Biomimetic Cationic Nanoparticles Based on Silica: Optimizing Bilayer Deposition from Lipid Films
Abstract
:1. Introduction
2. Materials and Methods
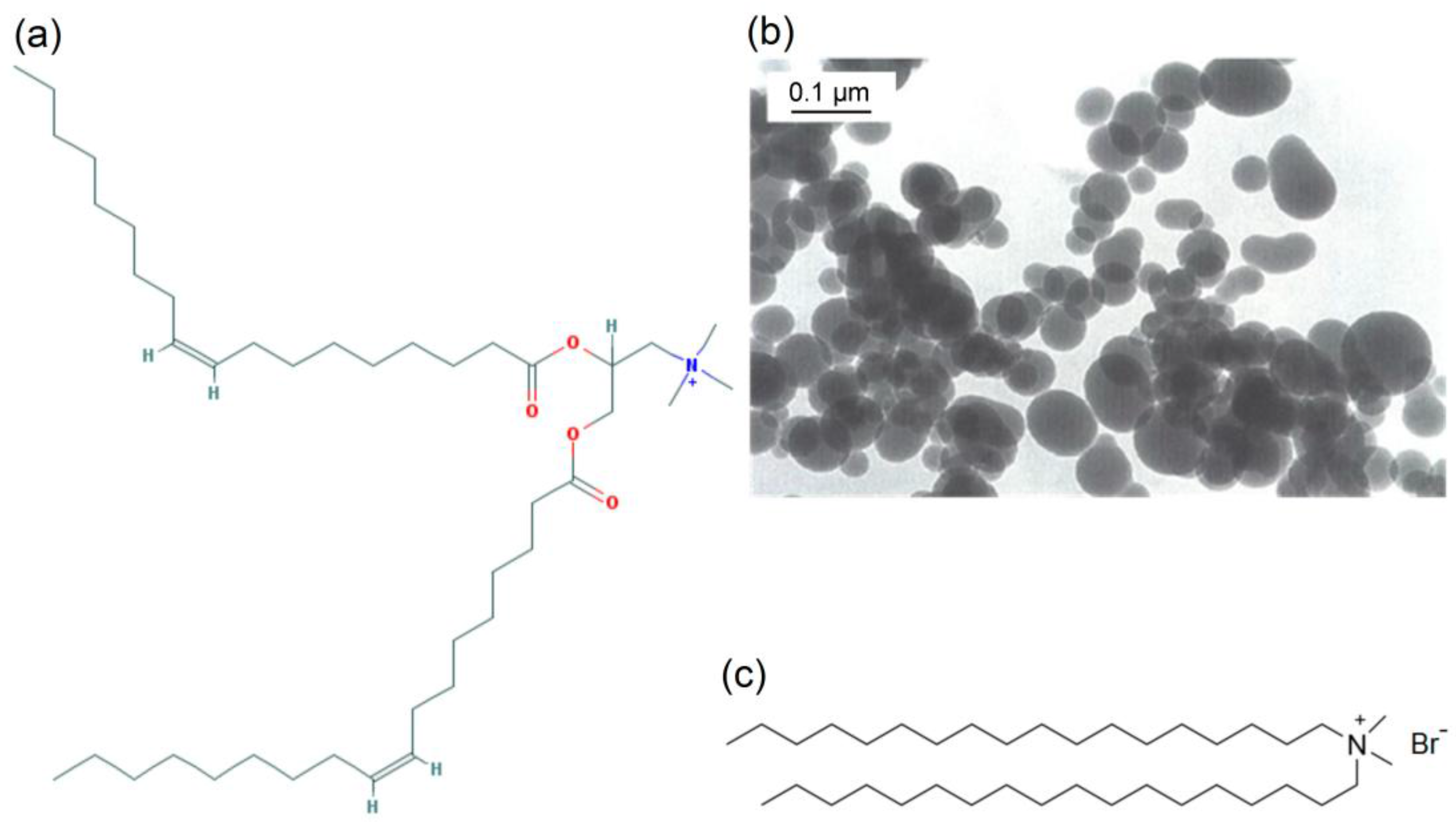
2.1. Materials
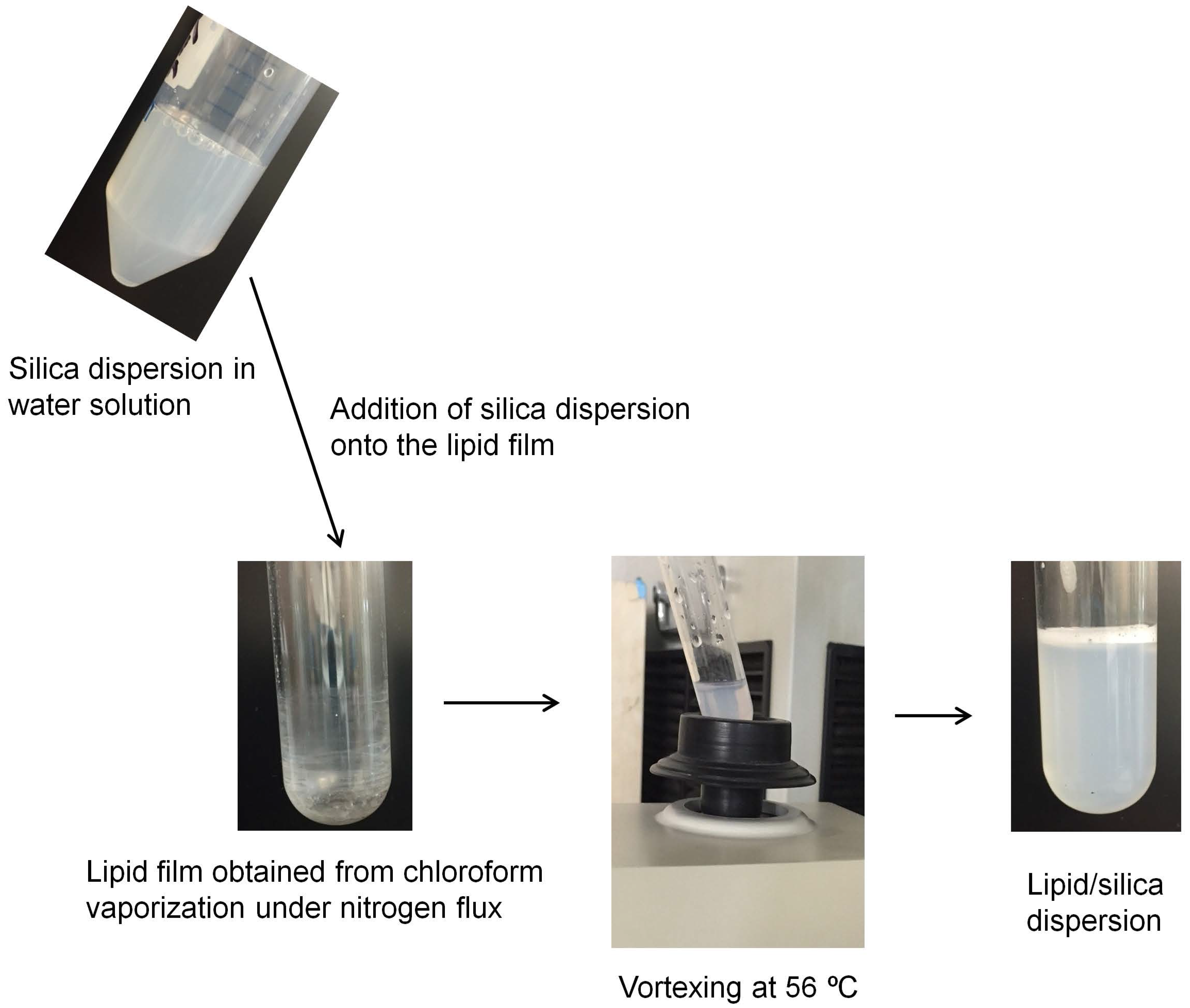
2.2. Preparation of Lipid Films
2.3. Preparation of DODAB Bilayer Fragments
2.4. Preparation and Characterization of the Biomimetic, Cationic Silica Particles
2.5. Determination of Lipid Adsorption onto Silica from Elemental Analysis of C, H and N
3. Results
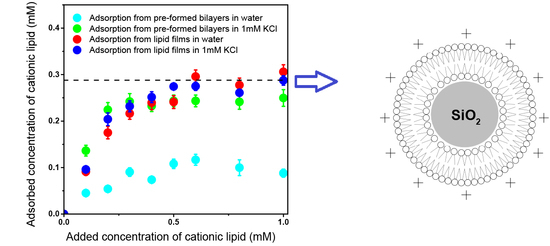
3.1. Colloidal Stability of Silica Nanoparticles in the Presence of Cationic Lipids
3.2. Physical Properties of Silica/DODAB and Silica/DOTAP Nanoparticles
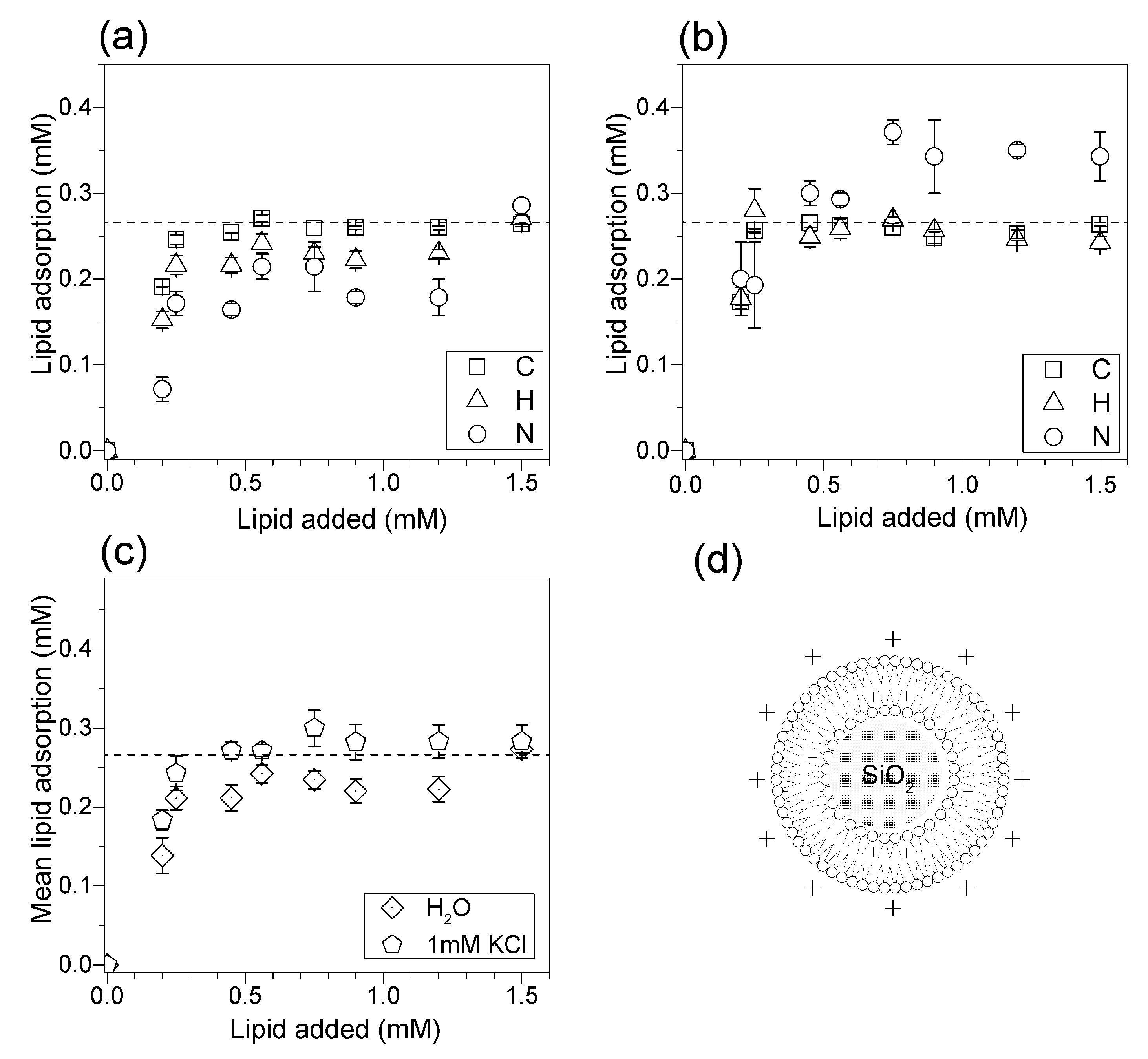
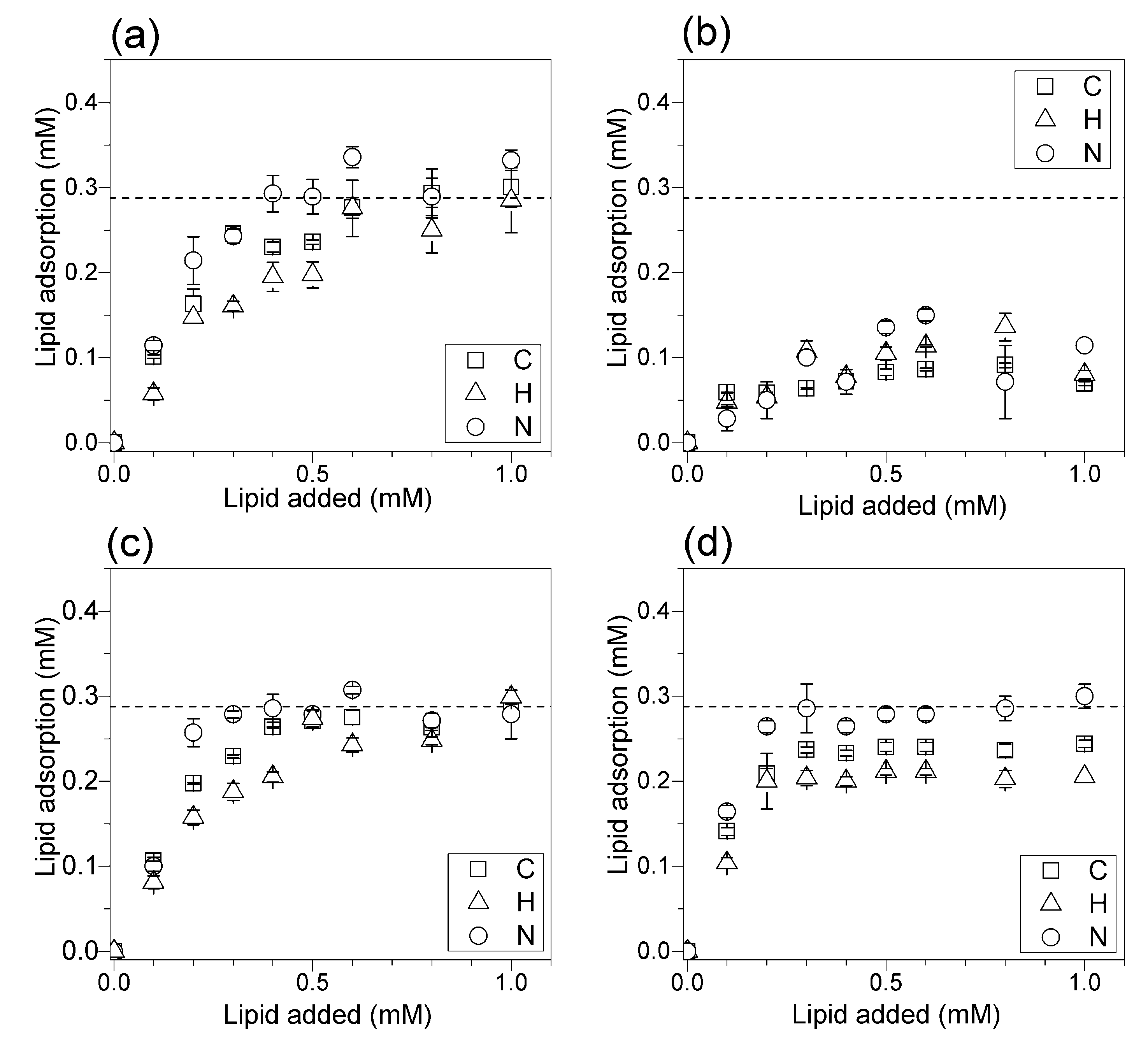
3.3. Adsorption Isotherms for DOTAP or DODAB onto Silica from Elemental Analysis In Situ
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mebert, A.M.; Baglole, C.J.; Desimone, M.F.; Maysinger, D. Nanoengineered silica: Properties, applications and toxicity. Food Chem. Toxicol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.K.; Dash, S.; Patel, S.; Mishra, B.K. Adsorption of organic molecules on silica surface. Adv. Colloid Interface Sci. 2006, 121, 77–110. [Google Scholar] [CrossRef] [PubMed]
- Raemdonck, K.; Braeckmans, K.; Demeester, J.; Smedt, S.C.D. Merging the best of both worlds: Hybrid lipid-enveloped matrix nanocomposites in drug delivery. Chem. Soc. Rev. 2013, 43, 444–472. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M.; Midmore, B.R. Synthetic bilayer adsorption onto polystyrene microspheres. Langmuir 1992, 8, 801–806. [Google Scholar] [CrossRef]
- Thevenot, J.; Troutier, A.-L.; David, L.; Delair, T.; Ladavière, C. Steric Stabilization of lipid/polymer particle assemblies by poly(ethylene glycol)-lipids. Biomacromolecules 2007, 8, 3651–3660. [Google Scholar] [CrossRef] [PubMed]
- Troutier, A.-L.; Delair, T.; Pichot, C.; Ladavière, C. Physicochemical and interfacial investigation of lipid/polymer particle assemblies. Langmuir 2005, 21, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wu, C.; Jiang, J.; Hao, Y.; Zhao, Y.; Xu, J.; Yu, T.; Ji, P. Lipid-coated hollow mesoporous silica nanospheres for co-delivery of doxorubicin and paclitaxel: Preparation, sustained release, cellular uptake and pharmacokinetics. Mater. Sci. Eng. C 2017, 71, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, R.; Rao, S.; Bremmell, K.; Prestidge, C. Synergistic role of solid lipid and porous silica in improving the oral delivery of weakly basic poorly water soluble drugs. Eur. J. Pharm. Sci. 2017, 96, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Rosa, H.; Petri, D.F.S.; Carmona-Ribeiro, A.M. Interactions between bacteriophage DNA and cationic biomimetic particles. J. Phys. Chem. B 2008, 112, 16422–16430. [Google Scholar] [CrossRef] [PubMed]
- Troutier-Thuilliez, A.-L.; Thevenot, J.; Delair, T.; Ladavière, C. Adsorption of plasmid DNA onto lipid/polymer particle assemblies. Soft Matter 2009, 5, 4739–4747. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M. Nanomaterials based on lipids for vaccine development. In Micro- and Nanotechnology in Vaccine Development; Skwarczynski, M., Toth, I., Eds.; Micro and Nano Technologies; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 241–257. ISBN 978-0-323-39981-4. [Google Scholar]
- Lincopan, N.; Espíndola, N.M.; Vaz, A.J.; da Costa, M.H.B.; Faquim-Mauro, E.; Carmona-Ribeiro, A.M. Novel immunoadjuvants based on cationic lipid: Preparation, characterization and activity in vivo. Vaccine 2009, 27, 5760–5771. [Google Scholar] [CrossRef] [PubMed]
- Skrastina, D.; Petrovskis, I.; Lieknina, I.; Bogans, J.; Renhofa, R.; Ose, V.; Dishlers, A.; Dekhtyar, Y.; Pumpens, P. Silica nanoparticles as the adjuvant for the immunisation of mice using hepatitis B core virus-like particles. PLoS ONE 2014, 9, e114006. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M. Biomimetic Nanoparticles: Preparation, Characterization and Biomedical Applications. Available online: https://www.dovepress.com/biomimetic-nanoparticles-preparation-characterization-and-biomedical-a-peer-reviewed-article-IJN (accessed on 13 July 2017).
- Sicchierolli, S.M.; Carmona-Ribeiro, A.M. Biomolecular recognition at phospholipid-covered polystyrene microspheres. J. Phys. Chem. 1996, 100, 16771–16775. [Google Scholar] [CrossRef]
- Baksh, M.M.; Jaros, M.; Groves, J.T. Detection of molecular interactions at membrane surfaces through colloid phase transitions. Nature 2004, 427, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Bayerl, T.M. A glass bead game. Nature 2004, 427, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid–polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Moura, S.P.; Carmona-Ribeiro, A.M. Biomimetic particles for isolation and reconstitution of receptor function. Cell Biochem. Biophys. 2006, 44, 446–452. [Google Scholar] [CrossRef]
- Richter, R.; Mukhopadhyay, A.; Brisson, A. Pathways of lipid vesicle deposition on solid surfaces: A combined QCM-D and AFM study. Biophys. J. 2003, 85, 3035–3047. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M. The Versatile Dioctadecyldimethylammonium Bromide; InTech: London, UK, 2017. [Google Scholar]
- Carmona-Ribeiro, A.M. Preparation and characterization of biomimetic nanoparticles for drug delivery. In Nanoparticles in Biology and Medicine; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; pp. 283–294. ISBN 978-1-61779-952-5. [Google Scholar]
- Moura, S.P.; Carmona-Ribeiro, A.M. Adsorption behavior of DODAB/DPPC vesicles on silica. J. Colloid Interface Sci. 2007, 313, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Moura, S.P.; Carmona-Ribeiro, A.M. Biomimetic particles: Optimization of phospholipid bilayer coverage on silica and colloid stabilization. Langmuir 2005, 21, 10160–10164. [Google Scholar] [CrossRef] [PubMed]
- Rapuano, R.; Carmona-Ribeiro, A.M. Physical adsorption of bilayer membranes on silica. J. Colloid Interface Sci. 1997, 193, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Moura, S.P.; Carmona-Ribeiro, A.M. Cationic bilayer fragments on silica at low ionic strength: Competitive adsorption and colloid stability. Langmuir 2003, 19, 6664–6667. [Google Scholar] [CrossRef]
- Nascimento, D.B.; Rapuano, R.; Lessa, M.M.; Carmona-Ribeiro, A.M. Counterion effects on properties of cationic vesicles. Langmuir 1998, 14, 7387–7391. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M. Lipid bilayer fragments and disks in drug delivery. Curr. Med. Chem. 2006, 13, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, E.; Morrison, I. Particle size distribution from analysis of quasi-elastic light scattering data. In Measurement of Suspended Particles by Quasi-Elastic Light Scattering; Dahneke, B.E., Ed.; Wiley: New York, NY, USA, 1983; Volume 21, pp. 199–236. [Google Scholar]
- Patterson, R.K. Automated Pregl–Dumas technique for determining total carbon, hydrogen, and nitrogen in atmospheric aerosols. Anal. Chem. 1973, 45, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Claesson, P.; Carmona-Ribeiro, A.M.; Kurihara, K. Dihexadecyl phosphate monolayers: Intralayer and interlayer interactions. J. Phys. Chem. 1989, 93, 917–922. [Google Scholar] [CrossRef]
- Zuidam, N.J.; Barenholz, Y. Electrostatic and structural properties of complexes involving plasmid DNA and cationic lipids commonly used for gene delivery. Biochim. Biophys. Acta BBA Biomembr. 1998, 1368, 115–128. [Google Scholar] [CrossRef]
- Jamróz, D.; Kepczynski, M.; Nowakowska, M. Molecular structure of the dioctadecyldimethylammonium bromide (DODAB) bilayer. Langmuir 2010, 26, 15076–15079. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, L.R.; Lessa, M.M.; Carmona-Ribeiro, A.M. Interactions between dioctadecyldimethylammonium chloride or bromide bilayers in water. Langmuir 1995, 11, 2938–2943. [Google Scholar] [CrossRef]
- Rapuano, R.; Carmona-Ribeiro, A.M. Supported bilayers on silica. J. Colloid Interface Sci. 2000, 226, 299–307. [Google Scholar] [CrossRef]
- Stelmo, M.; Chaimovich, H.; Cuccovia, I.M. Quantitative determination of alkylammonium amphiphiles using neutral detergents. J. Colloid Interface Sci. 1987, 117, 200–204. [Google Scholar] [CrossRef]
- Pereira, E.M.A.; Petri, D.F.S.; Carmona-Ribeiro, A.M. Adsorption of cationic lipid bilayer onto flat silicon wafers: Effect of ion nature and concentration. J. Phys. Chem. B 2006, 110, 10070–10074. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.B.; Carmona-Ribeiro, A.M. Synthetic bilayer fragments for solubilization of amphotericin B. J. Colloid Interface Sci. 2001, 244, 427–431. [Google Scholar] [CrossRef]
- Carvalho, C.A.; Olivares-Ortega, C.; Soto-Arriaza, M.A.; Carmona-Ribeiro, A.M. Interaction of gramicidin with DPPC/DODAB bilayer fragments. Biochim. Biophys. Acta BBA Biomembr. 2012, 1818, 3064–3071. [Google Scholar] [CrossRef] [PubMed]
- Ragioto, D.A.; Carrasco, L.D.; Carmona-Ribeiro, A.M. Novel gramicidin formulations in cationic lipid as broad-spectrum microbicidal agents. Int. J. Nanomed. 2014, 9, 3183–3192. [Google Scholar] [CrossRef]

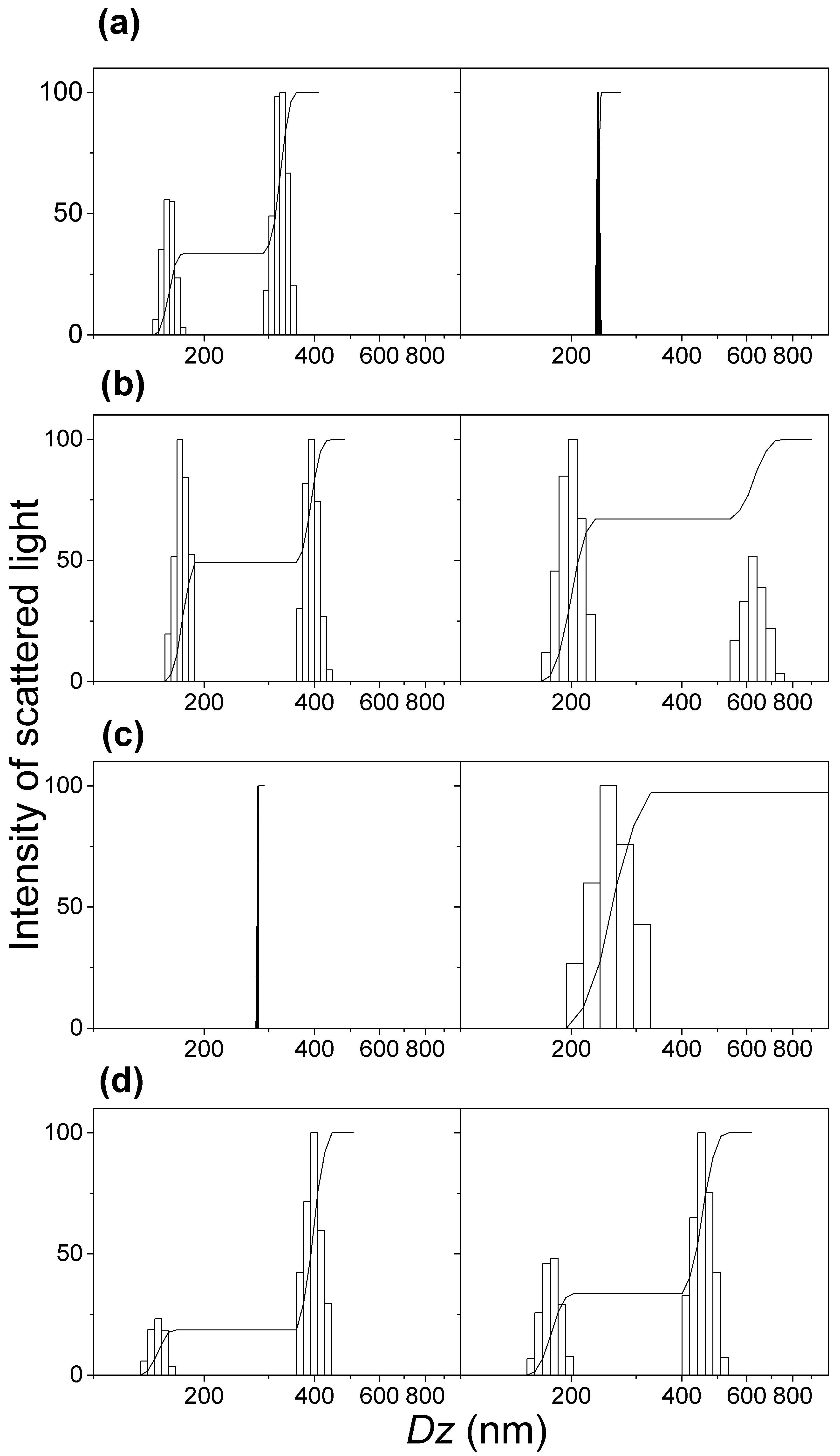

| Dispersion * | Dz (nm) | ζ (mV) | P |
|---|---|---|---|
| Silica 1 | 270 ± 4 | −24 ± 1 | 0.179 ± 0.012 |
| Silica 2 | 261 ± 3 | −20 ± 1 | 0.094 ± 0.020 |
| Silica 3 | 249 ± 2 | −21 ± 1 | 0.100 ± 0.024 |
| Silica 4 | 248 ± 2 | −25 ± 1 | 0.104 ± 0.027 |
| Silica/DOTAP film 1 | 284 ± 2 | +45 ± 1 | 0.123 ± 0.028 |
| Silica/DOTAP film 2 | 267 ± 3 | +55 ± 12 | 0.096 ± 0.022 |
| Silica/DOTAP film 3 | 287 ± 2 | +47 ± 1 | 0.050 ± 0.020 |
| Silica/DOTAP film 4 | 261 ± 3 | +47 ± 1 | 0.092 ± 0.024 |
| Silica/DODAB film 1 | 355 ± 19 | +55 ± 3 | 0.267 ± 0.013 |
| Silica/DODAB film 2 | 375 ± 4 | +56 ± 2 | 0.220 ± 0.005 |
| Silica/DODAB film 3 | 318 ± 3 | +43 ± 3 | 0.136 ± 0.020 |
| Silica/DODAB film 4 | 309 ± 4 | +44 ± 2 | 0.172 ± 0.017 |
| Silica/DODAB BF 1 | 251 ± 2 | +36 ± 1 | 0.128 ± 0.021 |
| Silica/DODAB BF 2 | 263 ± 3 | +38 ± 1 | 0.204 ± 0.019 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, R.T.; Braga, V.H.A.; Carmona-Ribeiro, A.M. Biomimetic Cationic Nanoparticles Based on Silica: Optimizing Bilayer Deposition from Lipid Films. Biomimetics 2017, 2, 20. https://doi.org/10.3390/biomimetics2040020
Ribeiro RT, Braga VHA, Carmona-Ribeiro AM. Biomimetic Cationic Nanoparticles Based on Silica: Optimizing Bilayer Deposition from Lipid Films. Biomimetics. 2017; 2(4):20. https://doi.org/10.3390/biomimetics2040020
Chicago/Turabian StyleRibeiro, Rodrigo T., Victor H. A. Braga, and Ana M. Carmona-Ribeiro. 2017. "Biomimetic Cationic Nanoparticles Based on Silica: Optimizing Bilayer Deposition from Lipid Films" Biomimetics 2, no. 4: 20. https://doi.org/10.3390/biomimetics2040020