tosoni, S.; Pacchioni, G. Hydrogen adsorption, dissociation, and spillover on Ru10 clusters supported on Anatase TiO2 and tetragonal ZrO2 (101) surfaces. ACS Catal. 2015, 5, 5486–5495. [Google Scholar] [CrossRef]
- Gajjela, S.R.; Ananthanarayanan, K.; Yap, C.; Grätzel, M.; Balaya, P. Synthesis of mesoporous titanium dioxide by soft template based approach: Characterization and application in dye-sensitized solar cells. Energy Environ. Sci. 2010, 3, 838–845. [Google Scholar] [CrossRef]
- Lekphet, W.; Ke, T.-C.; Su, C.; Kathirvel, S.; Sireesha, P.; Akula, S.B.; Li, W.-R. Morphology control studies of TiO2 microstructures via surfactant-assisted hydrothermal process for dye-sensitized solar cell applications. Appl. Surf. Sci. 2016, 382, 15–26. [Google Scholar] [CrossRef]
- Shan, A.Y.; Ghazi, T.I.M.; Rashid, S.A. Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: a review. Appl. Catal. A Gen. 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Kawahara, T.; Konishi, Y.; Tada, H.; Tohge, N.; Nishii, J.; Ito, S. A patterned TiO2 (anatase)/TiO2 (rutile) bilayer-type photocatalyst: effect of the anatase/rutile junction on the photocatalytic activity. Angew. Chemie 2002, 41, 2811–2813. [Google Scholar] [CrossRef]
- Tennakone, K.; Tilakaratne, C.T.K.; Kottegoda, I.R.M. Photocatalytic degradation of organic contaminants in water with TiO2 supported on polythene films. J. Photochem. Photobiol. A Chem. 1995, 87, 177–179. [Google Scholar] [CrossRef]
- Bouligand, Y. Twisted fibrous arrangement in biological materials and cholesteric mesophases. Tissue Cell 1972, 4, 189–217. [Google Scholar] [CrossRef]
- Shen, J.; Wang, H.; Zhou, Y.; Ye, N.; Wang, Y.; Wang, L. Hollow mesoporous frameworks without the annealing process for high-performance lithium–ion batteries: A case for anatase TiO2. Chem. Eng. J. 2013, 228, 724–730. [Google Scholar] [CrossRef]
- Belarmino, D.D.; Ladchumananandasivam, R.; Belarmino, L.D.; de M. Pimentel, J.R.; da Rocha, B.G.; Galvão, A.O.; de Andrade, S.M.B. Physical and morphological structure of chicken feathers (keratin biofiber) in natural, chemically and thermally modified forms. Mater. Sci. Appl. 2012, 3, 7. [Google Scholar] [CrossRef]
- Bella, J.; Eaton, M.; Brodsky, B.; Berman, M.H. Crystal and molecular structure of a collagen-like peptide at 1.9 A resolution. Science 1994, 266, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Pallela, R.; Janapala, V.R. Comparative ultrastructural and biochemical studies of four demosponges from Gulf of Mannar, India. Int. J. Mar. Sci. 2013, 3, 295–305. [Google Scholar] [CrossRef]
- Abderhalden, E.; Strauss, E. Die spaltprodukte des spongins mit säuren. Zeitschrift Für Physiol. Chemie 1906, 48, 49–53. [Google Scholar] [CrossRef]
- Clancey, V.J. The constitution of sponges. The common bath sponge, Hippospongia equina. Biochem. J. 1926, 20, 1186–1189. [Google Scholar] [CrossRef] [PubMed]
- Selmin, F.; Cilurzo, F.; Aluigi, A.; Franzè, S.; Minghetti, P. Regenerated keratin membrane to match the in vitro drug diffusion through human epidermis. Results Pharma Sci. 2012, 2, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Meilert, K.T.; Laub, D.; Kiwi, J. Photocatalytic self-cleaning of modified cotton textiles by TiO2 clusters attached by chemical spacers. J. Mol. Catal. A Chem. 2005, 237, 101–108. [Google Scholar] [CrossRef]
- Young, D.; Qi, L.; Ma, J. Eggshell membrane tamplating of hierarchically ordered macroporous networks composed of TiO2 tubes. Adv. Mater. 2002, 14, 1543–1546. [Google Scholar] [CrossRef]
- Koch, R.; Lipton, A.S.; Filipek, S.; Renugopalakrishnan, V. Arginine interactions with anatase TiO2 (100) surface and the perturbation of 49Ti NMR chemical shifts—A DFT investigation: Relevance to Renu-Seeram bio solar cell. J. Mol. Model. 2011, 11, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Baia, B.; Jing, D. Hydrothermal synthesis of TiO2–yeast hybrid microspheres with controllable structures and their application for the photocatalytic reduction of Cr (VI). J. Chem. Technol. Biotechnol. 2014, 90, 930–938. [Google Scholar] [CrossRef]
- Vergaro, V.; Carlucci, C.; Cascione, M.; Lorusso, C.; Conciauro, F.; Scremin, B.F.; Congedo, P.M.; Cannazza, G.; Cinzia Citti, C.; Ciccarella, G. Interaction between human serum albumin and different anatase TiO2 nanoparticles: A nano-bio interface study. Nanomater. Nanotechnol. 2015, 5, 5–30. [Google Scholar] [CrossRef]
- Mattioli, G.; Filippone, F.; Caminiti, R.; Amore Bonapasta, A. Short hydrogen bonds at the water/TiO2 (anatase). J. Phys. Chem. C 2008, 112, 13579–13586. [Google Scholar] [CrossRef]
- Kang, Y.; Li, X.; Tu, Y.; Wang, Q.; Ågren, H. On the mechanism of protein adsorption onto hydroxylated and nonhydroxylated TiO2 surfaces. J. Phys. Chem. C 2010, 114, 14496–14502. [Google Scholar] [CrossRef]
- Sirisuk, A.; Klansorn, E.; Praserthdam, P. Effects of reaction medium and crystallite size on Ti3+ surface defects in titanium dioxide nanoparticles prepared by solvothermal method. Catal. Commun. 2008, 9, 1810–1814. [Google Scholar] [CrossRef]
- Supphasrirongjaroen, P.; Praserthdam, P.; Panpranot, J.; Na-Ranong, D.; Mekasuwandumrong, O. Effect of quenching medium on photocatalytic activity of nano-TiO2 prepared by solvothermal method. Chem. Eng. J. 2008, 138, 622–627. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Bizani, E.; Fytianos, K.; Poulios, I.; Tsiridis, V. Photocatalytic decolorization and degradation of dye solutions and wastewaters in the presence of titanium dioxide. J. Hazard. Mater. 2006, 136, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Siwińska-Stefańska, K.; Przybylska, A.; Jesionowski, T.; Sójka-Ledakowicz, J.; Olczyk, J.; Walawska, A. The influence of TiO2-SiO2 oxide composite on the barrier properties of textile fabrics. Przem. Chem. 2010, 89, 1189–1194. [Google Scholar]
- Siwińska-Stefańska, K.; Ciesielczyk, F.; Jesionowski, T.; Sójka-Ledakowicz, J.; Lota, W.; Walawska, A. Evaluation of the photocatalytic properties of textile fabrics modified with titanium dioxide of anatase structure. Fibres Text. East. Eur. 2011, 19, 76–83. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szatkowski, T.; Siwińska-Stefańska, K.; Wysokowski, M.; Stelling, A.L.; Joseph, Y.; Ehrlich, H.; Jesionowski, T. Immobilization of Titanium(IV) Oxide onto 3D Spongin Scaffolds of Marine Sponge Origin According to Extreme Biomimetics Principles for Removal of C.I. Basic Blue 9. Biomimetics 2017, 2, 4. https://doi.org/10.3390/biomimetics2020004
Szatkowski T, Siwińska-Stefańska K, Wysokowski M, Stelling AL, Joseph Y, Ehrlich H, Jesionowski T. Immobilization of Titanium(IV) Oxide onto 3D Spongin Scaffolds of Marine Sponge Origin According to Extreme Biomimetics Principles for Removal of C.I. Basic Blue 9. Biomimetics. 2017; 2(2):4. https://doi.org/10.3390/biomimetics2020004
Chicago/Turabian StyleSzatkowski, Tomasz, Katarzyna Siwińska-Stefańska, Marcin Wysokowski, Allison L. Stelling, Yvonne Joseph, Hermann Ehrlich, and Teofil Jesionowski. 2017. "Immobilization of Titanium(IV) Oxide onto 3D Spongin Scaffolds of Marine Sponge Origin According to Extreme Biomimetics Principles for Removal of C.I. Basic Blue 9" Biomimetics 2, no. 2: 4. https://doi.org/10.3390/biomimetics2020004






