Effective Elastic Modulus of Structured Adhesives: From Biology to Biomimetics
Abstract
:1. Introduction
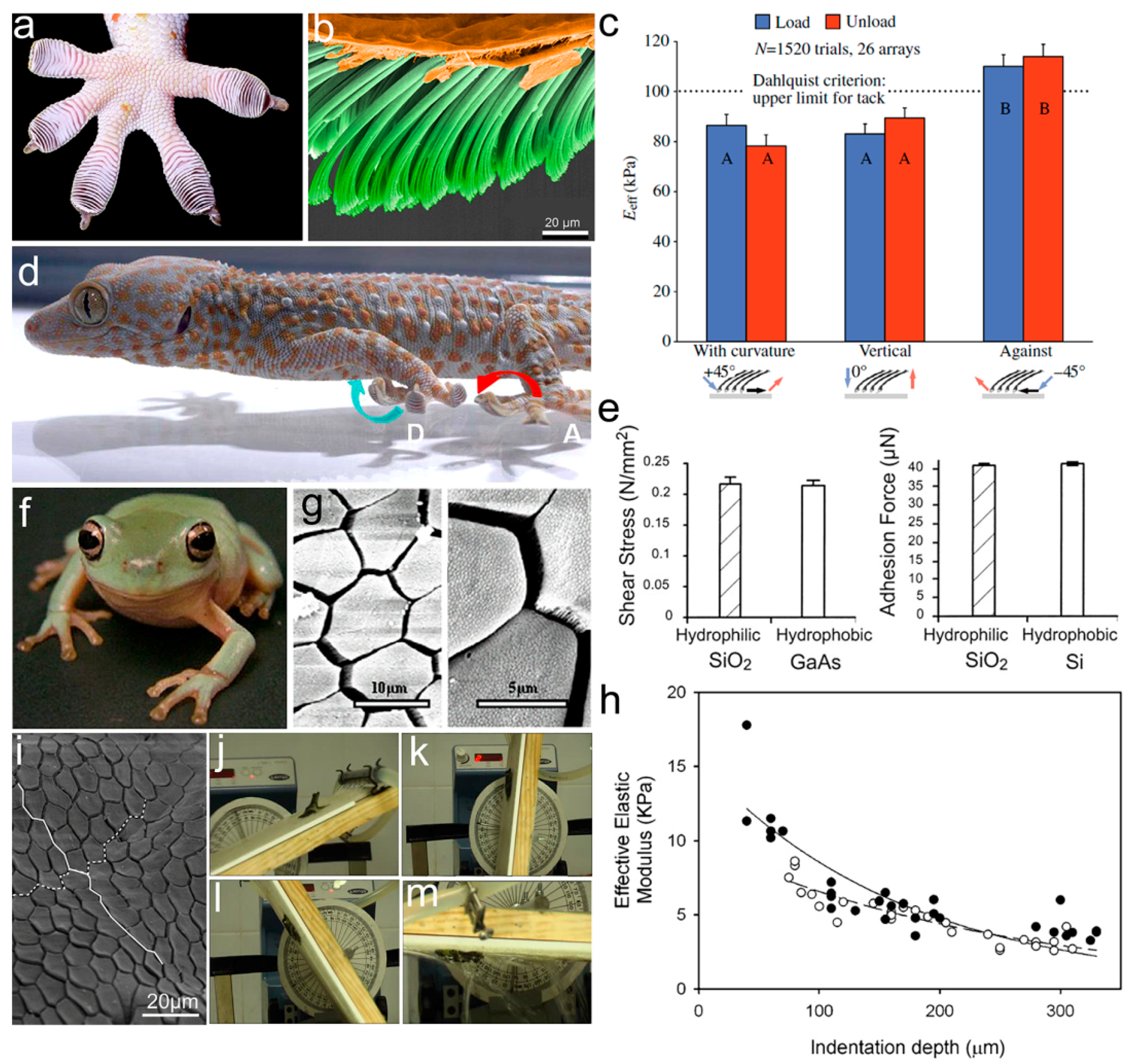
2. Naturally Occurring Hierarchical Structured Adhesives
3. Bioinspired Hierarchical Structured Adhesives
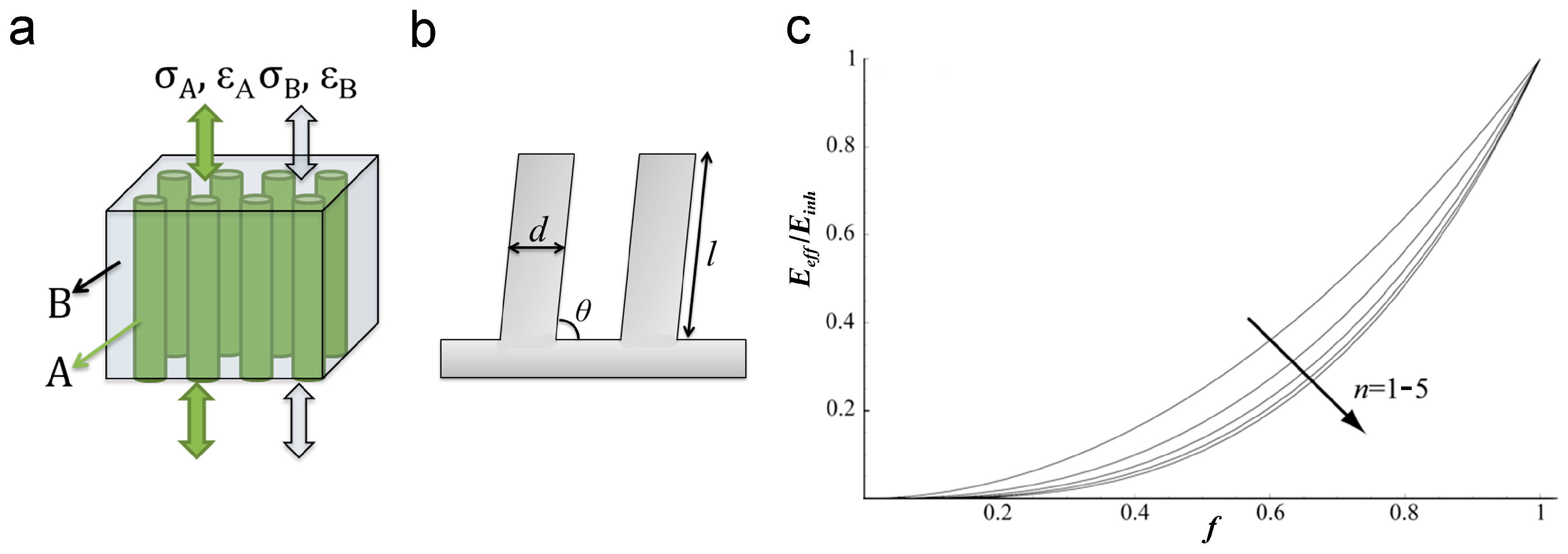
3.1. Self-Similar Pillar Structure
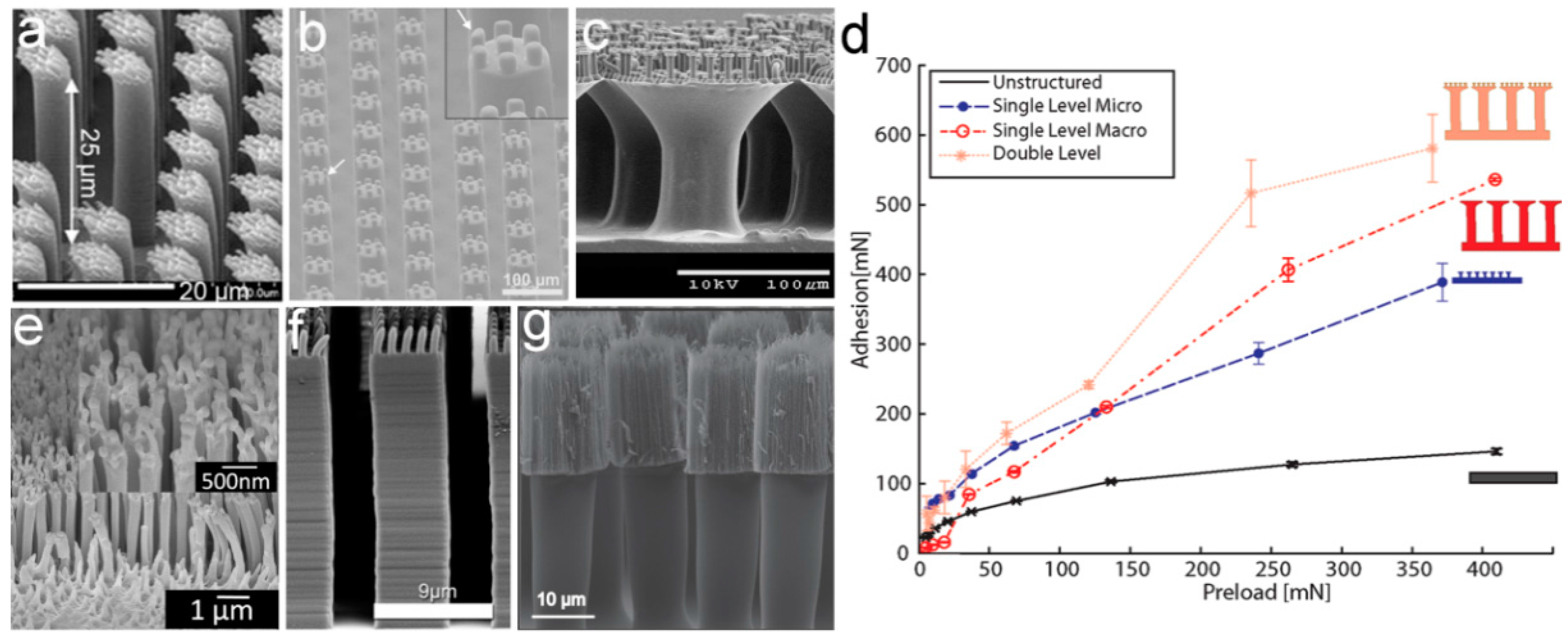
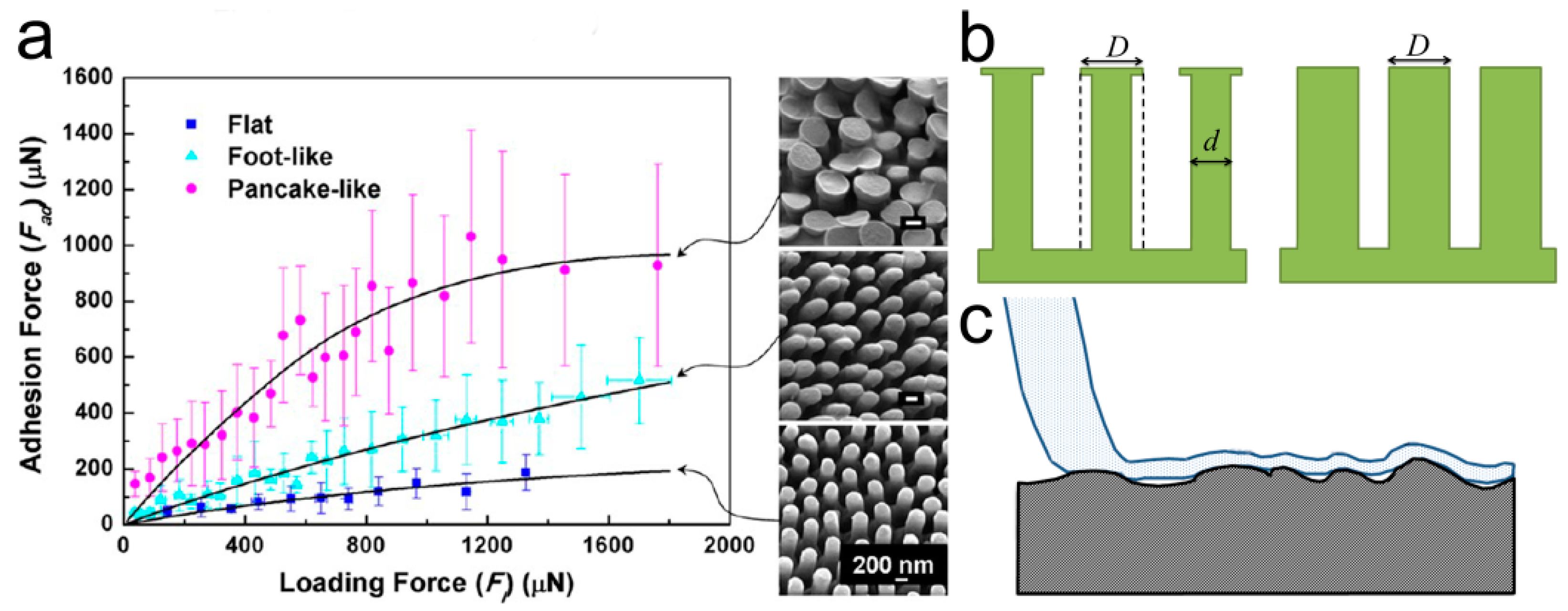
3.1.1. Self-Similar Hierarchical Pillar
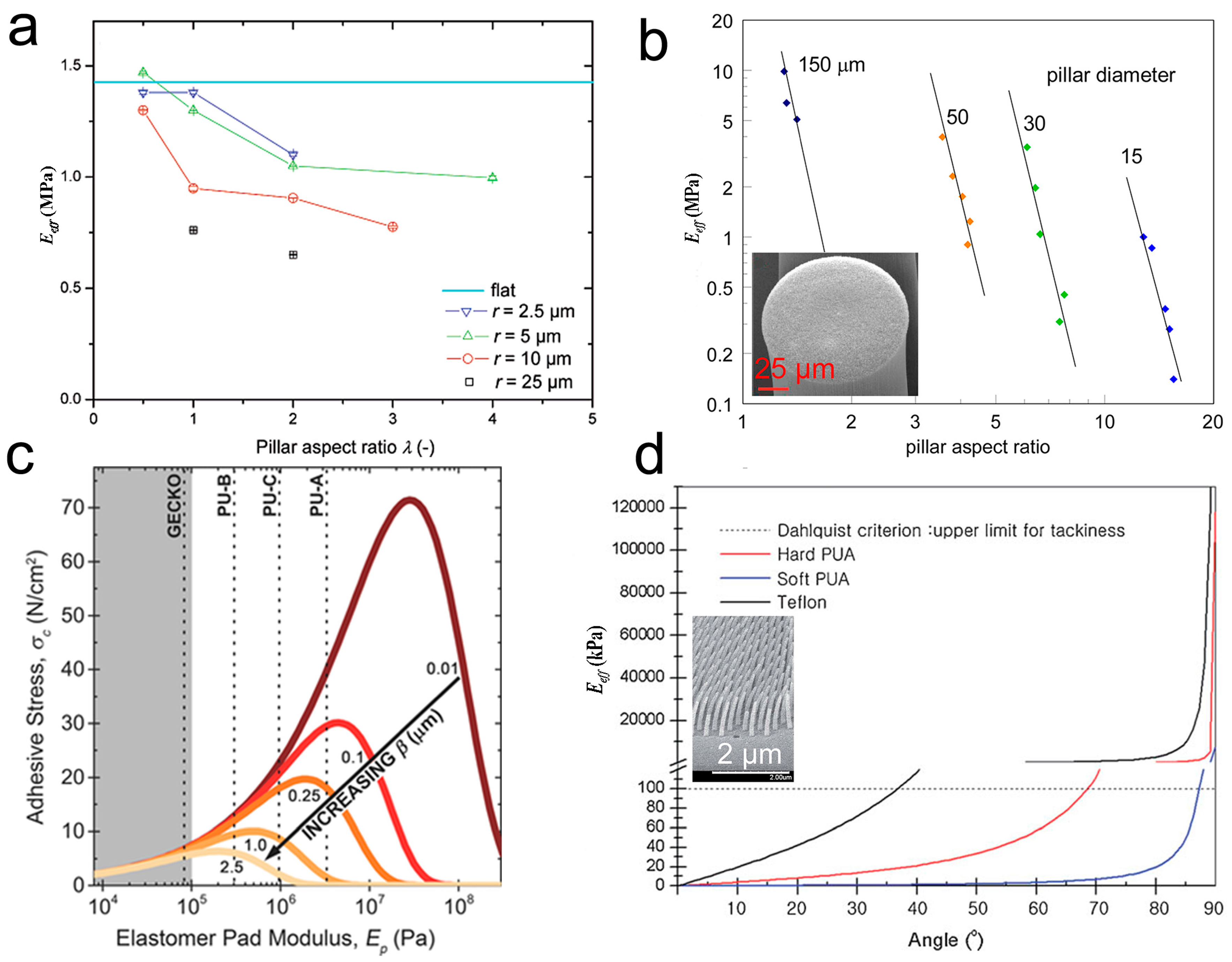
3.1.2. Structural Parameters of Pillar Arrays
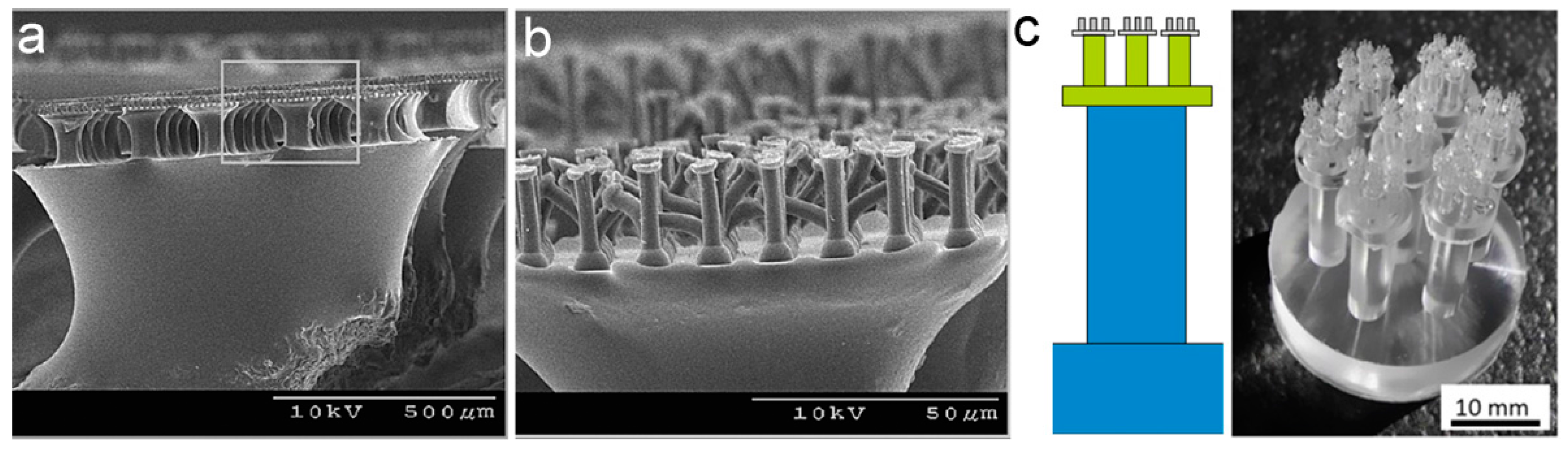
3.2. Lamella–Pillar Hybrid Structure
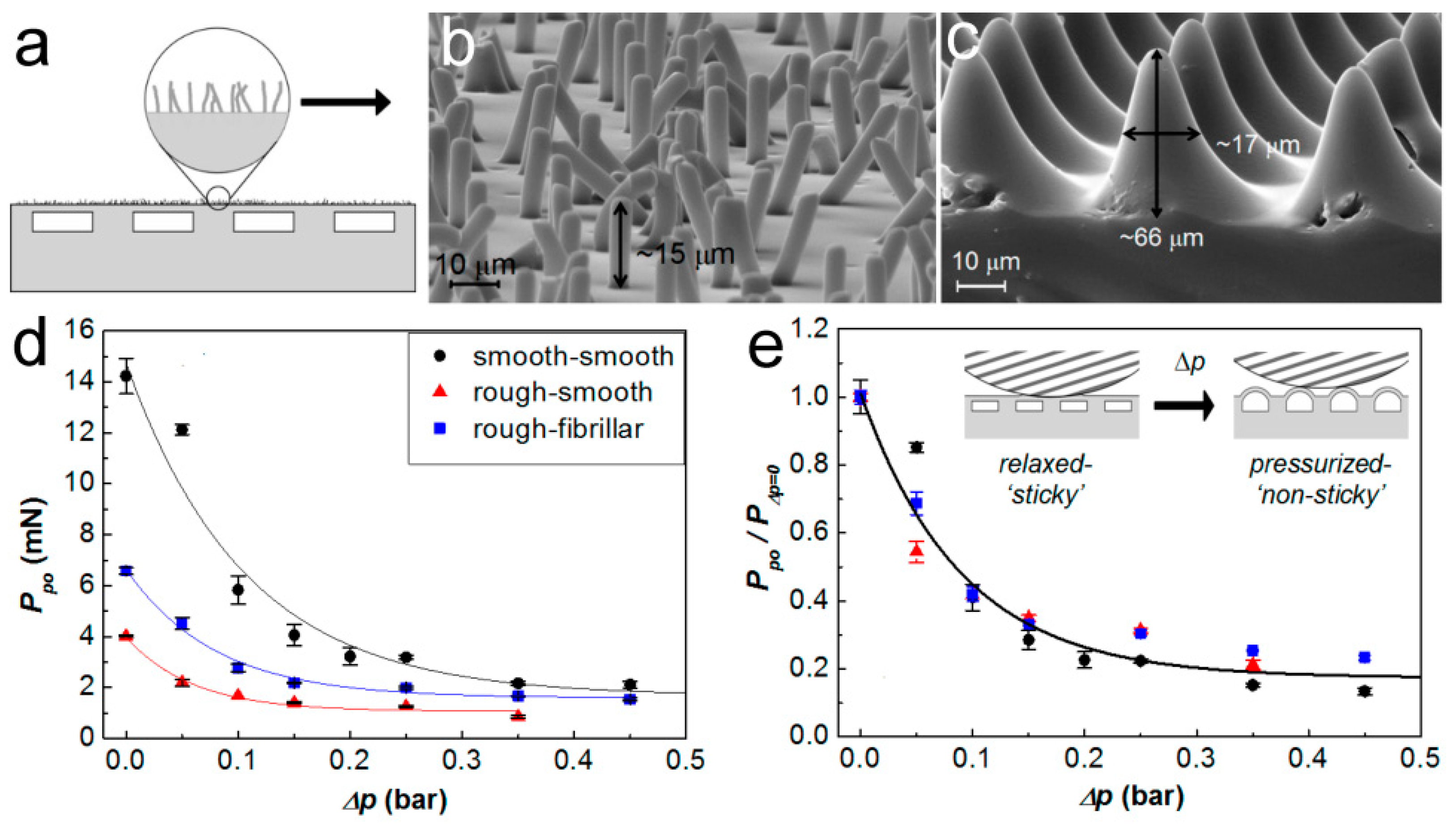
3.3. Porous Structure
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Boesel, L.F.; Greiner, C.; Arzt, E.; del Campo, A. Gecko-inspired surfaces: A path to strong and reversible dry adhesives. Adv. Mater. 2010, 22, 2125–2137. [Google Scholar] [CrossRef] [PubMed]
- Brodoceanu, D.; Bauer, C.T.; Kroner, E.; Arzt, E.; Kraus, T. Hierarchical bioinspired adhesive surfaces—A review. Bioinspir. Biomim. 2016, 11, 051001. [Google Scholar] [CrossRef] [PubMed]
- Labonte, D.; Clemente, C.J.; Dittrich, A.; Kuo, C.Y.; Crosby, A.J.; Irschick, D.J.; Federle, W. Extreme positive allometry of animal adhesive pads and the size limits of adhesion-based climbing. Proc. Natl. Acad. Sci. USA 2016, 113, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.W.; Kong, X.Q.; Wu, D. Micronanostructures of the scales on a mosquito’s legs and their role in weight support. Phys. Rev. E 2007, 76, 017301. [Google Scholar] [CrossRef] [PubMed]
- Hüsken, M.; Hufnagel, K.; Mende, K.; Appel, E.; Meyer, H.; Peisker, H.; Tögel, M.; Wang, S.; Wolff, J.; Gorb, S.N. Adhesive pad differentiation in Drosophila melanogaster depends on the Polycomb group gene Su(z)2. J. Exp. Biol. 2015, 218, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Heepe, L.; Petersen, D.S.; Tölle, L.; Wolff, J.O.; Gorb, S.N. Sexual dimorphism in the attachment ability of the ladybird beetle Coccinella septempunctata on soft substrates. Appl. Phys. A 2016, 123, 34. [Google Scholar] [CrossRef]
- Creton, C. Pressure-sensitive adhesives: An introductory course. MRS Bull. 2003, 28, 434–439. [Google Scholar] [CrossRef]
- Pocius, A.V. Adhesion and Adhesives Technology: An Introduction; Carl Hanser Verlag GmbH Co. KG: München, Germany, 2012. [Google Scholar]
- Dahlquist, C.A. Pressure-sensitive adhesives. Treatise Adhes. Adhes. 1969, 2, 219–260. [Google Scholar]
- Autumn, K.; Majidi, C.; Groff, R.E.; Dittmore, A.; Fearing, R. Effective elastic modulus of isolated gecko setal arrays. J. Exp. Biol. 2006, 209, 3558–3568. [Google Scholar] [CrossRef] [PubMed]
- Huber, G.; Orso, S.; Spolenak, R.; Wegst, U.G.K.; Enders, S.; Gorb, S.N.; Arzt, E. Mechanical properties of a single gecko seta. Int. J. Mater. Res. 2008, 99, 1113–1118. [Google Scholar] [CrossRef]
- Xue, L.; Steinhart, M.; Gorb, S.N. Biological and Bioinspired Micro-and Nanostructured Adhesives; Wiley-VCH: Weinheim, Germany, 2013; pp. 409–439. [Google Scholar]
- Autumn, K.; Niewiarowski, P.H.; Puthoff, J.B. Gecko adhesion as a model system for integrative biology, interdisciplinary science, and bioinspired engineering. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 445–470. [Google Scholar] [CrossRef]
- Autumn, K.; Sitti, M.; Liang, Y.A.; Peattie, A.M.; Hansen, W.R.; Sponberg, S.; Kenny, T.W.; Fearing, R.; Israelachvili, J.N.; Full, R.J. Evidence for van der Waals adhesion in gecko setae. Proc. Natl. Acad. Sci. USA 2002, 99, 12252–12256. [Google Scholar] [CrossRef] [PubMed]
- Autumn, K.; Dittmore, A.; Santos, D.; Spenko, M.; Cutkosky, M. Frictional adhesion: A new angle on gecko attachment. J. Exp. Biol. 2006, 209, 3569–3579. [Google Scholar] [CrossRef] [PubMed]
- Peisker, H.; Michels, J.; Gorb, S.N. Evidence for a material gradient in the adhesive tarsal setae of the ladybird beetle Coccinella septempunctata. Nat. Commun. 2013, 4, 1661. [Google Scholar] [CrossRef] [PubMed]
- Jagota, A. Mechanics of adhesion through a fibrillar microstructure. Integr. Comp. Biol. 2002, 42, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Hui, C.Y.; Glassmaker, N.J. Can a fibrillar interface be stronger and tougher than a non-fibrillar one? J. R. Soc. Interface 2005, 2, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Persson, B.N.J.; Gorb, S. The effect of surface roughness on the adhesion of elastic plates with application to biological systems. J. Chem. Phys. 2003, 119, 11437–11444. [Google Scholar] [CrossRef]
- Campolo, D.; Jones, S.; Fearing, R.S. Fabrication of gecko foot-hair like nano structures and adhesion to random rough surfaces. In Proceedings of the 2003 Third IEEE Conference on Nanotechnology 2003 (IEEE-NANO 2003), San Francisco, CA, USA, 12–14 August 2003; pp. 856–859. [Google Scholar]
- Persson, B.N.J. On the mechanism of adhesion in biological systems. J. Chem. Phys. 2003, 118, 7614–7621. [Google Scholar] [CrossRef]
- Schargott, M. A mechanical model of biomimetic adhesive pads with tilted and hierarchical structures. Bioinspir. Biomim. 2009, 4, 026002. [Google Scholar] [CrossRef] [PubMed]
- Schargott, M.; Popov, V.L.; Gorb, S. Spring model of biological attachment pads. J. Theor. Boil. 2006, 243, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Kovalev, A.; Thole, F.; Rengarajan, G.T.; Steinhart, M.; Gorb, S.N. Tailoring normal adhesion of arrays of thermoplastic, spring-like polymer nanorods by shaping nanorod tips. Langmuir 2012, 28, 10781–10788. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Peressadko, A.G.; Kim, T.-W. Adhesion analysis of two-level hierarchical morphology in natural attachment systems for ‘smart adhesion’. J. Adhes. Sci. Technol. 2006, 20, 1475–1491. [Google Scholar] [CrossRef]
- Kim, T.W.; Bhushan, B. Effect of stiffness of multi-level hierarchical attachment system on adhesion enhancement. Ultramicroscopy 2007, 107, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Bhushan, B. Adhesion analysis of multi-level hierarchical attachment system contacting with a rough surface. J. Adhes. Sci. Technol. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Autumn, K.; Liang, Y.A.; Hsieh, S.T.; Zesch, W.; Chan, W.P.; Kenny, T.W.; Fearing, R.; Full, R.J. Adhesive force of a single gecko foot-hair. Nature 2000, 405, 681–685. [Google Scholar] [PubMed]
- Arzt, E.; Gorb, S.; Spolenak, R. From micro to nano contacts in biological attachment devices. Proc. Natl. Acad. Sci. USA 2003, 100, 10603–10606. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, X.; Yao, H.; Gorb, S.; Arzt, E. Mechanics of hierarchical adhesion structures of geckos. Mech. Mater. 2005, 37, 275–285. [Google Scholar] [CrossRef]
- Xu, Q.; Wan, Y.; Hu, T.S.; Liu, T.X.; Tao, D.; Niewiarowski, P.H.; Tian, Y.; Liu, Y.; Dai, L.; Yang, Y.; et al. Robust self-cleaning and micromanipulation capabilities of gecko spatulae and their bio-mimics. Nat. Commun. 2015, 6, 8949. [Google Scholar] [CrossRef] [PubMed]
- Bullock, J.M.R.; Federle, W. The effect of surface roughness on claw and adhesive hair performance in the dock beetle Gastrophysa viridula. Insect Sci. 2011, 18, 298–304. [Google Scholar] [CrossRef]
- Gillies, A.G.; Henry, A.; Lin, H.; Ren, A.; Shiuan, K.; Fearing, R.S.; Full, R.J. Gecko toe and lamellar shear adhesion on macroscopic, engineered rough surfaces. J. Exp. Biol. 2014, 217, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Pugno, N.M.; Lepore, E. Observation of optimal gecko’s adhesion on nanorough surfaces. BioSystems 2008, 94, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Huber, G.; Gorb, S.N.; Hosoda, N.; Spolenak, R.; Arzt, E. Influence of surface roughness on gecko adhesion. Acta Biomater. 2007, 3, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Voigt, D.; Schuppert, J.M.; Dattinger, S.; Gorb, S.N. Sexual dimorphism in the attachment ability of the Colorado potato beetle Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) to rough substrates. J. Insect Physiol. 2008, 54, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.O.; Gorb, S.N. Surface roughness effects on attachment ability of the spider Philodromus dispar (Araneae, Philodromidae). J. Exp. Biol. 2012, 215, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Persson, B.N.J. Biological adhesion for locomotion on rough surfaces: Basic principles and a theorist’s view. MRS Bull. 2011, 32, 486–490. [Google Scholar] [CrossRef]
- Gillies, A.G.; Fearing, R.S. Simulation of synthetic gecko arrays shearing on rough surfaces. J. R. Soc. Interface 2014, 11, 20140021. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wan, J.; Pesika, N.; Zhou, M. Bridging nanocontacts to macroscale gecko adhesion by sliding soft lamellar skin supported setal array. Sci. Rep. 2013, 3, 1382. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Neuzil, P.; Kustandi, T.S.; Oh, S.; Samper, V.D. The nature of the gecko lizard adhesive force. Biophys. J. 2005, 89, L14–L17. [Google Scholar] [CrossRef] [PubMed]
- Niewiarowski, P.H.; Stephanie, L.; Ge, L.; Emily, H.; Ali, D. Sticky gecko feet: The role of temperature and humidity. PLoS ONE 2008, 3, e2192. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.Y.; Badge, I.; Wucinich, N.A.; Sullivan, T.W.; Niewiarowski, P.H.; Dhinojwala, A. Surface wettability plays a significant role in gecko adhesion underwater. Proc. Natl. Acad. Sci. USA 2013, 110, 6340–6345. [Google Scholar] [CrossRef] [PubMed]
- Puthoff, J.B.; Prowse, M.S.; Wilkinson, M.; Autumn, K. Changes in materials properties explain the effects of humidity on gecko adhesion. J. Exp. Biol. 2010, 213, 3699–3704. [Google Scholar] [CrossRef] [PubMed]
- Kovalev, A.E.; Gorb, S.N. Charge contribution to the adhesion performance of polymeric microstructures. Tribol. Lett. 2012, 48, 103–109. [Google Scholar] [CrossRef]
- Heepe, L.; Kovalev, A.E.; Filippov, A.E.; Gorb, S.N. Adhesion failure at 180,000 frames per second: Direct observation of the detachment process of a mushroom-shaped adhesive. Phys. Rev. Lett. 2013, 111, 104301. [Google Scholar] [CrossRef] [PubMed]
- Heepe, L.; Kovalev, A.E.; Gorb, S.N. Direct observation of microcavitation in underwater adhesion of mushroom-shaped adhesive microstructure. Beilstein J. Nanotechnol. 2014, 5, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Gorb, S.; Jiao, Y.; Scherge, M. Ultrastructural architecture and mechanical properties of attachment pads in Tettigonia viridissima (Orthoptera Tettigoniidae). J. Comp. Physiol. A 2000, 186, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Dirks, J.H.; Li, M.; Kabla, A.; Federle, W. In vivo dynamics of the internal fibrous structure in smooth adhesive pads of insects. Acta Biomater. 2012, 8, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Varenberg, M.; Gorb, S.N. Hexagonal surface micropattern for dry and wet friction. Adv. Mater. 2009, 21, 483–486. [Google Scholar] [CrossRef]
- Smith, J.M.; Barnes, W.J.; Downie, J.R.; Ruxton, G.D. Structural correlates of increased adhesive efficiency with adult size in the toe pads of hylid tree frogs. J. Comp. Physiol. A 2006, 192, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Barnes, W.J.P. Functional morphology and design constraints of smooth adhesive pads. MRS Bull. 2011, 32, 479–485. [Google Scholar] [CrossRef]
- Iturri, J.; Xue, L.; Kappl, M.; García-Fernández, L.; Barnes, W.J.P.; Butt, H.-J.; del Campo, A. Torrent frog-inspired adhesives: Attachment to flooded surfaces. Adv. Funct. Mater. 2015, 25, 1499–1505. [Google Scholar] [CrossRef]
- Drotlef, D.M.; Appel, E.; Peisker, H.; Dening, K.; Del Campo, A.; Gorb, S.N.; Barnes, W.J. Morphological studies of the toe pads of the rock frog, Staurois parvus (family: Ranidae) and their relevance to the development of new biomimetically inspired reversible adhesives. Interface Focus 2015, 5, 20140036. [Google Scholar] [CrossRef] [PubMed]
- Scholz, I.; Barnes, W.J.; Smith, J.M.; Baumgartner, W. Ultrastructure and physical properties of an adhesive surface, the toe pad epithelium of the tree frog, Litoria caerulea White. J. Exp. Biol. 2009, 212, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Crawford, N.; Endlein, T.; Pham, J.T.; Riehle, M.; Barnes, W.J. When the going gets rough—Studying the effect of surface roughness on the adhesive abilities of tree frogs. Beilstein J. Nanotechnol. 2016, 7, 2116–2131. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, L.; Zhang, D.; Zhang, P.; Han, Z. Bioinspired surface for surgical graspers based on the strong wet friction of tree frog toe pads. ACS Appl. Mater. Interfaces 2015, 7, 13987–13995. [Google Scholar] [CrossRef] [PubMed]
- Tsipenyuk, A.; Varenberg, M. Use of biomimetic hexagonal surface texture in friction against lubricated skin. J. R. Soc. Interface 2014, 11, 20140113. [Google Scholar] [CrossRef] [PubMed]
- Barnes, W.J.; Baum, M.; Peisker, H.; Gorb, S.N. Comparative cryo-SEM and AFM studies of hylid and rhacophorid tree frog toe pads. J. Morphol. 2013, 274, 1384–1396. [Google Scholar] [CrossRef] [PubMed]
- Barnes, W.J.; Goodwyn, P.J.; Nokhbatolfoghahai, M.; Gorb, S.N. Elastic modulus of tree frog adhesive toe pads. J. Comp. Physiol. A 2011, 197, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Barnes, W.J.; Oines, C.; Smith, J.M. Whole animal measurements of shear and adhesive forces in adult tree frogs: Insights into underlying mechanisms of adhesion obtained from studying the effects of size and scale. J. Comp. Physiol. A Neuroethol. 2006, 192, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Emerson, S.B.; Diehl, D. Toe pad morphology and mechanisms of sticking in frogs. Biol. J. Linnean Soc. 1980, 13, 199–216. [Google Scholar] [CrossRef]
- Hanna, G.; Jon, W.; Barnes, W.P.J. Adhesion and detachment of the toe pads of tree frogs. J. Exp. Biol. 1991, 155, 103–125. [Google Scholar]
- Federle, W.; Barnes, W.; Baumgartner, W.; Drechsler, P.; Smith, J. Wet but not slippery: Boundary friction in tree frog adhesive toe pads. J. R. Soc. Interface 2006, 3, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Barnes, W.J.P.; Samuel, D.S.; Crawford, N.A.; Bee, B.A.; Ulmar, G. Sticking under wet conditions: The remarkable attachment abilities of the torrent frog, Staurois guttatus. PLoS ONE 2013, 8, e73810. [Google Scholar]
- Ohler, A. Digital pad morphology in torrent-living ranid frogs. Asiat. Herpetol. Res. 1995, 6, 85–96. [Google Scholar]
- Geim, A.K.; Dubonos, S.V.; Grigorieva, I.V.; Novoselov, K.S.; Zhukov, A.A.; Shapoval, S.Y. Microfabricated adhesive mimicking gecko foot-hair. Nat. Mater. 2003, 2, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Drotlef, D.-M.; Stepien, L.; Kappl, M.; Barnes, W.J.P.; Butt, H.-J.; del Campo, A. Insights into the adhesive mechanisms of tree frogs using artificial mimics. Adv. Funct. Mater. 2013, 23, 1137–1146. [Google Scholar] [CrossRef]
- Frensemeier, M.; Kaiser, J.S.; Frick, C.P.; Schneider, A.S.; Arzt, E.; Fertig, R.S.; Kroner, E. Temperature-induced switchable adhesion using nickel-titanium-polydimethylsiloxane hybrid surfaces. Adv. Funct. Mater. 2015, 25, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Purtov, J.; Frensemeier, M.; Kroner, E. Switchable adhesion in vacuum using bio-inspired dry adhesives. ACS Appl. Mater. Interfaces 2015, 7, 24127–24135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, H.; Shao, J.; Sameoto, D.; Li, X.; Wang, L.; Hu, H.; Ding, Y.; Lu, B. Switchable dry adhesion with step-like micropillars and controllable interfacial contact. ACS Appl. Mater. Interfaces 2016, 8, 10029–10037. [Google Scholar] [CrossRef] [PubMed]
- Frost, S.J.; Mawad, D.; Higgins, M.J.; Ruprai, H.; Kuchel, R.; Tilley, R.D.; Myers, S.; Hook, J.M.; Lauto, A. Gecko-inspired chitosan adhesive for tissue repair. NPG Asia Mater. 2016, 8, e280. [Google Scholar] [CrossRef]
- Shahsavan, H.; Salili, S.M.; Jakli, A.; Zhao, B. Thermally active liquid crystal network gripper mimicking the self-peeling of gecko toe pads. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Roca-Cusachs, P.; Rico, F.; Martínez, E.; Toset, J.; Farré, R.; Navajas, D. Stability of microfabricated high aspect ratio structures in poly(dimethylsiloxane). Langmuir 2005, 21, 5542–5548. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.Y.; Jagota, A.; Lin, Y.Y.; Kramer, E.J. Constraints on microcontact printing imposed by stamp deformation. Langmuir 2002, 18, 1394–1407. [Google Scholar] [CrossRef]
- Spolenak, R.; Gorb, S.; Arzt, E. Adhesion design maps for bio-inspired attachment systems. Acta Biomater. 2005, 1, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Goldberg Oppenheimer, P.; Shean, T.A.V.; Wirth, C.T.; Hofmann, S.; Robertson, J. Adhesive properties of gecko-inspired mimetic via micropatterned carbon nanotube forests. J. Phys. Chem. C 2012, 116, 20047–20053. [Google Scholar] [CrossRef]
- Li, Y.; Gates, B.D.; Menon, C. Improved adhesion and compliancy of hierarchical fibrillar adhesives. ACS Appl. Mater. Interfaces 2015, 7, 16410–16417. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.E.; Lee, J.K.; Kim, H.N.; Moon, S.H.; Suh, K.Y. A nontransferring dry adhesive with hierarchical polymer nanohairs. Proc. Natl. Acad. Sci. USA 2009, 106, 5639–5644. [Google Scholar] [CrossRef] [PubMed]
- Greiner, C.; Arzt, E.; del Campo, A. Hierarchical gecko-like adhesives. Adv. Mater. 2009, 21, 479–482. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, L.; Jia, S.; Guo, D.; Dai, Z. Fabrication and adhesion of hierarchical micro-seta. Chin. Sci. Bull. 2012, 57, 1343–1349. [Google Scholar] [CrossRef]
- Murphy, M.P.; Kim, S.; Sitti, M. Enhanced adhesion by gecko-inspired hierarchical fibrillar adhesives. ACS Appl. Mater. Interfaces 2009, 1, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Kustandi, T.S.; Samper, V.D.; Ng, W.S.; Chong, A.S.; Gao, H. Fabrication of a gecko-like hierarchical fibril array using a bonded porous alumina template. J. Micromech. Microeng. 2007, 17, N75–N81. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, D.H.; Lee, S.G.; Cho, K. Hierarchical gecko-inspired nanohairs with a high aspect ratio induced by nanoyielding. Soft Matter 2012, 8, 4905–4910. [Google Scholar] [CrossRef]
- Jo, H.; Haberkorn, N.; Pan, J.A.; Vakili, M.; Nielsch, K.; Theato, P. Fabrication of chemically tunable, hierarchically branched polymeric nanostructures by multi-branched anodic aluminum oxide templates. Langmuir 2016, 32, 6437–6444. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.Y.Y.; Yeo, L.P.; Lam, Y.C.; Rodríguez, I. Fabrication and analysis of gecko-inspired hierarchical polymer nanosetae. ACS Nano 2011, 5, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Izadi, H.; Golmakani, M.; Penlidis, A. Enhanced adhesion and friction by electrostatic interactions of double-level Teflon nanopillars. Soft Matter 2013, 9, 1985–1996. [Google Scholar] [CrossRef]
- Zhang, E.; Liu, Y.; Yu, J.; Lv, T.; Li, L. Fabrication of hierarchical gecko-inspired microarrays using a three-dimensional porous nickel oxide template. J. Mater. Chem. B 2015, 3, 6571–6575. [Google Scholar] [CrossRef]
- Rohrig, M.; Thiel, M.; Worgull, M.; Holscher, H. 3D direct laser writing of nano- and microstructured hierarchical gecko-mimicking surfaces. Small 2012, 8, 3009–3015. [Google Scholar] [CrossRef] [PubMed]
- Rong, Z.; Zhou, Y.; Chen, B.; Robertson, J.; Federle, W.; Hofmann, S.; Steiner, U.; Goldberg-Oppenheimer, P. Bio-inspired hierarchical polymer fiber-carbon nanotube adhesives. Adv. Mater. 2014, 26, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Lee, H. Fabrication and characterization of multi-level hierarchical surfaces. Faraday Discuss. 2012, 156, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Bhushan, B. Fabrication and characterization of hierarchical nanostructured smart adhesion surfaces. J. Colloid Interface Sci. 2012, 372, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.T.; Kroner, E.; Fleck, N.A.; Arzt, E. Hierarchical macroscopic fibrillar adhesives: In situ study of buckling and adhesion mechanisms on wavy substrates. Bioinspir. Biomim. 2015, 10, 066002. [Google Scholar] [CrossRef] [PubMed]
- Greiner, C.; del Campo, A.; Arzt, E. Adhesion of bioinspired micropatterned surfaces: Effects of pillar radius, aspect ratio, and preload. Langmuir 2007, 23, 3495–3502. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Dai, L. Gecko-foot-mimetic aligned single-walled carbon nanotube dry adhesives with unique electrical and thermal properties. Adv. Mater. 2007, 19, 3844–3849. [Google Scholar] [CrossRef]
- Qu, L.; Dai, L.; Stone, M.; Xia, Z.; Wang, Z.L. Carbon nanotube arrays with strong shear binding-on and easy normal lifting-off. Science 2008, 322, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Barreau, V.; Hensel, R.; Guimard, N.K.; Ghatak, A.; McMeeking, R.M.; Arzt, E. Fibrillar elastomeric micropatterns create tunable adhesion even to rough surfaces. Adv. Funct. Mater. 2016, 26, 4687–4694. [Google Scholar] [CrossRef]
- Canas, N.; Kamperman, M.; Volker, B.; Kroner, E.; McMeeking, R.M.; Arzt, E. Effect of nano- and micro-roughness on adhesion of bioinspired micropatterned surfaces. Acta Biomater. 2012, 8, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.; Lim, C.T.; Natarajan, S.; Ho, A.Y.Y.; Van, E.L.; Elmouelhi, N.; Low, H.Y.; Vyakarnam, M.; Cooper, K. Shear adhesion strength of gecko-inspired tapes on surfaces with variable roughness. J. Adhes. 2013, 89, 921–936. [Google Scholar] [CrossRef]
- Yu, J.; Chary, S.; Das, S.; Tamelier, J.; Turner, K.L.; Israelachvili, J.N. Friction and adhesion of gecko-inspired PDMS flaps on rough surfaces. Langmuir 2012, 28, 11527–11534. [Google Scholar] [CrossRef] [PubMed]
- King, D.R.; Bartlett, M.D.; Gilman, C.A.; Irschick, D.J.; Crosby, A.J. Creating gecko-like adhesives for “real world” surfaces. Adv. Mater. 2014, 26, 4345–4351. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-I.; Jeong, H.E.; Suh, K.Y.; Lee, H.H. Stooped nanohairs: Geometry-controllable, unidirectional, reversible, and robust gecko-like dry adhesive. Adv. Mater. 2009, 21, 2276–2281. [Google Scholar] [CrossRef]
- Yu, J.; Chary, S.; Das, S.; Tamelier, J.; Pesika, N.S.; Turner, K.L.; Israelachvili, J.N. Gecko-inspired dry adhesive for robotic applications. Adv. Funct. Mater. 2011, 21, 3010–3018. [Google Scholar] [CrossRef]
- Aksak, B.; Murphy, M.P.; Sitti, M. Adhesion of biologically inspired vertical and angled polymer microfiber arrays. Langmuir 2007, 23, 3322–3332. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Jeong, H.E.; Kim, T.-I.; Kang, T.J.; Tahk, D.; Char, K.; Suh, K.Y. Adhesion hysteresis of Janus nanopillars fabricated by nanomolding and oblique metal deposition. Nano Today 2009, 4, 385–392. [Google Scholar] [CrossRef]
- Del Campo, A.; Greiner, C.; Álvarez, I.; Arzt, E. Patterned surfaces with pillars with controlled 3D tip geometry mimicking bioattachment devices. Adv. Mater. 2007, 19, 1973–1977. [Google Scholar] [CrossRef]
- Hossfeld, C.K.; Schneider, A.S.; Arzt, E.; Frick, C.P. Detachment behavior of mushroom-shaped fibrillar adhesive surfaces in peel testing. Langmuir 2013, 29, 15394–15404. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, A.; Greiner, C.; Arzt, E. Contact shape controls adhesion of bioinspired fibrillar surfaces. Langmuir 2007, 23, 10235–10243. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.Y.; Glassmaker, N.J.; Tang, T.; Jagota, A. Design of biomimetic fibrillar interfaces: 2. Mechanics of enhanced adhesion. J. R. Soc. Interface 2004, 1, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Carbone, G.; Pierro, E.; Gorb, S.N. Origin of the superior adhesive performance of mushroom-shaped microstructured surfaces. Soft Matter 2011, 7, 5545–5552. [Google Scholar] [CrossRef]
- Xue, L.; Iturri, J.; Kappl, M.; Butt, H.J.; del Campo, A. Bioinspired orientation-dependent friction. Langmuir 2014, 30, 11175–11182. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Lee, J.; Kim, K.S.; Ko, K.H.; Lee, J.H.; Lee, J. Anisotropic adhesion of micropillars with spatula pads. ACS Appl. Mater. Interfaces 2014, 6, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Tamelier, J.; Chary, S.; Turner, K.L. Vertical anisotropic microfibers for a gecko-inspired adhesive. Langmuir 2012, 28, 8746–8752. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.S.; Kim, T.W. Simulation of the attachment system with various tip shapes contacting rough surface. J. Adhes. Sci. Technol. 2013, 27, 1755–1766. [Google Scholar] [CrossRef]
- Lee, J.; Bush, B.; Maboudian, R.; Fearing, R.S. Gecko-inspired combined lamellar and nanofibrillar array for adhesion on nonplanar surface. Langmuir 2009, 25, 12449–12453. [Google Scholar] [CrossRef] [PubMed]
- Northen, M.T.; Greiner, C.; Arzt, E.; Turner, K.L. A gecko-inspired reversible adhesive. Adv. Mater. 2008, 20, 3905–3909. [Google Scholar] [CrossRef]
- Glass, P.; Chung, H.; Washburn, N.R.; Sitti, M. Enhanced reversible adhesion of dopamine methacrylamide-coated elastomer microfibrillar structures under wet conditions. Langmuir 2009, 25, 6607–6612. [Google Scholar] [CrossRef] [PubMed]
- Nadermann, N.; Ning, J.; Jagota, A.; Hui, C.Y. Active switching of adhesion in a film-terminated fibrillar structure. Langmuir 2010, 26, 15464–15471. [Google Scholar] [CrossRef] [PubMed]
- Glassmaker, N.J.; Jagota, A.; Hui, C.Y.; Noderer, W.L.; Chaudhury, M.K. Biologically inspired crack trapping for enhanced adhesion. Proc. Natl. Acad. Sci. USA 2007, 104, 10786–10791. [Google Scholar] [CrossRef] [PubMed]
- Asbeck, A.; Dastoor, S.; Parness, A.; Fullerton, L.; Esparza, N.; Soto, D.; Heyneman, B.; Cutkosky, M. Climbing rough vertical surfaces with hierarchical directional adhesion. In Proceedings of the IEEE International Conference on Robotics and Automation, 2009 (ICRA 2009), Kobe, Japan, 12–17 May 2009; pp. 2675–2680. [Google Scholar]
- Northen, M.T.; Turner, K.L. A batch fabricated biomimetic dry adhesive. Nanotechnology 2005, 16, 1159–1166. [Google Scholar] [CrossRef]
- Wang, J.C. Young’s modulus of porous materials. J. Mater. Sci. 1984, 19, 809–814. [Google Scholar] [CrossRef]
- Xue, L.; Kovalev, A.; Dening, K.; Eichler-Volf, A.; Eickmeier, H.; Haase, M.; Enke, D.; Steinhart, M.; Gorb, S.N. Reversible adhesion switching of porous fibrillar adhesive pads by humidity. Nano Lett. 2013, 13, 5541–5548. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Kovalev, A.; Eichler-Volf, A.; Steinhart, M.; Gorb, S.N. Humidity-enhanced wet adhesion on insect-inspired fibrillar adhesive pads. Nat. Commun. 2015, 6, 6621. [Google Scholar] [CrossRef] [PubMed]
- Greiner, C.; Buhl, S.; del Campo, A.; Arzt, E. Experimental parameters controlling adhesion of biomimetic fibrillar surfaces. J. Adhes. 2009, 85, 646–661. [Google Scholar] [CrossRef]
- Pham, J.T.; Xue, L.; Del Campo, A.; Salierno, M. Guiding cell migration with microscale stiffness patterns and undulated surfaces. Acta Biomater. 2016, 38, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Ghatak, A.; Sharma, A. Microfluidic adhesion induced by subsurface microstructures. Science 2007, 318, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Prieto-López, L.O.; Williams, J.A. Using microfluidics to control soft adhesion. J. Adhes. Sci. Technol. 2016, 30, 1555–1573. [Google Scholar] [CrossRef]
- Prieto-López, L.; Williams, J. Switchable adhesion surfaces with enhanced performance against rough counterfaces. Biomimetics 2016, 1, 2. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Tan, D.; Zhang, X.; Lei, Y.; Xue, L. Effective Elastic Modulus of Structured Adhesives: From Biology to Biomimetics. Biomimetics 2017, 2, 10. https://doi.org/10.3390/biomimetics2030010
Wang X, Tan D, Zhang X, Lei Y, Xue L. Effective Elastic Modulus of Structured Adhesives: From Biology to Biomimetics. Biomimetics. 2017; 2(3):10. https://doi.org/10.3390/biomimetics2030010
Chicago/Turabian StyleWang, Xin, Di Tan, Xinyu Zhang, Yifeng Lei, and Longjian Xue. 2017. "Effective Elastic Modulus of Structured Adhesives: From Biology to Biomimetics" Biomimetics 2, no. 3: 10. https://doi.org/10.3390/biomimetics2030010





