Biomechanical Modeling of Human Skin Tissue Surrogates
Abstract
:1. Introduction
2. Materials and Methods
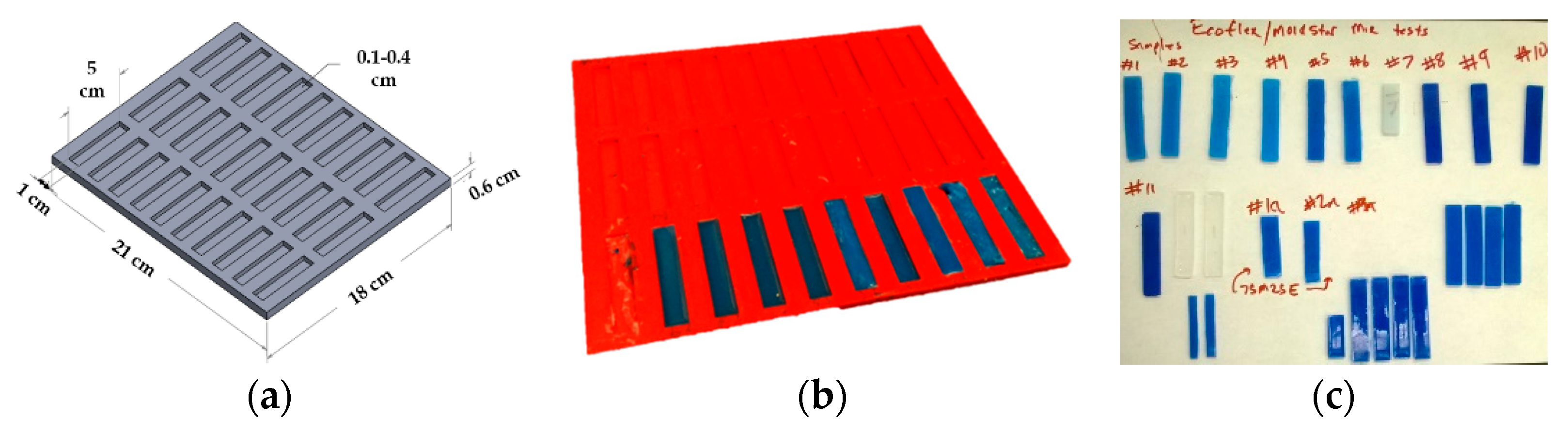
2.1. Fabrication of Human Skin Tissue Surrogates
2.2. Mechanical Testing of Human Skin Tissue Surrogates
2.3. Nonlinear Material Modeling
3. Results and Discussion
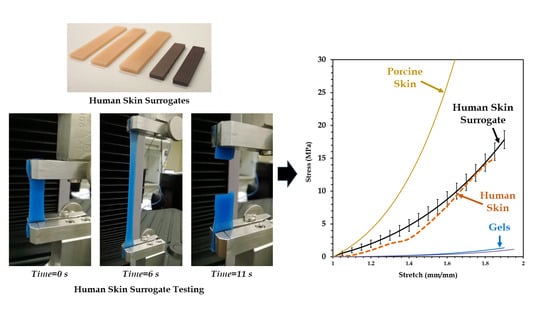
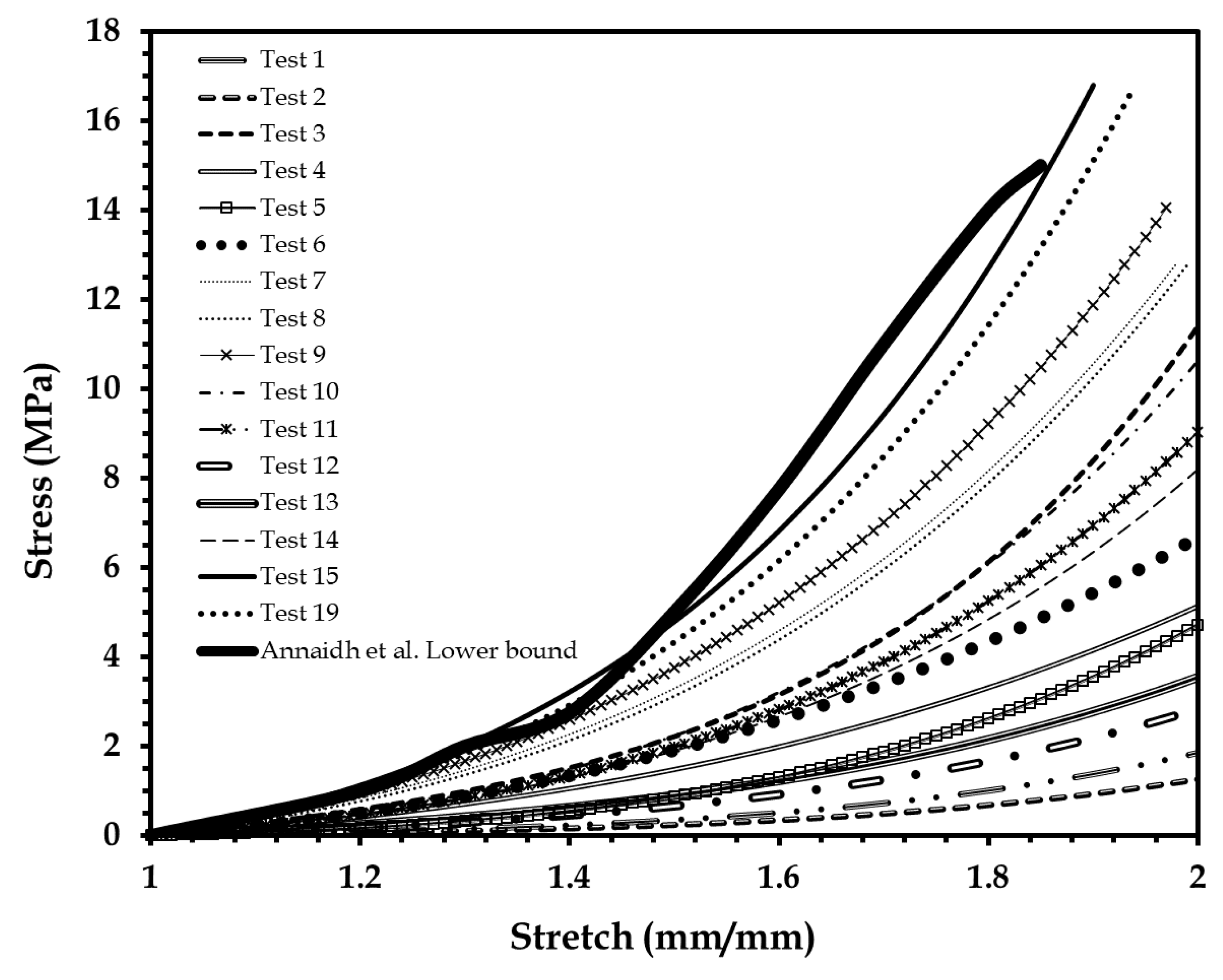
3.1. Mechanical Test of Human Skin Tissue Surrogates
3.2. Mechanical Modeling of Human Skin Tissue Surrogates at Different Body Sites
3.3. Comparison of the Mechanical Properties of the 90-10 Skin Tissue Surrogate and Human Skin
4. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- McGrath, J.; Uitto, J. Anatomy and Organization of Human Skin. Rook’s Textbook of Dermatology, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 1–53. [Google Scholar]
- Yannas, I.; Burke, J.F. Design of an artificial skin. I. Basic design principles. J. Biomed. Mater. Res. 1980, 14, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Douven, L.F.; Meijer, R.; Oomens, C.W. Characterization of Mechanical Behavior of Human Skin In Vivo. In Proceedings of the International Symposium on Biomedical Optics BiOS 2000, San Jose, CA, USA, 22–28 January 2000; International Society for Optics and Photonics: Bellingham, WA, USA. [Google Scholar]
- Hendriks, F.; Brokken, D.; Oomens, C.; Baaijens, F.; Horsten, J. Mechanical Properties of Different Layers of Human Skin; Department of Biomedical Engineering, Eindhoven University of Technology: Eindhoven, The Netherlands, 2000. [Google Scholar]
- Heinrich, T.; Lunderstaedt, R.A. Quantification of Mechanical Properties of Human Skin In Vivo. In Proceedings of the International Symposium on Optical Science and Technology, San Diego, CA, USA, 29 July–3 August 2001; International Society for Optics and Photonics: Bellingham, WA, USA. [Google Scholar]
- Hendriks, F. Mechanical Behaviour of Human Skin In Vivo—A Literature Review; Nat. Lab. Unclassified Report 820; Koninklijke Philips Electronics N.V.: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Silver, F.H.; Freeman, J.W.; DeVore, D. Viscoelastic properties of human skin and processed dermis. Skin Res. Technol. 2001, 7, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Abas, W.W.; Barbenel, J. Uniaxial tension test of human skin in vivo. J. Biomed. Eng. 1982, 4, 65–71. [Google Scholar] [CrossRef]
- Chanda, A.; Curry, K. Patient-specific biofidelic human coronary artery surrogates. J. Mech. Med. Biol. 2018, 18. [Google Scholar] [CrossRef]
- Chanda, A.; Flynn, Z.; Unnikrishnan, V. Biomechanical characterization of normal and prolapsed vaginal tissue surrogates. J. Mech. Med. Biol. 2018, 18. [Google Scholar] [CrossRef]
- Chanda, A.; Unnikrishnan, V.; Lackey, K. Biofidelic Conductive Synthetic Skin Composites. In Proceedings of the American Society for Composites—Thirty-Second Technical Conference, West Lafayette, IN, USA, 23–25 October 2017. [Google Scholar]
- Cook, T.; Alexander, H.; Cohen, M. Experimental method for determining the 2-dimensional mechanical properties of living human skin. Med. Biol. Eng. Comput. 1977, 15, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Agache, P.; Monneur, C.; Leveque, J.; De Rigal, J. Mechanical properties and Young’s modulus of human skin in vivo. Arch. Dermatol. Res. 1980, 269, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Manschot, J.F.M. The Mechanical Properties of Human Skin In Vivo. Ph.D. Thesis, Radboud University Nijmegen, Nijmegen, The Netherlands, 10 October 1985. [Google Scholar]
- Manschot, J.; Brakkee, A. The measurement and modelling of the mechanical properties of human skin in vivo—II. Model. J. Biomech. 1986, 19, 517–521. [Google Scholar] [CrossRef]
- Edwards, C.; Marks, R. Evaluation of biomechanical properties of human skin. Clin. Dermatol. 1995, 13, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Kvistedal, Y.; Nielsen, P. Investigating Stress-Strain Properties of In-Vivo Human Skin Using Multiaxial Loading Experiments and Finite Element Modeling. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004. [Google Scholar]
- Boyer, G.; Zahouani, H.; Le Bot, A.; Laquieze, L. In Vivo Characterization of Viscoelastic Properties Of Human Skin Using Dynamic Micro-Indentation. In Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007. [Google Scholar]
- Tran, H.; Charleux, F.; Rachik, M.; Ehrlacher, A.; Ho Ba Tho, M. In vivo characterization of the mechanical properties of human skin derived from MRI and indentation techniques. Comput. Methods Biomech. Biomed. Eng. 2007, 10, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Delalleau, A.; Josse, G.; Lagarde, J.M.; Zahouani, H.; Bergheau, J.M. A nonlinear elastic behavior to identify the mechanical parameters of human skin in vivo. Skin Res. Technol. 2008, 14, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Pailler-Mattei, C.; Bec, S.; Zahouani, H. In vivo measurements of the elastic mechanical properties of human skin by indentation tests. Med. Eng. Phys. 2008, 30, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Finlay, B. Dynamic mechanical testing of human skin ‘in vivo’. J. Biomech. 1970, 3, 557–568. [Google Scholar] [CrossRef]
- Potts, R.O.; Chrisman, D.A.; Buras, E.M. The dynamic mechanical properties of human skin in vivo. J. Biomech. 1983, 16, 365–372. [Google Scholar] [CrossRef]
- Chanda, A. Biofidelic Soft Composites—Experimental and Computational Modeling; University of Alabama Libraries: Tuscaloosa, AL, USA, 2017. [Google Scholar]
- Chanda, A.; Unnikrishnan, V. Human Tissue Simulants for Study of Traumatic Brain Injury (TBI). In Proceedings of the American Society for Composites: Thirty-First Technical Conference, Williamsburg, VA, USA, 19–22 September 2016. [Google Scholar]
- Evans, S.L.; Holt, C.A. Measuring the mechanical properties of human skin in vivo using digital image correlation and finite element modelling. J. Strain Anal. Eng. Des. 2009, 44, 337–345. [Google Scholar] [CrossRef]
- Kearney, S.P.; Dai, Z.; Royston, T.J. Wideband Optical Elastography of In Vivo Human Skin Using Geometrically Focused Surface Waves. In Proceedings of the SPIE BiOS, San Francisco, CA, USA, 1–6 February 2014; Larin, K.V., Sampson, D.D., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2014; Volume 8946. [Google Scholar]
- Payne, T.; Mitchell, S.; Bibb, R. Design of human surrogates for the study of biomechanical injury: A review. Crit. Rev. Biomed. Eng. 2013, 41, 51–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tai, B.L.; Yu, H.; Shih, A.J. Silicone-based tissue-mimicking phantom for needle insertion simulation. J. Med. Devices 2014, 8, 021001. [Google Scholar] [CrossRef]
- Lualdi, M.; Colombo, A.; Farina, B.; Tomatis, S.; Marchesini, R. A phantom with tissue-like optical properties in the visible and near infrared for use in photomedicine. Lasers Surg. Med. 2001, 28, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Madsen, E.L.; Frank, G.R. Tissue Mimicking Elastography Phantoms. U.S. Patent US7462488B2, 9 December 2008. [Google Scholar]
- Kremer, M.; Lang, E.; Berger, A. Organotypical engineering of differentiated composite-skin equivalents of human keratinocytes in a collagen-GAG matrix (INTEGRA Artificial Skin) in a perfusion culture system. Langenbeck’s Arch. Surg. 2001, 386, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Payne, T.; Mitchell, S.; Bibb, R.; Waters, M. Initial validation of a relaxed human soft tissue simulant for sports impact surrogates. Procedia Eng. 2014, 72, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Payne, T.; Mitchell, S.; Bibb, R.; Waters, M. The evaluation of new multi-material human soft tissue simulants for sports impact surrogates. J. Mech. Behav. Biomed. Mater. 2015, 41, 336–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bir, C.A.; Resslar, M.; Stewart, S. Skin penetration surrogate for the evaluation of less lethal kinetic energy munitions. Forensic Sci. Int. 2012, 220, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Jussila, J.; Leppäniemi, A.; Paronen, M.; Kulomäki, E. Ballistic skin simulant. Forensic Sci. Int. 2005, 150, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Amick, D. Gel Compositions as Muscle Tissue Simulant and Related Articles and Methods. U.S. Patent US20070116766A1, 24 May 2006. [Google Scholar]
- Giurintano, D.; Callais, R.; Cancienne, J.P.; Lousteau, J.; Tumlin, J. Torso Simulator for Ballistics Testing. U.S. Patent US8215165B2, 10 July 2012. [Google Scholar]
- Oldham, S. Less-lethal munitions. Law Order 2002, 50, 54–56. [Google Scholar]
- Arthur, B.; Janssen, P.A. Silicone breast implants. AWHONN Lifelines 2000, 4, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Callaway, C. Tissue anisotropy modeling using soft composite materials. Appl. Bionics Biomech. 2018, 2018, 4838157. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Unnikrishnan, V. Numerical modeling of skin wound closure with interrupted sutures. In NanoBio Summit; The University of Alabama at Birmingham (UAB): Birmingham, AL, USA, 2015. [Google Scholar]
- Chanda, A.; Unnikrishnan, V. Interrupted Suture Force Estimation for Skin Wound Closure: A Computational Approach. In Proceedings of the Society of Engineering Science 52nd Annual Technical Meeting, College Station, TX, USA, 29 October 2015. [Google Scholar]
- Chanda, A.; Unnikrishnan, V. Effect of bladder and rectal loads on the vaginal canal and levator ani in varying pelvic floor conditions. Mech. Adv. Mater. Struct. 2017. just-accepted. [Google Scholar] [CrossRef]
- Chanda, A.; Unnikrishnan, V.; Flynn, Z.; Lackey, K. Experimental study on tissue phantoms to understand the effect of injury and suturing on human skin mechanical properties. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2017, 231, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Annaidh, A.N.; Bruyère, K.; Destrade, M.; Gilchrist, M.D.; Otténio, M. Characterization of the anisotropic mechanical properties of excised human skin. J. Mech. Behav. Biomed. Mater. 2012, 5, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annaidh, A.N.; Destrade, M.; Ottenio, M.; Bruyere, K.; Gilchrist, M.D. Strain Rate Effects on the Failure Characteristics of Excised Human Skin. In Proceedings of the 9th International Conference on the Mechanics of Time Dependent Materials (MTL), Montréal, QC, Canada, 27–30 May 2014. [Google Scholar]
- Groves, R.B.; Coulman, S.A.; Birchall, J.C.; Evans, S.L. An anisotropic, hyperelastic model for skin: Experimental measurements, finite element modelling and identification of parameters for human and murine skin. J. Mech. Behav. Biomed. Mater. 2013, 18, 167–180. [Google Scholar] [CrossRef] [PubMed]
- ASTM D2240-15e1 Standard Test Method for Rubber Property—Durometer Hardness; ASTM International: West Conshohocken, PA, USA, 2015; Available online: https://doi.org/10.1520/D2240-15E01 (accessed on 23 July 2018).
- Chanda, A.; Callaway, C.; Clifton, C.; Unnikrishnan, V. Biofidelic human brain tissue surrogates. Mech. Adv. Mater. Struc. 2016. [Google Scholar] [CrossRef]
- Martins, P.; Natal Jorge, R.; Ferreira, A. A comparative study of several material models for prediction of hyperelastic properties: Application to silicone-rubber and soft tissues. Strain 2006, 42, 135–147. [Google Scholar] [CrossRef]
- Shergold, O.A.; Fleck, N.A.; Radford, D. The uniaxial stress versus strain response of pig skin and silicone rubber at low and high strain rates. Int. J. Impact Eng. 2006, 32, 1384–1402. [Google Scholar] [CrossRef]
- Sasso, M.; Palmieri, G.; Chiappini, G.; Amodio, D. Characterization of hyperelastic rubber-like materials by biaxial and uniaxial stretching tests based on optical methods. Polym. Test. 2008, 27, 995–1004. [Google Scholar] [CrossRef]
- Chanda, A.; Graeter, R.; Unnikrishnan, V. Effect of blasts on subject-specific computational models of skin and bone sections at various locations on the human body. AIMS Mater. Sci. 2015, 2, 425–447. [Google Scholar] [CrossRef]
- Chanda, A.; Unnikrishnan, V.; Richter, H.E.; Lockhart, M.E. A biofidelic computational model of the female pelvic system to understand effect of bladder fill and progressive vaginal tissue stiffening due to prolapse on anterior vaginal wall. Int. J. Numer. Methods Biomed. Eng. 2016, 32. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Meyer, I.; Richter, H.E.; Lockhart, M.E.; Moraes, F.R.; Unnikrishnan, V. Vaginal changes due to varying degrees of rectocele prolapse: A computational study. J. Biomech. Eng. 2017, 139, 101001. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Unnikrishnan, V.; Richter, H.E.; Lockhart, M.E. Computational Modeling of Anterior and Posterior Pelvic Organ Prolapse (POP). In Proceedings of the ASME 2016 International Mechanical Engineering Congress and Exposition, Phoenix, AZ, USA, 11–17 November 2016; American Society of Mechanical Engineers: New York, NY, USA, 2016. [Google Scholar]
- Chanda, A.; Ghoneim, H. Pumping potential of a two-layer left-ventricle-like flexible-matrix-composite structure. Compos. Struct. 2015, 122, 570–575. [Google Scholar] [CrossRef]
- Chanda, A.; Unnikrishnan, V.; Roy, S.; Richter, H.E. Computational modeling of the female pelvic support structures and organs to understand the mechanism of pelvic organ prolapse: A review. Appl. Mech. Rev. 2015, 67, 040801. [Google Scholar] [CrossRef]
- Gonzalez, L.Y.S.; Botero, M.G.; Betancur, M. Hyperelastic Material Modeling; Universidad EAFIT: Medellín, Colombia, 2005. [Google Scholar]
- Holzapfel, G.A. Nonlinear Solid Mechanics; Wiley Chichester: Hoboken, NJ, USA, 2000; Volume 24. [Google Scholar]
- Lapeer, R.; Gasson, P.; Karri, V. Simulating plastic surgery: From human skin tensile tests, through hyperelastic finite element models to real-time haptics. Prog. Biophys. Mol. Biol. 2010, 103, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, J.; Holt, C.; Evans, S.; Manan, N.F.A.; Chizari, M. A parametric study and simulations in quantifying human skin hyperelastic parameters. Procedia Eng. 2012, 41, 1580–1586. [Google Scholar] [CrossRef]
- Gallagher, A.; Ní Annaidh, A.; Bruyère, K. Dynamic Tensile Properties of Human Skin. In Proceedings of the 2012 IRCOBI Conference Proceedings—International Research Council on the Biomechanics of Injury, Dublin, Ireland, 12–14 September 2012. [Google Scholar]





| Test Specimen No. | Shore 00-10 | Shore 30 A | ||
|---|---|---|---|---|
| Part A | Part B | Part A | Part B | |
| 1 | 45 | 45 | 5 | 5 |
| 2 | 45 | 45 | 5 | 5 |
| 3 | 15 | 15 | 35 | 35 |
| 4 | 42 | 42 | 8 | 8 |
| 5 | 42 | 42 | 8 | 8 |
| 6 | 35 | 35 | 15 | 15 |
| 7 | 12.5 | 12.5 | 37.5 | 37.5 |
| 8 | 12.5 | 12.5 | 37.5 | 37.5 |
| 9 | 10 | 10 | 40 | 40 |
| 10 | 15 | 15 | 35 | 35 |
| 11 | 15 | 15 | 35 | 35 |
| 12 | 45 | 45 | 5 | 5 |
| 13 | 42 | 42 | 8 | 8 |
| 14 | 25 | 25 | 25 | 25 |
| 15 | 5 | 5 | 45 | 45 |
| 16 | 3 | 3 | 47 | 47 |
| 17 | 3 | 3 | 57 | 37 |
| 18 | 3 | 3 | 47 | 47 |
| 19 | 5 | 5 | 45 | 45 |
| 20 | 5 | 5 | 47 | 43 |
| 21 | 3 | 3 | 54 | 40 |
| 22 | 3 | 3 | 52 | 42 |
| 23 | 4 | 4 | 46 | 46 |
| Sample Location | Shore 00-10 | Shore 30 A | Veronda–Westmann Hyperelastic Model Coefficients | |
|---|---|---|---|---|
| Part A–Part B | Part A–Part B | c1 | c2 | |
| 1 | 3:7 | 51:39 | 27 | 0.29 |
| 2 | 8:2 | 44:46 | 16 | 0.26 |
| 3 | 7:3 | 61:29 | 35 | 0.33 |
| 4 | 4:6 | 52:38 | 28 | 0.3 |
| 5 | 7:3 | 45:45 | 15 | 0.23 |
| 6 | 5:5 | 45:45 | 13.1 | 0.21 |
| 7 | 2:8 | 48:42 | 18 | 0.20 |
| 8 | 5:5 | 47:43 | 22 | 0.26 |
| 9 | 6:4 | 58:32 | 33 | 0.30 |
| 10 | 8:2 | 42:48 | 14.4 | 0.27 |
| 11 | 7:3 | 48:42 | 24 | 0.28 |
| Test Specimen | Elastic Modulus (E) (Low Stretch Ratio) | Elastic Modulus (E) (High Stretch Ratio) | Ultimate Tensile Stress (MPa) |
|---|---|---|---|
| 90-10 Human Skin Surrogate | 2.163 ± 0.196 | 29.411 ± 2.261 | 16.23 ± 1.15 |
| Human Skin 1 | 1.18 ± 0.88 | 83.3 ± 34.9 | 21.6 ± 8.4 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanda, A. Biomechanical Modeling of Human Skin Tissue Surrogates. Biomimetics 2018, 3, 18. https://doi.org/10.3390/biomimetics3030018
Chanda A. Biomechanical Modeling of Human Skin Tissue Surrogates. Biomimetics. 2018; 3(3):18. https://doi.org/10.3390/biomimetics3030018
Chicago/Turabian StyleChanda, Arnab. 2018. "Biomechanical Modeling of Human Skin Tissue Surrogates" Biomimetics 3, no. 3: 18. https://doi.org/10.3390/biomimetics3030018