Although there are a number of nanomaterials that have been artificially synthesized by mimicking the nanostructures present in nature, we will cover here some of the most important materials with great future application potential. We begin by looking at the double nanostructured pillars on the surface of a lotus leaf showing superhydrophobicity and study their design pattern for artificial synthesis. This is followed by the study of nanostructured spatulae of gecko feet and their dual characteristic of superhydrophobicity and high water adhesivity. We then look at how the biological wound healing process is mimicked to follow a synthetic self-healing process for producing self-repairing materials. Next, we jump over to flora, studying the process of photosynthesis in detail and its mimicry for the generation of hydrogen fuel. Going back to the animal kingdom once again, we look at the nanostructures of moth eye, butterfly wings and frog toe pads. Simultaneously, we also look at some of the fabrication techniques for these nanostructures and their potential applications.
3.1. The Lotus Effect
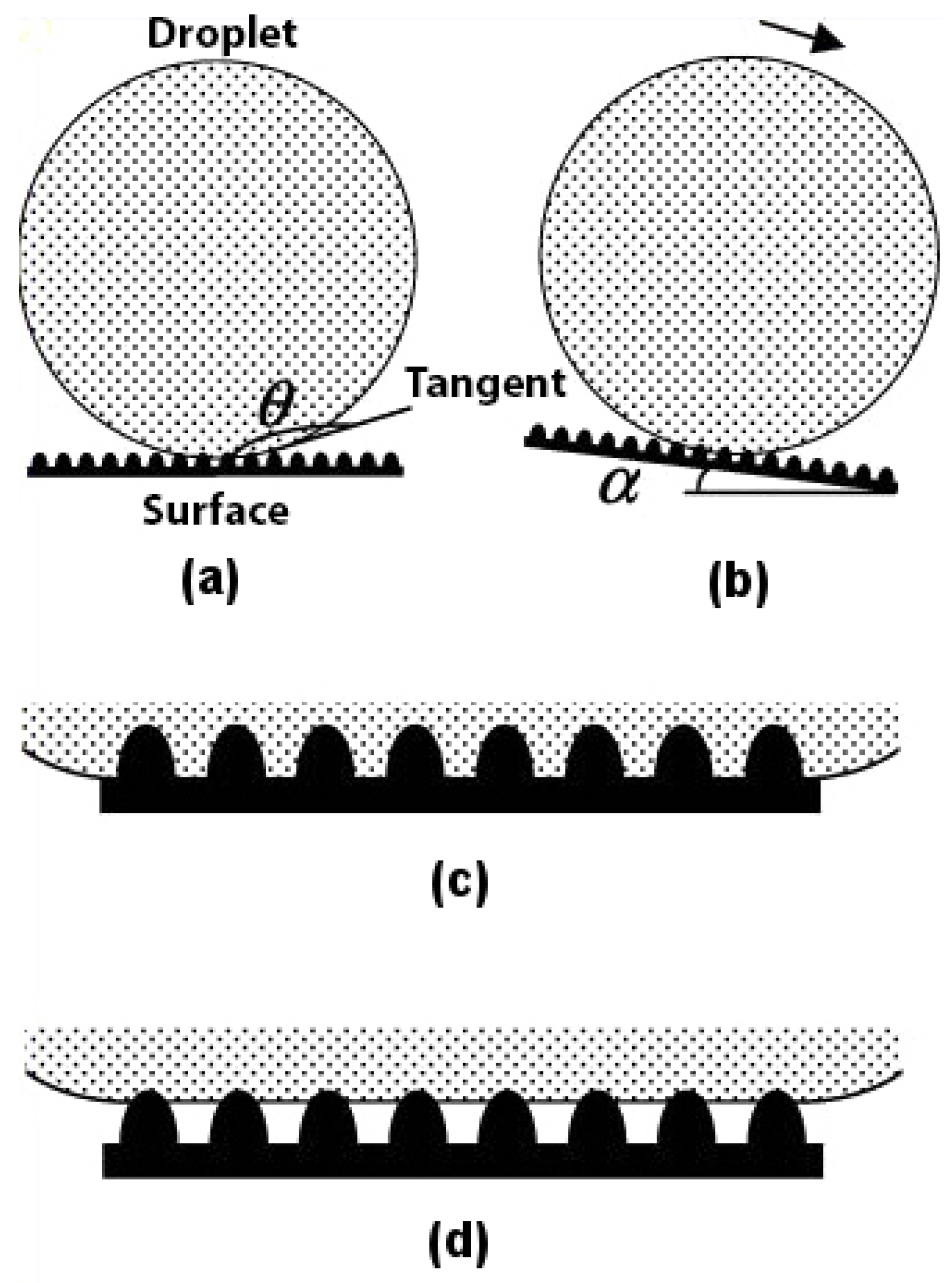
Superhydrophobicity is based on the concept of contact angle and roll-off angle. For a surface to be called superhydrophobic, the contact angle (
) between the water droplet and the surface should be as high as possible, and the roll-off angle (
) should be as low as possible, as shown in
Figure 1a,b. Any rough surface can become wet in two ways. Firstly, if the liquid droplet penetrates completely into the rough surface to reach its troughs, it is known as homogeneous wetting; see
Figure 1c. Secondly, if the troughs of the rough surface have any other fluid or air trapped inside them, then there is heterogeneous wetting; see
Figure 1d.
The type of wetting is a major contributing factor to the superhydrophobicity of the material. It has been found [
17] that the lotus leaf follows a heterogeneous type of wetting. The reason why nature prefers heterogeneous wetting over homogeneous wetting is because in the former, the wet area is always less as compared to the latter, as shown in
Figure 1c,d. This ensures that the contact angle for a water droplet on the surface of a lotus leaf following the heterogeneous wetting regime is greater and not lower, as in the case of the homogeneous wetting regime. This makes for an easier roll-off of the water droplet from the lotus leaf and, thus, has a lower roll-off angle. The type of wetting plays such an important role in superhydrophobicity that if water is made to condense in the troughs of a lotus leaf [
18], the surface of the leaf starts demonstrating a homogeneous wetting character, thus becoming hydrophilic with a contact angle much lower than 90.
Barthlott and Neinhuis [
19] first collected data on how dirt-contaminated lotus leaves self-cleaned themselves due to their superhydrophobic characteristics. It was years later that the researchers found that it was the effect of surface roughness and the wetting regime of the lotus leaf surface that resulted in the self-cleaning effect. Furthermore, it was found [
20] that the surface roughness of a lotus leaf was due to multiple nanostructured pillars (
Figure 2a) (with sizes in the range of 200 nm to 1
m) on its surface.
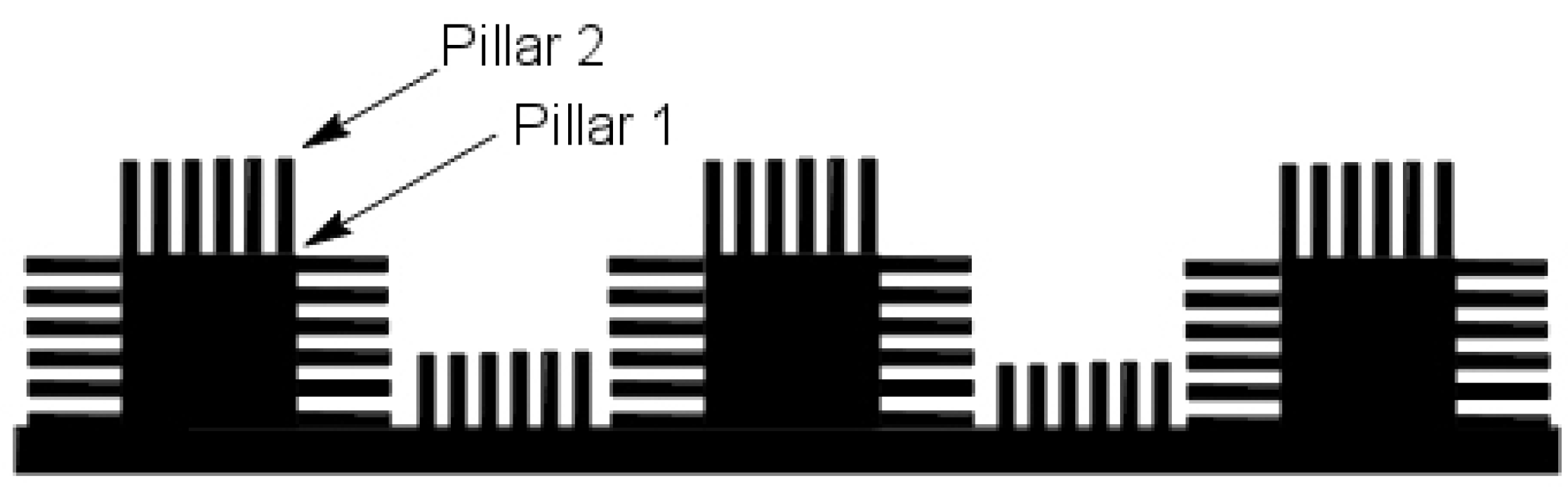
Figure 2b illustrates a drop of mercury on the surface of a lotus leaf. Thus, it was now possible to fabricate a design that would mimic the superhydrophobic behavior of a lotus leaf. A double-pillared structure design as shown in
Figure 3 is found to be best suitable for fabricating superhydrophobic self-cleaning surfaces by greatly amplifying the contact angle.
The double pillared nanostructures can be artificially fabricated using the soft lithographic imprinting technique [
21]. In this method, the lotus leaf is used as a natural template, and a liquid polymer is cast on the surface of this lotus leaf. When solidified, this polymer layer is peeled off to form a negative template. Another layer of the same or a different polymer is then cast on this negative template and peeled off in a similar fashion to transfer the nanostructures, mimicking the surface morphology of the lotus leaf on this second layer. Many polymers like poly(dimethylsiloxane) (PDMS) [
22,
23], poly(vinyl chloride) (PVC) [
24] and poly(ethylene oxide) (PEO) [
25] have been used to form negative templates and thus, superhydrophobic surfaces.
Much work has been done in the field of superhydrophobic surfaces in the last decade. However, still, we do not see them readily available in the market. This is because, although superhydrophobic surfaces have been fabricated in the lab with very high contact angles and very low roll-off angles, these are not durable enough to be commercialized. They are susceptible to dynamic impact, mechanical deterioration, environmental and chemical degradation and organic or bacterial contamination. Moreover, the tests that are conducted for durability by different researchers are also not standardized and, thus, fail to support industrial-level manufacturing of superhydrophobic surfaces. Inevitably, today, the area of interest in this field is the fabrication of superhydrophobic surfaces with high durability [
26]. Furthermore, as these coatings are implemented in areas where they are subjected to various kinds of environments, additional properties like anticorrosion, antifouling, self-healing capabilities, UV-B blocking, flame retardancy and oil repellency are actively researched [
27,
28].
3.2. Gecko Feet
Gecko lizards are found throughout the world in places with warm climates. They are of particular importance due to their feet, which allow them to climb any surface whether smooth or rough, inverted or vertical, without any problem. This characteristic feature of gecko feet results from their unique microstructured setae (3–7
m in diameter and 20–70
m in length), which are keratinous hairs present in millions on the surface of their feet, and each seta is further subdivided into hundreds of nanostructured spatulae (100–200 nm in diameter). The setae are superhydrophobic in nature, making a contact angle of 160 with water [
29]. However, it has also been found that not only these setae showed superhydrophobicity, but also an adhesive property towards water. This is a very unique dual characteristic behavior of gecko feet, which make them superhydrophobic, as well as highly adhesive towards water at will. The adhesion property is temperature-dependent as observed by Niewiarowski et al. [
30], who found that adhesion of gecko feet towards a water droplet was halved at 32 C than at 12 C.
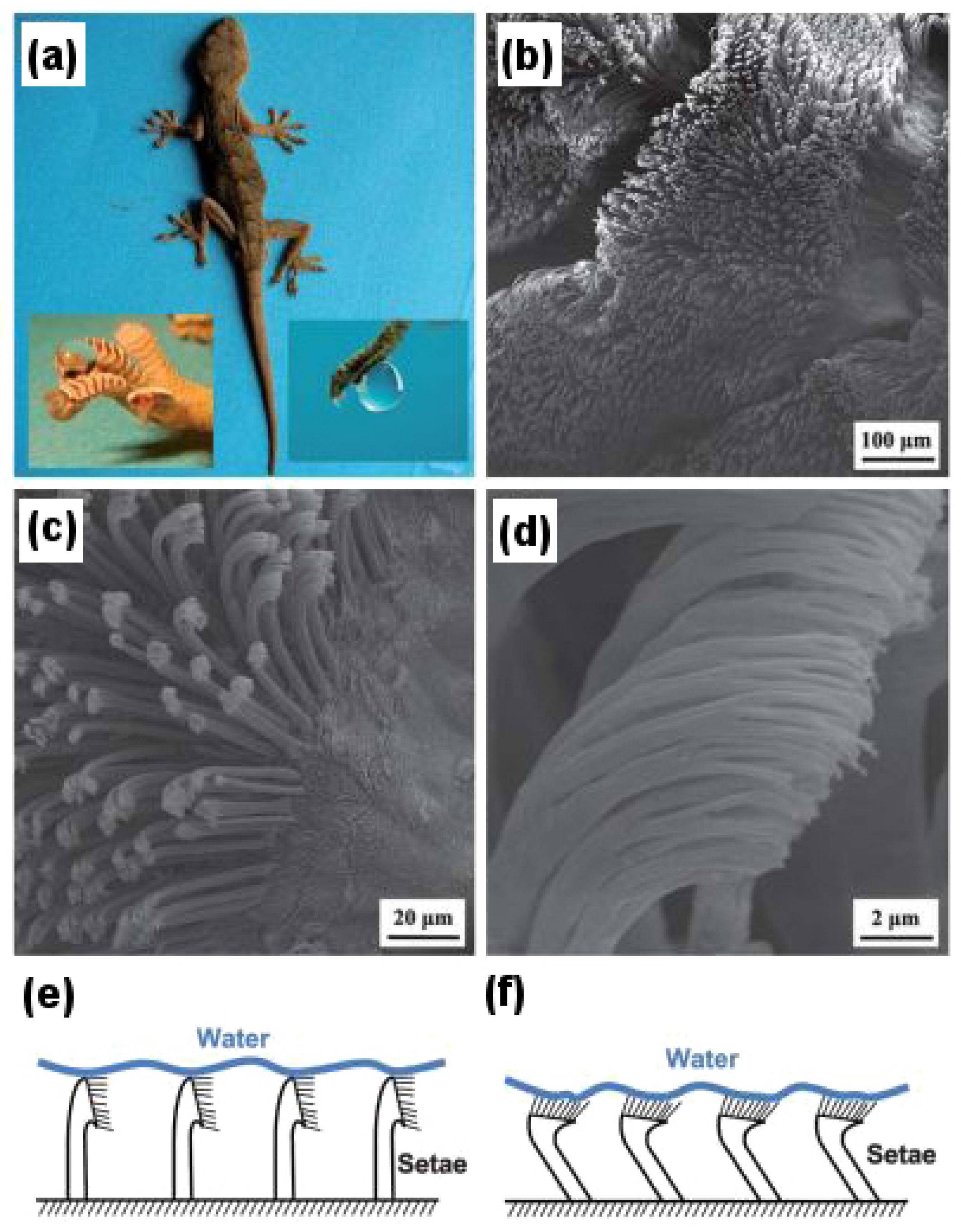
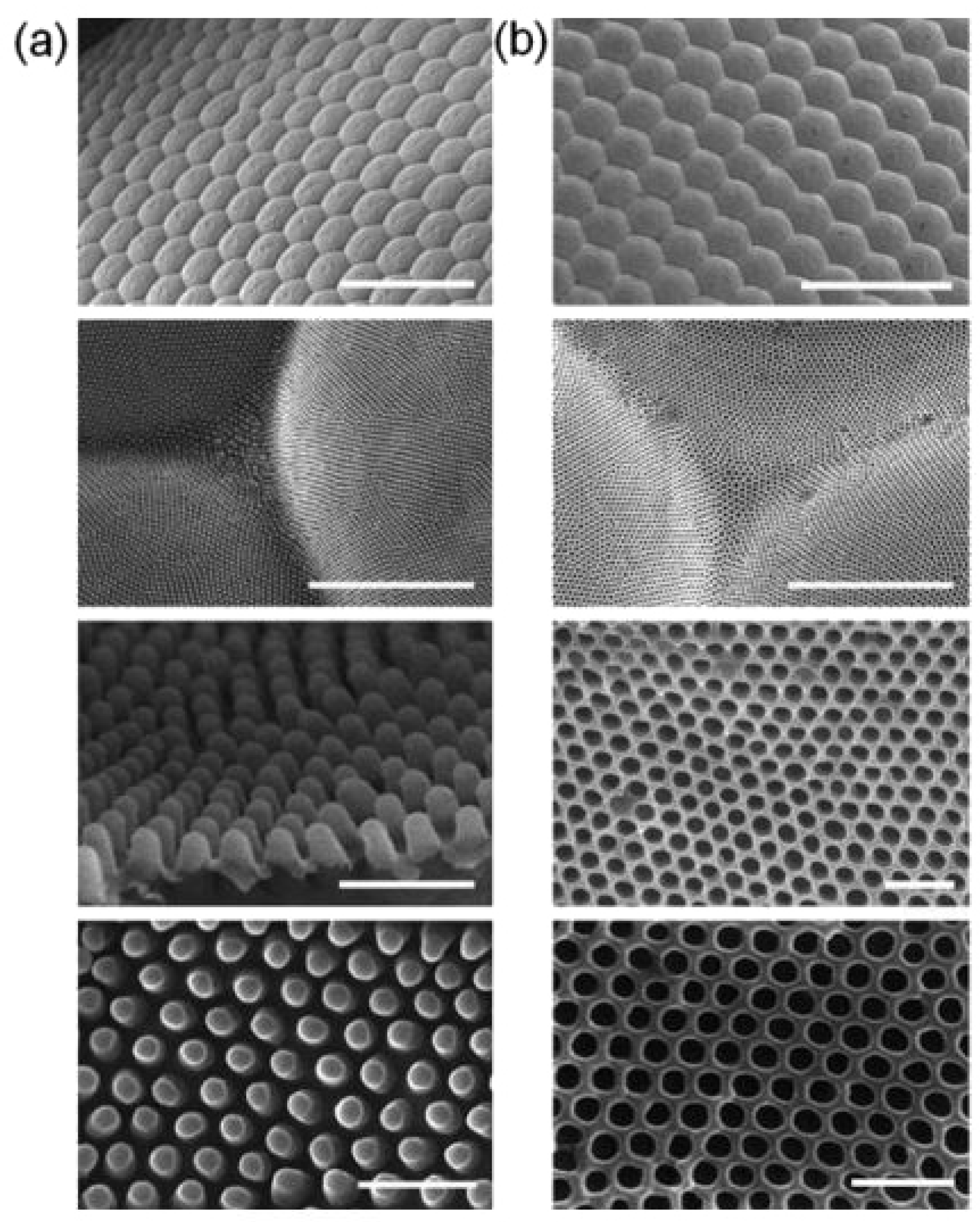
Figure 4 demonstrates the microstructures and nanostructures present on the surface of gecko feet and their characteristic features in detail. The inset to the left of
Figure 4a shows a drop of water almost perfectly spherical in shape on the foot of an anesthetized gecko revealing its superhydrophobic character, whereas the inset to the right demonstrates how a water droplet clinches to the surface of the gecko foot even when it is turned 90, showing its highly adhesive behavior towards the droplet.
Figure 4b–d shows scanning electron microscopy (SEM) images of setae and spatulae, whereas
Figure 4e,f illustrates the schematic as to how setae and spatulae are responsible for providing a superhydrophobic, as well as highly adhesive dual character to gecko feet. Autumn and Hansen [
31] first demonstrated the superhydrophobicity of setae by isolating them on a glass substrate and mimicking the arrangement as shown in
Figure 4e. However, to demonstrate its highly adhesive behavior due to the very large number of nanopillared spatulae coming in contact with the water droplet, the arrangement as shown in
Figure 4f can be used.
Such a high adhesive force of gecko feet is said to be the result of van der Waals interactions [
29,
33] as in the case of solid–solid adhesion, but here, it is between the water droplet and the numerous nanopillared spatulae. It has also been speculated that the actual contact area between the water droplet and spatulae is much larger than we predict [
34], which provides the gecko feet with this kind of highly adhesive behavior. The role of capillary-induced adhesion also cannot be ruled out [
35].
Liu et al. [
32] fabricated superhydrophobic, as well as highly adhesive polyimide films with densely packed microclusters and nanopillars mimicking gecko feet via anodic aluminum oxide (AAO) templates. A value of about 66 ± 3
N was obtained for the adhesion force between liquid–solid (i.e., water droplet–polyimide film) measured using the highly sensitive microelectromechanical balance system. Qu and Dai [
14] mimicked gecko feet using vertically aligned single-walled carbon nanotubes (VA-SWCNTs), which they synthesized using the plasma enhanced chemical vapor deposition (PE-CVD) technique and a fast heating process. The micro- and nanostructures mimicking gecko feet fabricated using this technique also demonstrated excellent thermal and electrical properties making it possible for them to be used at higher temperature (∼125 as compared to scotch tape and super glue) and in semiconductor devices, respectively.
More recently, inspired from the self-cleaning capabilities of gecko feet without the use of water, focus has been shifted to the fabrication of artificial dry self-cleaning membranes [
36]. Furthermore, due to their highly adhesive nature, gecko feet-inspired mimics have demonstrated their use for the pick up and drop off microparticles, which has further led to the manufacturing of complex micropatterns. This property of gecko feet-inspired artificial adhesives is now being used in many potential applications like in micromanipulation for the fabrication of microelectromechanical systems (MEMS) and in biomedical devices [
37].
3.3. Self-Healing
The skin that covers the body of all vertebrates is composed of two main layers: the epidermis (outer layer) and the dermis (inner layer) [
38]. In case we sustain an injury, these two layers of skin tear apart or are removed, exposing internal body tissues to the environment. This sets in the natural process of wound healing by our body, which forms the basis of our next biomimetic approach. To grasp the concept of self-healing in materials, it is essential to discuss the process of wound healing in belief, which is done next.
The wound healing process takes place in three steps: (i) hemostasis or blood clotting and inflammation; (ii) proliferation or tissue growth; and (iii) maturation or tissue remodeling [
39]. Hemostasis is the cessation of blood flow at the site of injury to prevent blood loss. This happens by first sticking of blood platelets at the injury site, which then takes an amorphous shape to further promote clotting. Simultaneously, a chemical response is generated and sent, activating fibrin, which glues all of these platelets together to form a clot that stops the blood flow [
40,
41]. After this, the white blood cells engulf all of the bacteria and pathogens along with dead and damaged cells that are present at the injured site by a process known as phagocytosis [
42]. This happens during the inflammation phase. In the next step, i.e., proliferation, new blood vessels form [
43] along with the structural support provided by extracellular matrix [
44]. Epithelial cells proliferate, as well providing cover for the newly formed tissue [
45], and finally, wound contraction ensures decreased wound size. In the third and last step, collagen is realigned along wound boundaries, and unneeded cells undergo programmed cell death or apoptosis.
Figure 5 presents a pictorial representation of the wound healing process.
Inspired by the self-healing capabilities of the human body, we are on our way to create smart materials that are able to self-heal based on a process similar to wound healing.
Figure 6 shows demonstrates similarities between the two processes of biological wound healing and synthetic self-healing. As can be seen from the figure, the two processes are strikingly similar in nature.
In synthetic self-healing, the first step is an actuation or triggering mechanism, which takes place if a crack develops. This is followed by a second step in which the material is transferred to the affected site leading to a third and final step of carrying out the repair operation of the affected area as per the healing mechanism applied. There are a number of healing mechanisms that can be implemented as discussed in subsequent paragraphs. It is also worth noting that not only humans and animals, but plants [
46] also demonstrate self-healing capabilities by fighting against infection from pathogenic plants and preventing themselves from desiccation [
47], i.e., extreme dryness.
Different types of materials like polymers and their composites [
48], cementitious materials [
49], ceramics [
50] and metals [
51] are being actively researched to provide them with self-healing capabilities. For example, smart polymeric materials possessing self-healing capabilities are being synthesized in three different ways, namely: capsule-based healing, vascular self-healing and the intrinsic approach to self-healing [
48]. Various other techniques are continuously being discovered for materials to have self-healing properties.
In the capsule-based healing approach, the healing agent is packed in tiny capsules. As the crack or damage propagates and reaches these encapsulations, they rupture, releasing the healing agent into the crack, which repairs the damage. This is however a one-time repair mechanism, as once ruptured, the capsule is useless if the crack reappears at the same spot. In the vascular self-healing process, hollow tubes and capillaries are filled with healing agent and are connected in a network. This system of healing agent-containing capillaries can form one-, two- or three-dimensional networks, and their density depends on the amount of cracks, which can develop in the material over time. Once a crack propagates to these channels, the capillaries break, releasing the healing agent and repairing the crack. A major advantage to this approach is that it is a multiple time healing process. If a crack reappears at the same spot, capillaries releasing the healing agent can once again repair the crack. Empty capillaries from previous repair cycles can be refilled with healing agent externally or from other capillaries connected in the network. In the intrinsic type self-healing, there is no healing agent, rather a latent characteristic of the material, which promotes self-healing. This characteristic can be attributed to the chemical bonding within the material and physical interactions in the crack interfaces. Intrinsic self-healing is based on reversible reactions, like hydrogen bonding, ionic interactions, reversible polymerization, etc. Yet, an external trigger is often required, like heat, mechanical stress and electrical stimulus, to initiate the self-healing process [
52]. Since reversible reactions are involved, this type of healing can occur many times at the same spot in case a crack appears.
Figure 7 shows the self-healing process using the capsule-based approach.
In more recent studies, research is no longer focused on the refilling of gaps after crack development. Rather, emphasis is laid on self-healing capabilities of the various functionalities of the material [
53]. These include self-repair of properties like resistance, conductivity and charge carrier mobility in conductors, emission, reflectivity and absorption in optoelectronic materials and various functions of novel films and coatings, like superhydrophobicity, flame resistance, surface energy, aesthetics and superamphiphobicity. Moreover, advanced trigger systems are also being researched, like stimulus by chemical, thermal, electrochemical, mechanical or photochemical impetus, as against the conventional crack propagation. In the coming years, hybrid self-healing materials will be the center of attention, which will involve an amalgamation of self-repairing capabilities for different properties of the material.
3.4. Photosynthesis
Photosynthesis is the process by which plants use carbon dioxide, water and energy to synthesize glucose, the building blocks of plants, and give away oxygen as the by-product. This process is represented by the equation as given below:
Understanding the process of photosynthesis provides us with better insight into the working of plant mechanisms and helps us to reap fruitful results by its biomimicry. Subsequent paragraphs describe the process of photosynthesis in brief [
54].
To carry out the photosynthetic process, plants obtain carbon dioxide from the atmosphere; water is derived from the ground through roots; and energy is obtained from the sun. Light from the sun contains photons from a wide range of the electromagnetic spectrum, but for plants, only photons in the range of the visible spectrum (400–700 nm) are required. To capture these photons, plants have special pigments called chlorophylls. The color of this pigment is a result of the light reflected. For example, reflecting green and yellow wavelengths of light and absorbing blue and red wavelengths are what give plants their characteristic yellowish-green color. The blue and red wavelengths of light are sufficient to provide the required energy to plants for photosynthesis.
Inside autotrophs, i.e., self-feeding plants, photosynthesis is carried out in special structures of the plant cell called chloroplasts. Photosynthesis is a two reaction set mechanism: (i) light-dependent reactions [
55] and (ii) the Calvin cycle [
55], as depicted in
Figure 8 and
Figure 9, respectively. Chloroplasts contain small disc-like structures called thylakoids [
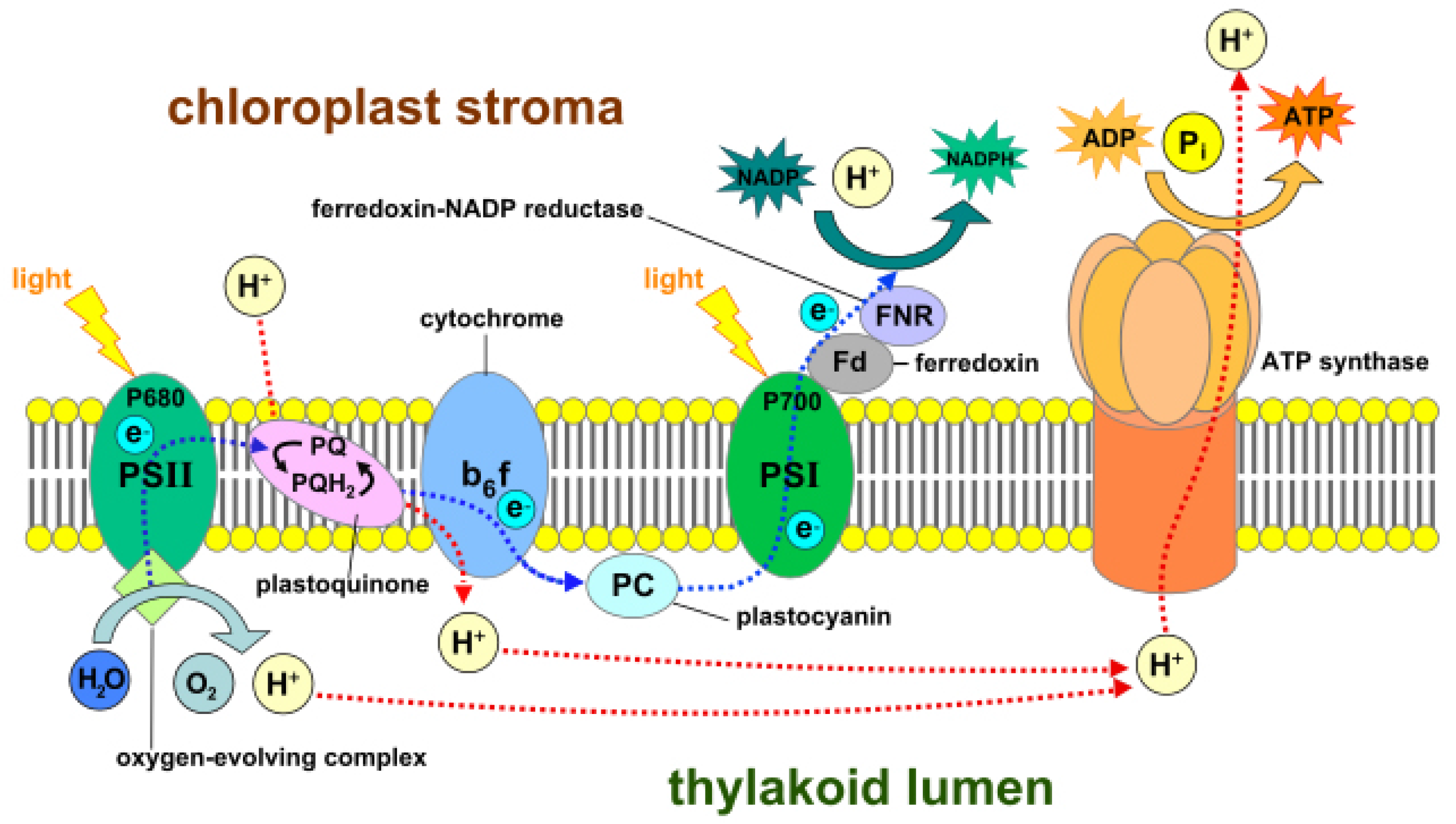
56], which are surrounded by colorless fluid stroma. Light-dependent reactions occur in thylakoid, whereas light independent reactions or the Calvin cycle occur in stroma. Thylakoid converts light energy from the sun to chemical energy, which ultimately drives the synthesis of glucose. Each thylakoid contains a pair of photosystems called photosystem-I and photosystem-II, which work together to achieve this goal. These photosystems contain chlorophyll molecules, which capture incoming photons of light, thereby exciting their electrons to a higher state. These excited electrons are emitted and channeled to a single chlorophyll molecule called the reaction center chlorophyll. Photons strike both photosystems simultaneously.
In photosystem-II, the reaction center transports the excited electrons via an electron transport chain. The electron transport chain is a group of proteins situated on the thylakoid membrane. In case the photosystem-II loses any excited electrons, they are replaced by a process called photolysis in which water is oxidized to produce free electrons and oxygen gas as a by-product. The high energy electrons in the electron transport chain are used to pump hydrogen ions from the stroma to the thylakoid via the cytochrome b6f complex enzyme. This develops a concentration gradient between the stroma and thylakoid membrane, which is used to power a protein called ATP synthase that converts adenosine diphosphate (ADP) to adenosine triphosphate (ATP). The low-energy electrons remaining in the electron transport chain are pushed to photosystem-I where they are re-energized and again carried via a second electron transport chain to ferredoxin-NADP+ reductase (FNR), an enzyme that mediates the transfer of electrons from reduced ferredoxin to nicotinamide adenine dinucleotide phosphate (NADP+) for production of NADPH.
The ATP and NADPH molecules produced and released continuously in the stroma during these light-dependent reactions power the Calvin cycle, which is a set of light-independent reactions reducing carbon dioxide to produce carbohydrate glyceraldehyde 3-phosphate (G3P). The Calvin cycle is a three-step process consisting of: (i) carbon fixation; (ii) reduction; and (iii) regeneration of ribulose-1,5-bisphosphate (RuBP). During carbon fixation, one molecule of CO2 combines with one molecule of RuBP to produce two molecules of 3-phosphoglycerate (PGA). Then, during the reduction, one molecule of ATP reduces one molecule of PGA to one molecule of 1,3-bisphosphoglycerate (1,3-BPG), which is further reduced by a single molecule of NADPH to one molecule of G3P. Thus, a single turn of the Calvin cycle produces two molecules of G3P. When the cycle runs three times, it produces six G3P molecules, five of which are used for the regeneration of RuBP in the third and final step of the Calvin cycle. The remaining G3P molecules once accumulated are then used to form glucose, glycerol and fatty acids.
Two molecules of G3P form one molecule of glucose 6-phosphate, which can get rid of its phosphate to combine with fructose and form sucrose. Thus, it take six turns for the Calvin cycle to produce one glucose molecule. Glucose 6-phosphate is also used to synthesize starch and cellulose. This completes our description of the process of photosynthesis as it is carried out in nature.
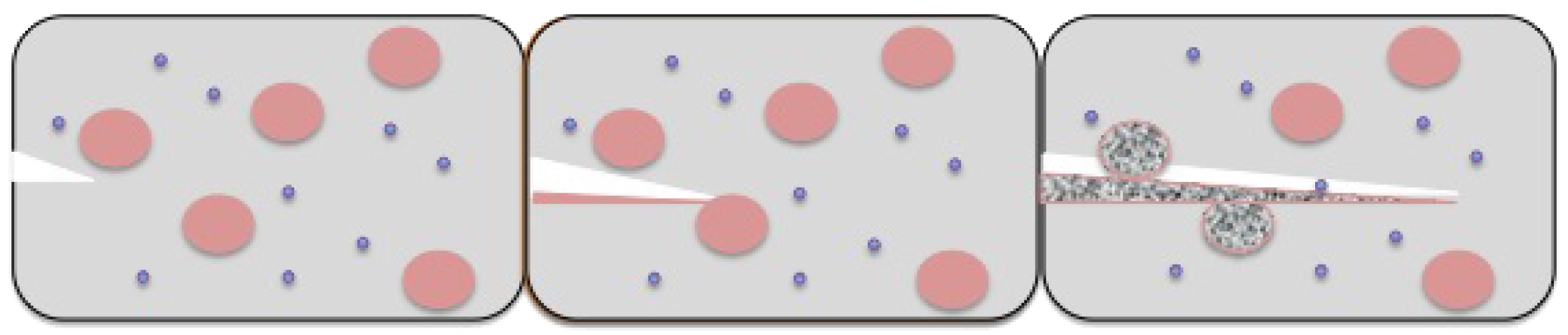
To bring about artificial photosynthesis in the lab, we need various components, like: (i) antennas that have the capability to capture incoming photons of light; (ii) a reaction center where all electrons pile up to be transported by an electron transport chain; and (iii) some kind of electron transport mechanism, which would lead to the development of the potential gradient for pumping protons.
It is now possible to synthesize artificial antennas that can harvest light [
15]. These when linked with reaction centers are able to convert the excitation energy of photons to chemical potential by generating a long-lived charge separation. If however, the charge separation is short-lived, the potential energy of photons, which has been stored, does not last long enough to be harvested, thereby rendering the reaction center redundant. Artificial reaction centers when incorporated in a lipid bilayer of an artificial vesicular setup are responsible for generating transmembrane proton motive force by acting as light-driven proton pumps. This proton gradient generated is used by the ATP synthase enzyme to synthesize ATP, which is the desired high energy biofuel molecule.
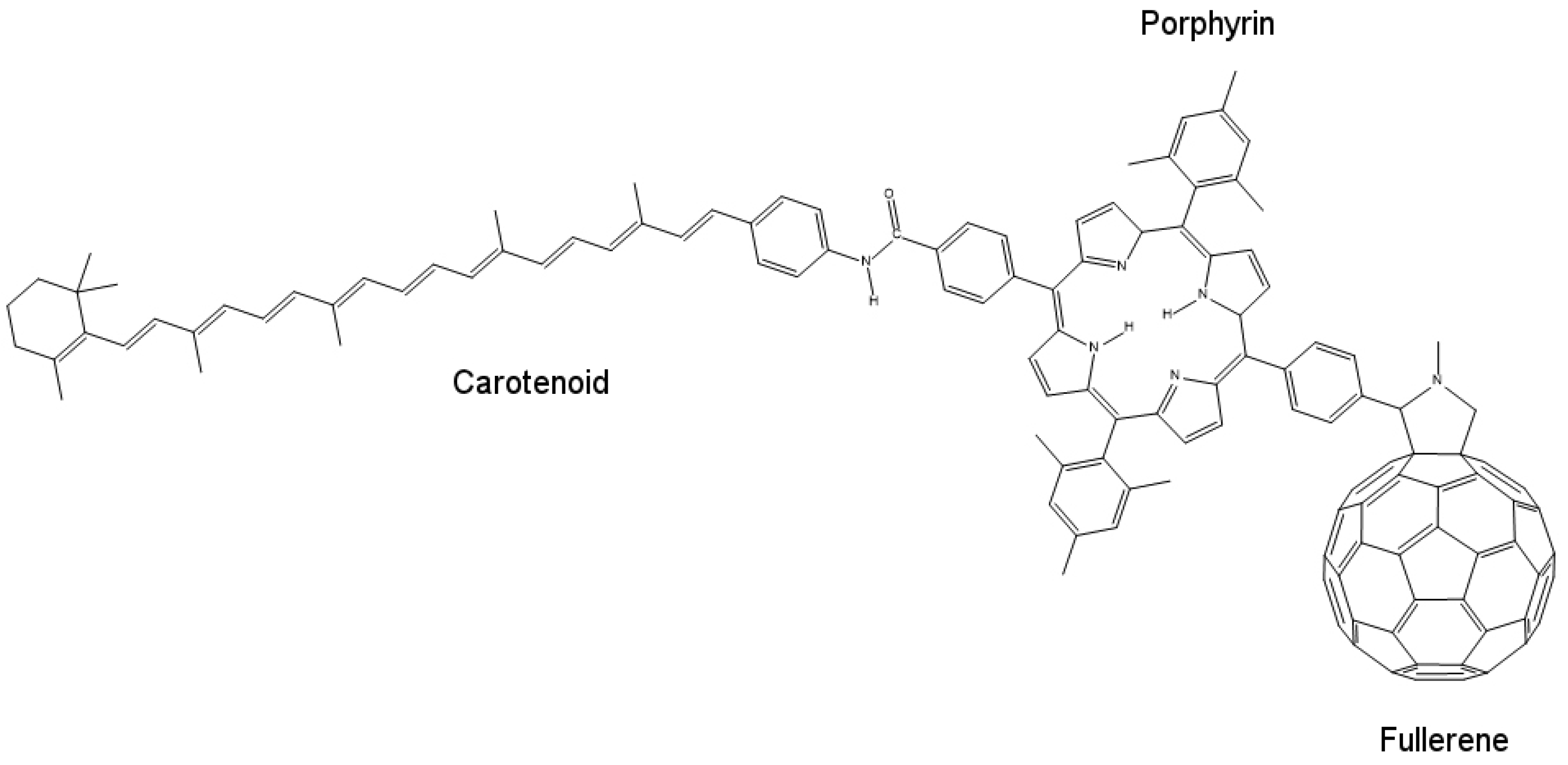
To fabricate a model mimicking the photo-induced electron transfer mechanism or an artificial reaction center, we require an electron donor/acceptor chromophore, which is able to absorb visible light and can be bonded to another electron donor/acceptor moiety for generating the necessary charge generation. One such system can be designed using molecules of porphyrin, fullerene and carotenoid, as shown in
Figure 10. Porphyrin, being less stable, mimics the chlorophyll present in plants. Fullerene (C
60) behaves as the electron acceptor. Porphyrin (P) and fullerene when bonded covalently provide electronic coupling, which mimics the photo-induced electron transfer mechanism of the natural reaction center.
When excited by a photon of light, porphyrin and fullerene form a P
+–
charge separation pair, which recombines in 478 ps. This time interval is too short to harvest stored potential energy generated by this charge separation. To increase this time interval, electronic coupling between the two moieties should be weakened, which can be done by increasing the distance between donor and acceptor molecules, thus obtaining slow charge recombination. This is where carotenoid (C) plays its role. When carotenoid is linked covalently to porphyrin on another side, it completes the artificial reaction center model by overcoming the charge separation problem. Carotenoid increases the charge recombination time by 1000-fold to around 57 ns [
57] acting as an electron donor in the process. When a photon strikes and charge separation occurs (C–P
+–
), instead of recombining, this charge separation state forms another intermediate with carotenoid (C
+–P–
), which then recombines very slowly, thus allowing the stored potential energy to be harvested.
To successfully mimic photosynthesis, however, only artificial reaction centers are not enough. They should work in combination with artificial antennas to fully develop a working model of an artificial photosynthetic system. An ideal artificial antenna would be able to absorb light and immediately transfer the electrons to constituent chromophores for generating charge separations. Sunlight is absorbed mostly by antenna systems and not reaction centers. Thus, it is extremely important to have excellent antenna systems to increase the efficiency of a lab-made, artificial photosynthetic system as much as possible. As natural photosynthesis can occur in a wide range of environmental and weather conditions, there are a number of antenna morphologies that exist in nature.
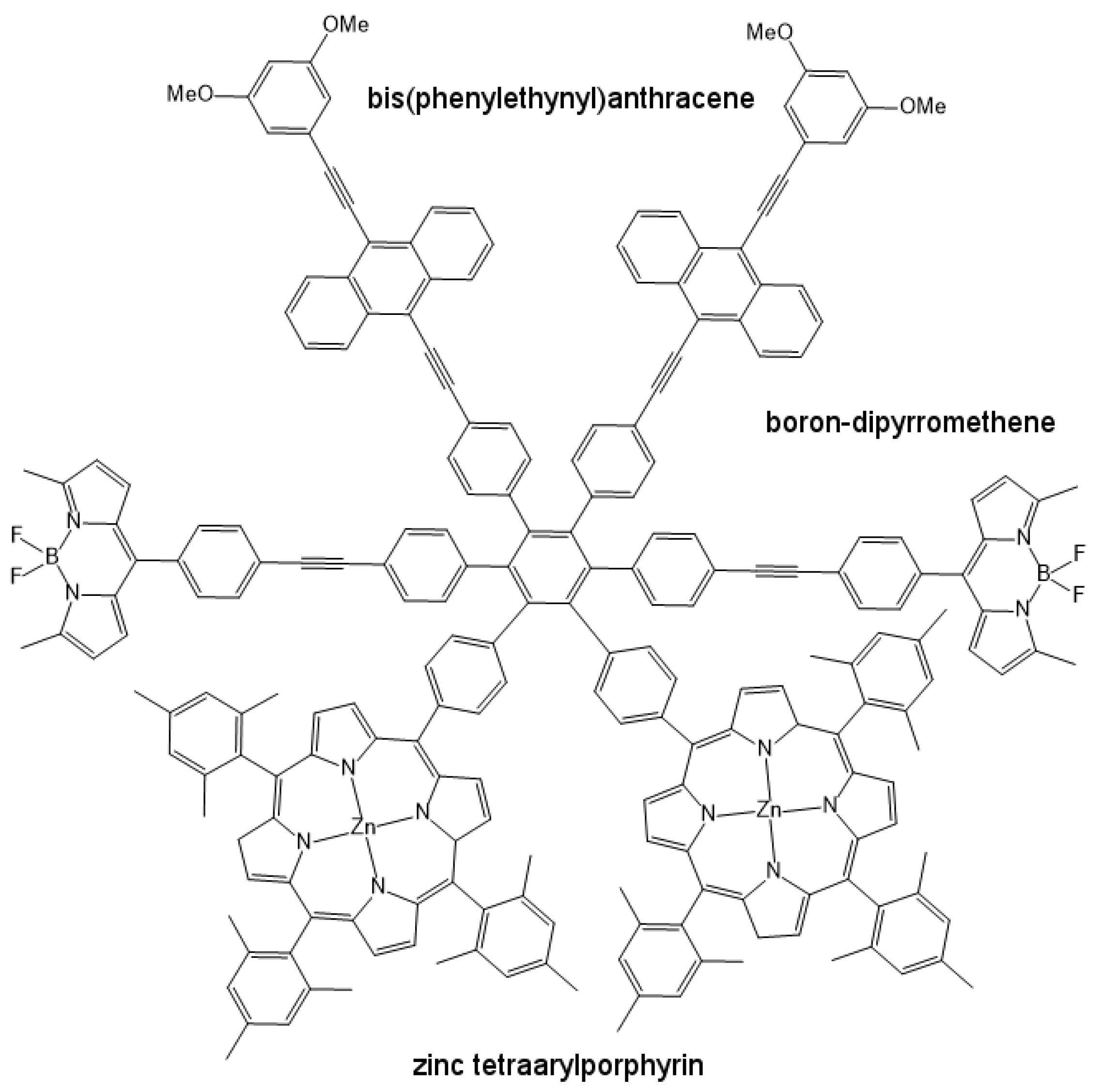
One such type of artificial antenna morphology is illustrated in
Figure 11 [
58]. In this model, bis(phenylethynyl)anthracene (BPEA), boron-dipyrromethene (BDPY) and zinc tetraarylporphyrin (ZTAP) act as primary light absorbing components. BPEA absorbs strongly at around 450 nm, which represents the blue region of the electromagnetic spectrum; BDPY absorbs at 513 nm, which is close to green; and ZTAP absorbs at 418 nm (Soret region), and also at 557 nm and 598 nm, corresponding to the orange and red wavelengths of the electromagnetic spectrum, respectively. Thus, this model absorbs photons from almost the entire visible spectrum of light, which is usually the case with natural antenna systems. This antenna unit can then be attached to a charge separation unit like fullerene [
57], which is a part of the artificial reaction center, thus completing the artificial photosynthetic system.
As an example of a working model of the proton pump [
59], artificial reaction centers involving carotenoid–porphyrin–quinone and independent quinone molecules can be incorporated in the lipid bilayer, such that the quinone (Q) of carotenoid–porphyrin–quinone, being hydrophilic in nature, faces the external side of the lipid bilayer, whereas carotenoid being hydrophobic faces the inside of the membrane. The pump follows a mechanism of redox loop, in which, when excited by light, the reaction center forms a charge separation state C
+–P–Q
−. The quinone anion (Q
−) of the artificial reaction center reduces independent quinone molecules to semiquinone anions, which can then grab a proton from the outside aqueous environment, becoming neutral themselves in the process. These neutral semiquinone molecules travel freely in the lipid bilayer, where, upon encountering the carotenoid ion (C
+), they get reconverted into quinones, thereby releasing the proton in the interior side of the lipid bilayer. Many such carotenoid–porphyrin–quinone and quinone molecule pairs can transfer many protons simultaneously from the exterior of lipid bilayer membrane to its interior surface, thus generating a proton gradient, required for the synthesis of ATP.
Although we are now able to successfully fabricate artificial antennas and reaction centers to mimic the process of photosynthesis in the lab, there is always room for improvement. If we need more efficient hydrogen fuel generators, we have to increase the amount of light that is harvested by increasing the number of artificial antennas or by designing them more efficiently. This will ensure an increase in the amount of potential energy stored. Furthermore, the stored energy should be available to harvest for a longer duration of time, which can be achieved by increasing the charge separation period.
Some recent studies are a step in this direction. Artificial reaction centers based on porphyrin have been developed, which showed properties very similar to natural reaction centers of purple bacteria [
60]. The charge separation distance in the reaction center of purple bacteria is around 7.0 Å, which was very closely mimicked in the artificial reaction center with a center-to-center distance of 6.2 Å. The electron-transfer reaction distances were also close, indicating the similarity between the electron-transport properties of the natural and artificial reaction centers. Moreover, the primary and secondary charge separation and recombination times were also strikingly similar, expect for the secondary charge recombination time, which was on a microsecond time-scale instead of the millisecond time-scale of the natural counterpart. Yet, further improvement is expected in the coming years. Other more advanced methods are now being researched, which involve the use of semiconducting photoelectrodes [
61] or photobioelectrochemical systems with enzymatic cathodes and photoanodes [
62] for mimicking photosynthesis and for fabricating photosynthetic systems on a large scale.
3.5. Moth Eyes
Another interesting type of nanostructure for bioinspiration are the eyes of a moth insect. Bernhard [
63], first in 1967, observed a unique characteristic of these nocturnal flying insects, where they played a game of camouflage with their predators by reducing reflection of light from their corneas. Later it was discovered that this anti-reflecting behavior was due to the regular arrangement of conical protuberances, which covered the entire surface of their eye. This anti-reflection property can be mimicked in the lab if the spacing between protuberances is much less than the incident wavelength of light, whereas the depth is significantly larger. A similar principle is applicable in acoustics as in the case of anechoic chambers. For fabricating artificial moth eye-like structures, this spacing between protuberances and the depth was found to be around 200 nm.
Figure 12a depicts conical protuberance nanostructures of the moth eye.
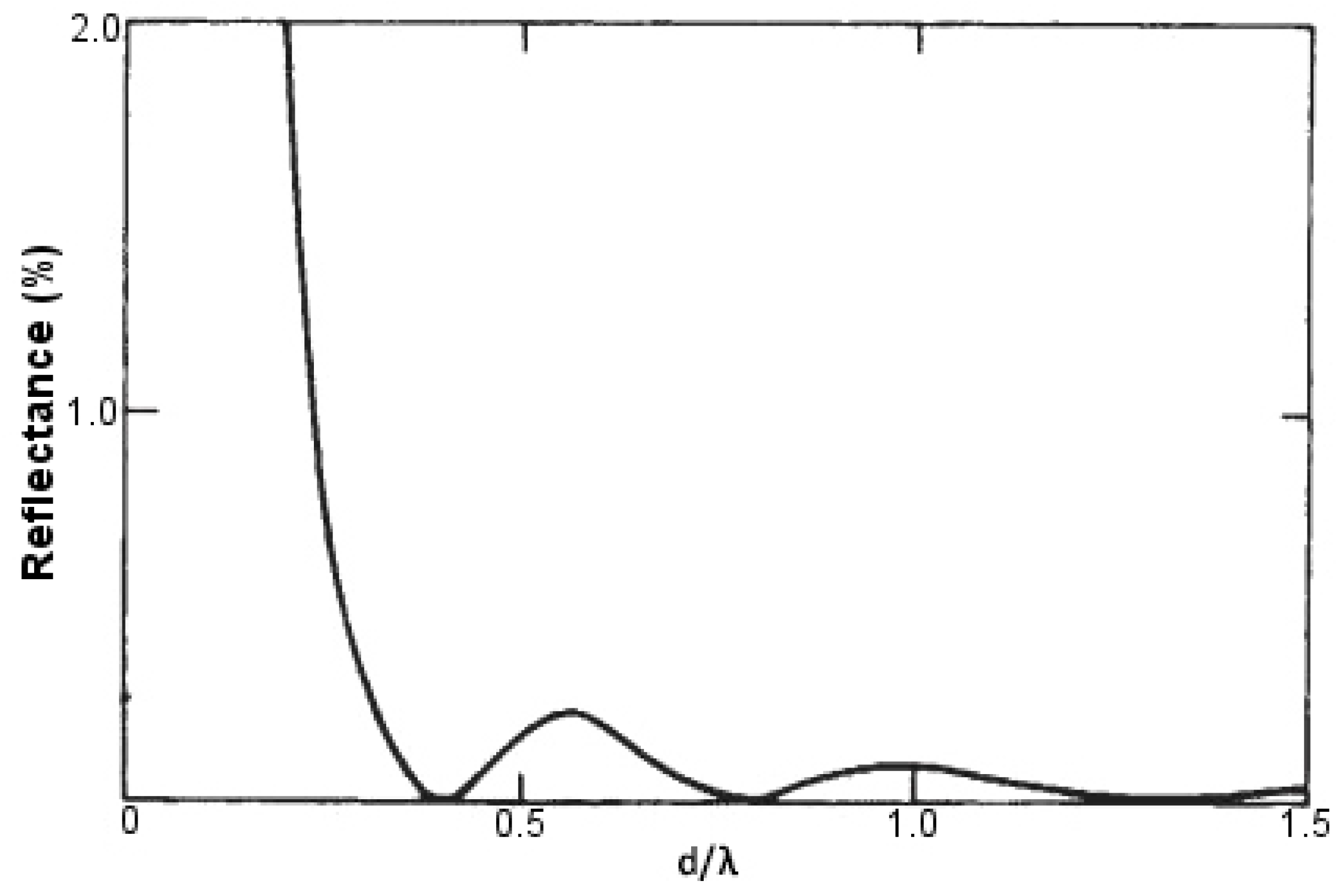
The height of these conical protuberances (
d) shares a special relationship with the incident wavelength (
) of light, which is responsible for developing the anti-reflection property mimicking the moth eye. The principle was first given by Rayleigh. When
d is extremely small, as compared to
, the surface is sharp and shows maximum reflectance. As
d increases, it is accompanied by a decrease in reflectance levels, with a minimum value of zero attained at
d/
= 0.4. If we further keep on increasing the height
d of these conical protuberances, we obtain successive maxima and minima of reflectance levels, none though reaching a value as high as when
d was extremely small as compared to
. When
d reaches a value that is very large as compared to
, we obtain practically zero reflectance. This behavior has been demonstrated in
Figure 13. Thus, to obtain anti-reflecting properties in the visible spectrum, we have to make conical protuberances with a height greater than or equal to 250 nm [
64].
To fabricate moth eye-like nanostructures artificially, the soft imprint lithographic technique can be used to generate a negative mold with materials like perfluoropolyether (PFPE), as shown in
Figure 12b, which can then be used to generate another positive mold that mimics the morphology of the eye surface. Another method, based on a bioinspired templating technique, allows us to fabricate anti-reflection coatings on single crystalline silicon substrate. In doing this, a silica colloidal crystal-polymer nanocomposite is fabricated using spin coating, in which polymer is etched with oxygen plasma, Si is etched with SF
6 reactive ion etching (SF
6 RIE) and silica is etched with hydrofluoric acid to obtain silicon nipple arrays mimicking moth eye [
66].
Figure 14 demonstrates this fabrication technique. A slightly altered route is followed by Linn et al. [
67] to fabricate similar nanostructures. A third method involves fabrication using the roller nanoimprint technique [
16]. Here, a master layer containing nanoscale structures is pressed sequentially on a UV-sensitive resist which has been spin coated on a silicon substrate. This UV-sensitive resist acts as an etching mask when moth-eye like patterns are transferred to the substrate using inductively coupled plasma etching or reactive ion etching to form conical protuberances.
The anti-reflective properties of the bioinspired moth eye-like structures have recently been demonstrated to possess numerous potential applications, for example in eyeglasses, flat-displays, solar panels, etc. They have been shown to increase the efficiency of both perovskite, as well as organic solar cells. In one study, mesoporous TiO
2 film mimicking moth eye-like nanostructures were prepared using the lithography, nanoimprinting and PDMS stamping technique, which increased the power conversion efficiency by ≈11% as against conventional perovskite solar cells [
68]. In another study, organic polymers were used to prepare moth eye-mimicking organic solar cells [
69], which are economical and may one day solve our energy crisis by providing solar energy-converted electricity efficiently to our homes and offices. In yet another study, moth eye-based filters were tested for various optical properties, like wavefront quality, transmission and the light scattered, by deploying them in infrared instruments at normal and at cryogenic temperatures for ground- and space-based applications [
70].
3.6. Butterfly Wings
Nature works in sophisticated ways, and time and again, it is proven by studying it closely. For example, even small insects like butterflies exhibit highly complicated physical properties by possessing unique optical characteristics. The wings of a butterfly demonstrate high or low reflectivity at particular wavelengths of light while simultaneously diffusing reflected light in a wide angular spectrum. These properties are extremely important for their survival via camouflage, for mating and for marking territorial boundaries. Biomimeticians are particularly interested in the iridescence property of butterfly wings, which causes them to change color when viewed from different angles, sometimes even making the wing look metallic [
71]. This property is a direct result of the micro- and nano-structural composition of butterfly wings as discussed in subsequent paragraphs.
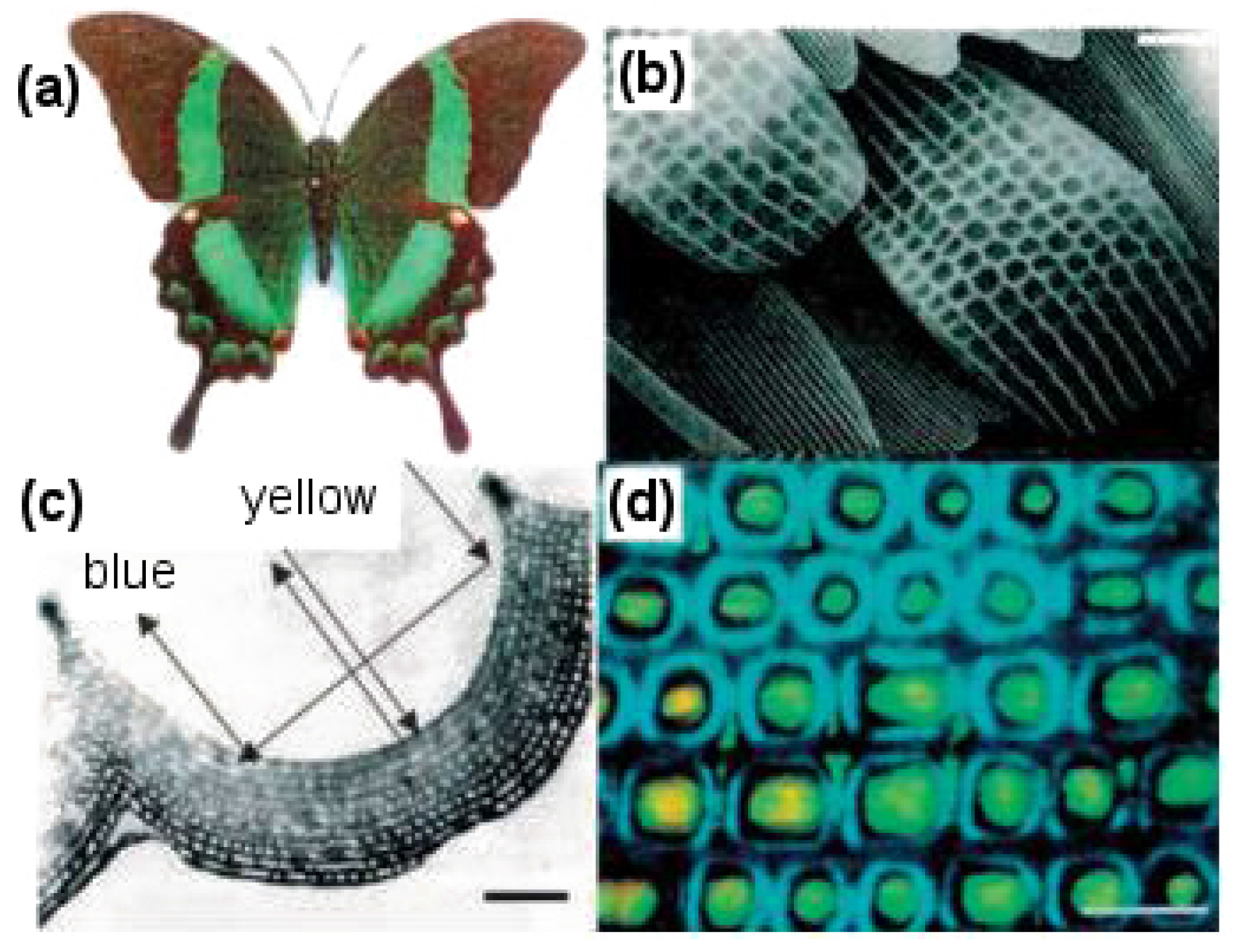
Butterflies are of particular interest for studying nanostructures because they have a rich diversity of scales coating their wings that bring about beautiful colors and patterns (see
Figure 15). The arrangement of scales follows an overlapping roof tile pattern with a scale density of 200–600 per mm
2 depending on the species. Apart from the simple roof tile pattern, scales further possess voids, complicated groove shapes and stratification, which allow them to display complex optical effects, like light scattering, diffraction and interference [
72]. It is interesting to note that color is usually linked with chromophore pigments, which are responsible for chemically displaying the color, but in the case of butterfly wings, various optical phenomena of light taking place on physically-patterned scales are responsible for color generation [
2] (see
Figure 15c).
By looking more closely at these scales, it can be observed that on a single wing, they are present in the thousands and have dimensions of about 200 m in length and 50 m in width. The wings consist of two sets of scales with an alternate row arrangement, a set of long scales acting as covers, which overlap and hide a set of shorter ground-level scales. A raised grid of longitudinal quasi-parallel ridges (lamellae) constituting the upper surface of the scale run along its complete length with a spacing of about 2.5 m. Fine tubes forming a net-like latticework (reticulum) cover the spacing between two adjacent lamellae. These lamellae and reticulum, forming arrays of repeated structures, work together to provide the observed characteristic colors of butterfly wings.
To fabricate butterfly wing-like structures in the lab, several techniques have been reported in the literature. Conformal evaporated film by rotation (CEFR) is one such specialized physical vapor deposition method [
73]. It involves thermal evaporation of chalcogenide glasses constituted of materials like GeSbSe, to form collimated vapor flux in a low pressure chamber, which provides a covering over a biotemplate. The biotemplate is rotated in a complex manner such that vapor flux material forms a uniform coating on its surface. Glass composition of GeSbSe is selected due to its excellent optical and mechanical properties. Another method used for fabricating artificial butterfly wings is a three-step dipping process [
74]. In doing this, wings undergo an initial pre-treatment process, after which they are dipped in a closed vessel-containing precursor solution of zinc oxide. They are then taken out, dried and further treated at high temperature to remove the wing template and obtain a ceramic surface morphology mimicking the butterfly wings.
Other methods used to fabricate artificial butterfly wings include deposition of silicate minerals in the inner spaces of wing scales by chemical vapor deposition to form a thick coating of about 100–150 nm on its surface, to obtain silicified butterfly wings [
75]. The sol–gel technique to fabricate highly ordered structures of lead lanthanum zirconate titanate (PLZT) mimicking butterfly wings uses dip coating [
76]. Titanium dioxide-, tin dioxide (SnO
2)- and silicon dioxide (SiO
2)-based wing morphology uses the sonochemical method followed by a calcination process [
77]. Butterfly wings react readily to external stimuli, like temperature and humidity. Needless to say, biomimetic structures inspired by butterfly wings have recently found application in a number of sensors, like temperature and infrared sensors, vapor and solvent sensors and pH sensors [
78].
3.7. Frog Toe Pads
The adhesive systems used by various animals have intrigued researchers to investigate the dynamic mechanisms of adhesion. Studies show that the reason adhesive forces occur is due to a bond formed between the animal and the surface, which opposes the gravitational pull of the Earth [
79]. In
Section 3.2, we already saw the dual characteristic of superhydrophobicity and the high adhesivity of gecko feet towards wet surfaces. However, other structural adaptations need to be studied as adhesion in animals can occur in multiple ways. In fact, there are four different adhesion mechanisms that we can observe in animals, namely gluing, suction, wet adhesion and dry adhesion [
80]. Echinoderms (sea urchins and starfish) use gluing for attachment and locomotion, whereas suction is used by disk-winged bats by reducing internal pressure. In dry adhesion, the formation of intermolecular/van der Waals forces between the animal and the surface leads to adhesion. As a result, geckos have the ability to climb smooth vertical surfaces and run on ceilings. Wet adhesion observed in amphibians, ants and grasshoppers results from the combined forces of surface tension and viscosity produced by the fluid layer between the pad and the substrate.
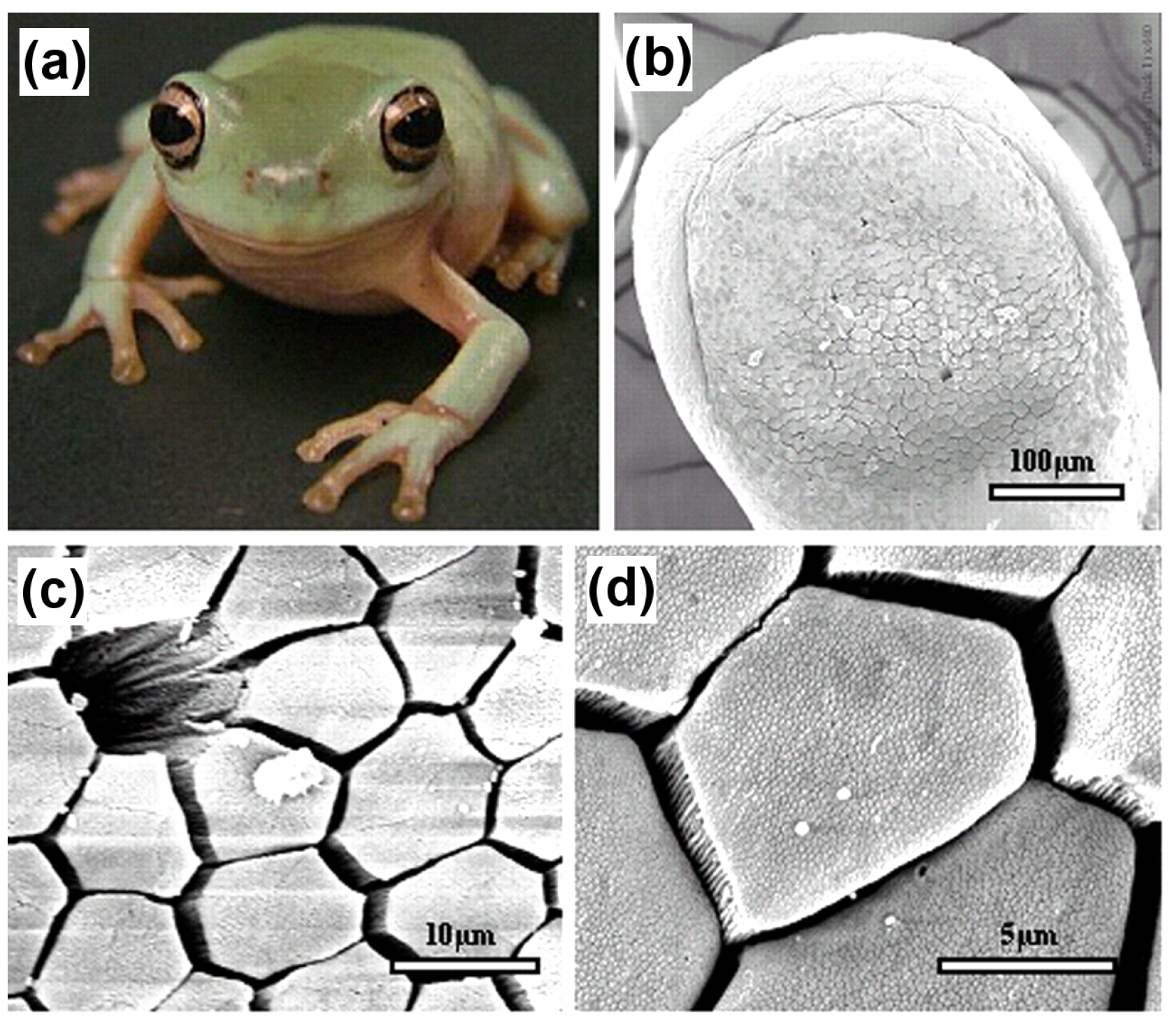
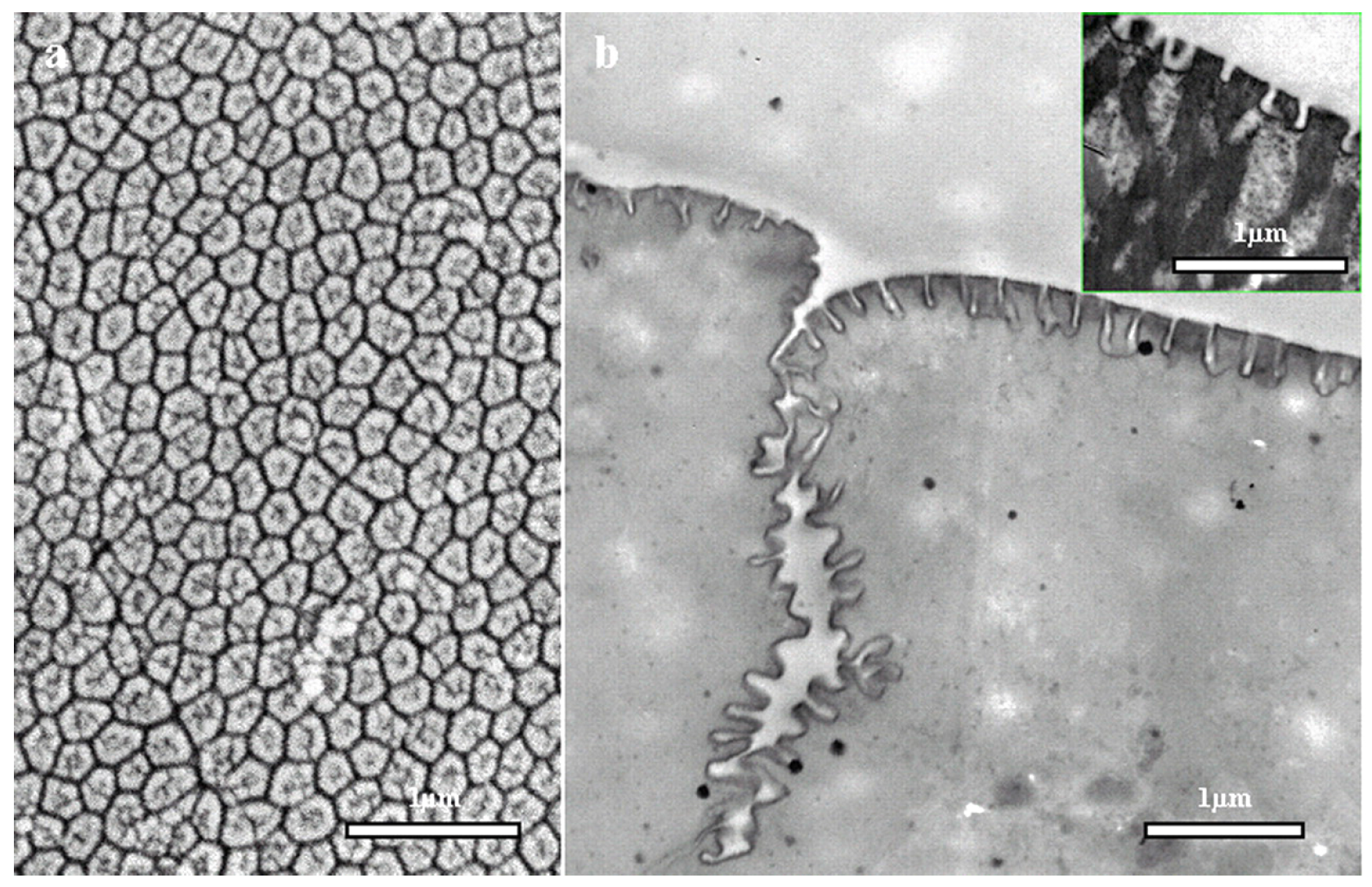
In amphibians, adhesive toe pads can be found in seven families of frogs, including torrent or tree frogs and arboreal salamanders. The toe pads are comprised of columnar epithelial cells with regular hexagonal microstructures of about 10
m in diameter. These are separated from each other at their tips by 1
m-wide channels [
81].
Figure 16 depicts the hexagonal microstructures and separation channels. The channels consist of mucous-secreating glands, forming a thin layer between the pad and the substrate. Earlier researchers presumed that mucous acts as glue with which the animal is able to stick itself to the substrate [
82]. After years of intensive research, it was evident that a visible meniscus lies at the area of contact between the pad and the substrate [
83], and the shear forces of toe pad responsible for adhesion were also found to be velocity dependent [
84]. Further, the sticking ability of frogs was observed to decrease in the case of wet toes [
85], but at the same time, fresh water frogs demonstrated capabilities to climb on wet rocks with water flowing over the substrate [
86].
With the recent development of microscopic techniques like SEM, transmission electron microscopy (TEM) and AFM, it has been found that the top of the toe pad epithelial cells is not flat, but comprised of a dense array of nanopillars, which are 300–400 nm both in diameter and height. These nanopillars come in direct contact with the substrate producing considerable shear force and are responsible for the adhesive characteristic of the frog toe pad via wet adhesion.
Figure 17 illustrates the nanopillars resting on top of hexagonal microstructures along with a higher magnification view of the separation channels.
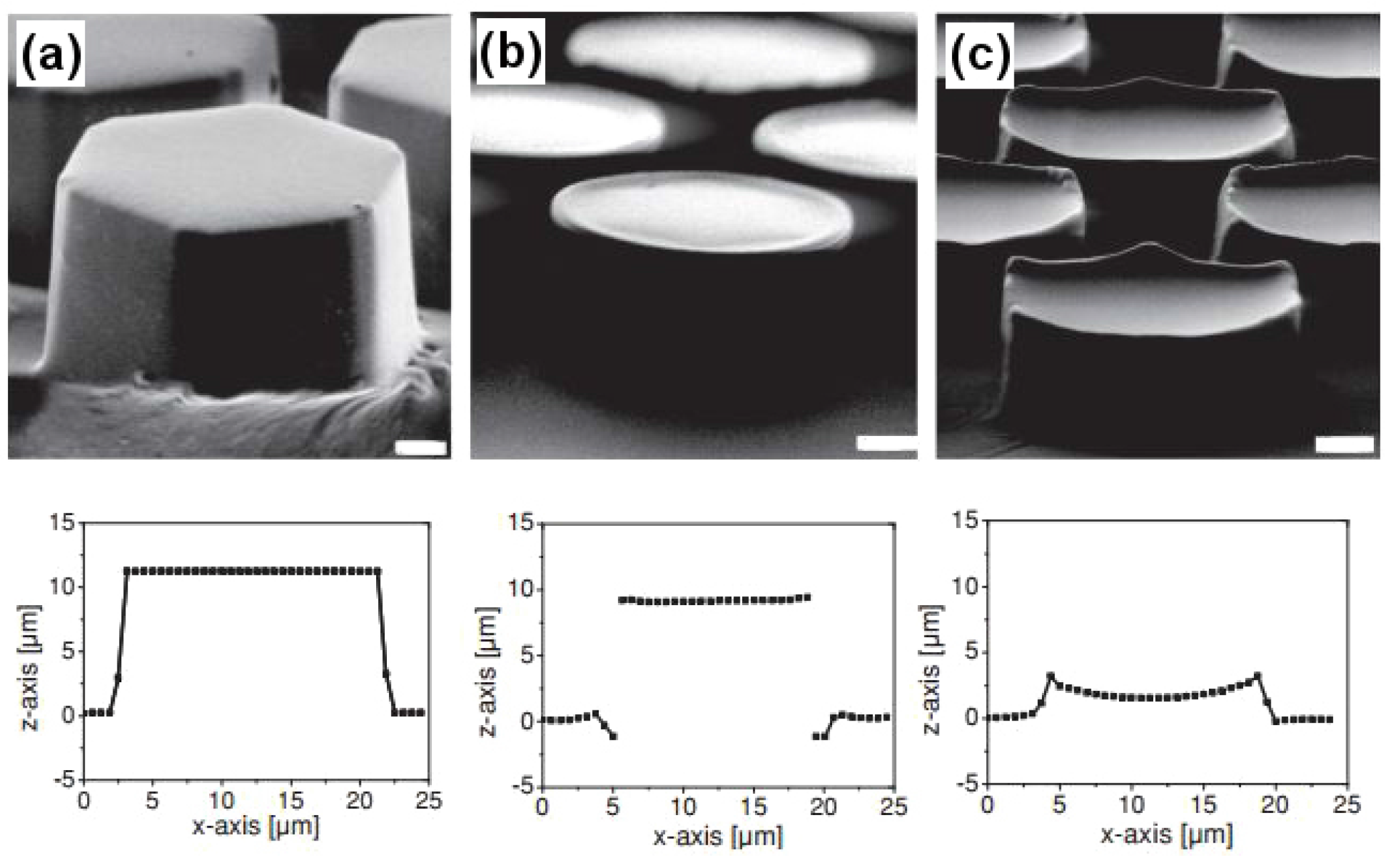
Not much has been done to mimic the frog toe pad structure, partly due to its complicated design, which needs to be studied further in order to be fabricated in the lab. However, in one study similar structures were fabricated having different microstructured surface designs using photolithography [
87]. Polydimethylsiloxane being a soft elastomer and with a Young’s modulus of 2 MPa lying in close proximity with the frog toe pad (∼5 MPa) was the preferred choice as the fabricating material [
81].
Figure 18 demonstrates the hexagonal micropillared arrays with flat, T-shaped and concave surface designs along with their dimensional profiles obtained using confocal microscopy. In more recent studies, the frog toe pad structure has been used to inspire the design of safety razors [
88] and surgical graspers [
89]. Polyvinyl siloxane (PVS) was the preferred material choice for designing the hexagonal toe pad-like surface texture of safety razors. The razors thus developed gave almost double sliding friction as against the presently available disposable safety razors when tested on lubricated human skin. In the case of the surgical grasper, hexagonal micropillared patterns fabricated using PDMS were compared to the jaw of currently sold surgical graspers. The biomimetic structure exhibited stronger and more stable friction on the surface of soft tissue while incurring minimal damage or deformation of tissue.