A Cost-Effective and Efficient Chick Ex-Ovo CAM Assay Protocol to Assess Angiogenesis
Abstract
:1. Introduction
2. Experimental Design
2.1. Materials
- 3-day old chicken embryonated eggs (from Central Poultry Development Organization, Bhubaneswar, India).
- Wheat husks, for maintaining temperature and humidity for the egg during transportation.
- Alcohol (Changshu City Hongsheng Fine Chemical Co., Ltd., Changshu City, China).
- An egg-tray, for holding the eggs before culturing in the cups.
- Plastic cups (better if transparent).
- A cuboid metal bar, for cracking the eggshell.
- Scissors, for cutting the eggshell after cracking—to ensure proper transfer of embryonic contents into the cups and for cutting the cling wrap.
- Cling wrap, for covering the shell-less culture.
- Rubber bands, for holding the cling wrap on the plastic cups.
- Toothpicks, for making pores on the cling wrap to provide ventilation for the embryo.
- Whatman filter papers (thickness 1 mm), for making circular discs impregnated over the CAM.
- Forceps, for dropping the discs over the CAM.
- Dulbecco’s Modified Eagle Medium (DMEM; Hi-Media, Mumbai, India, Cat. No.: AL219A).
2.2. Equipment
- Cell culture Dishes (60 × 15 mm; Eppendorf, Hamburg, Germany, Cat. No.: 30701119).
- A CO2 Incubator (New Brunswick. Galaxy® 170R; Eppendorf).
- A stereomicroscope (Leica–EZ,; Leica Microsystems (UK) Ltd., Milton Keynes, UK).
- A Charge-coupled Device (CCD) Camera (ChemiDoc™ XRS; Bio-Rad, Hercules, CA, USA).
3. Procedure
3.1. Incubation of the Eggs. Time for Completion: 2–3 h
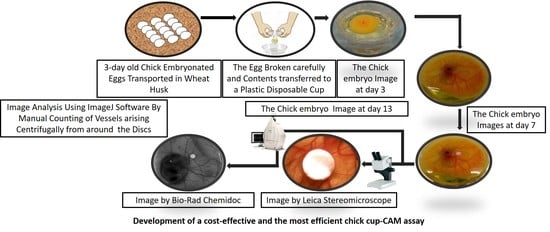
- 3-day old specific pathogen-free embryonated eggs should be obtained from the hatchery and kept inside the husks during transportation to the lab. (Note: We observed significantly reduced survival rate of embryos when 0-day-old eggs were purchased and stored at lower temperature before proceeding to the experiment.)
- The presence of an embryo and its location inside the eggs should be checked using an egg candler.
- The eggs should be placed horizontally in a suitable egg tray and incubated for an hour at 37 °C and 50% humidity without rotation, inside an incubator. (Note: The eggs should be kept horizontally in the incubator for an hour to bring the CAM to the upper side of the egg. This helps in the transfer of an intact and viable embryo to the plastic cups for the ex-ovo culture.)
3.2. The Ex-Ovo Culture. Time for Completion: 96–100 h
- The egg tray containing the eggs should be taken out from the incubator and kept inside a laminar flow hood.
- The eggs should be kept in a horizontal position and cracked open using the moderately sharp edge of a sterilized hard cube/rectangle shaped structure, lying perpendicular to the horizontal axis of the eggs. (Note: We used a metallic surface for this step to provide a hard and durable material for the cracking of the egg.)
- The cracked eggs should be placed just above the surface of the cups to avoid any leakage of the egg white. (Note: As the egg white came from the eggs, it is essential that it stays connected to the inner contents of the egg for us to obtain a viable and intact embryo. Additionally, it is essential that the plastic cups used are transparent for better view and should be thoroughly cleaned with 70% ethanol.)
- The contents of the eggs should be transferred into the cup in a continuous flow by carefully applying the pressure on the crack—without breaking the connection of the inner and the outer contents of the egg or damaging the embryo and the vessels around it. (Note: The contents were efficiently transferred when both halves of the eggshell were separated simultaneously.)
- The cups should be covered with sterile cling wrap with the help of rubber bands. (Note: Rubber bands were used to ensure that the cling wrap stayed in place, covering the cups.)
- Small pores should be made on the wrap with the help of sterilized toothpicks to provide ventilation for the chick embryo inside the cup. (Note: Pores should be made carefully to avoid larger pores which could allow the movement of contaminant particles along with air. We ensured that there was no damage to the embryo while making these pores.)
- The ex-ovo cultures should be kept back in the incubator for the next 96 h and should be maintained at 37 °C and 50% humidity. (Note: We used cell culture incubators for incubating the eggs. We maintained the humidity by keeping a water tray at the bottom of the incubator.)
3.3. Preparation of the Conditioned Media. Time for Completion: 120–144 h
- The tumor cells—of interest from all experimental groups, should be cultured in complete media.
- To prepare the conditioned media, equal number of cells should be plated in 60 or 100 mm dishes for all experimental groups.
- Upon reaching 50–70% confluency, the complete medium from the dishes should be replaced with low-serum or serum-free DMEM (Dulbecco’s Modified Eagle’s Medium). (Note: The amount of serum for each cell line needed optimization.)
- At 24–72 h post incubation, the conditioned media should be collected from the dishes and centrifuged at 4000 rpm for 3 min. (Note: We might have alternatively filtered the conditioned media using a 0.45 µm syringe filter to get rid of cell debris.)
- The conditioned media can be snap-chilled prior to use for later experiments or lyophilization. (Note: The lyophilized media from each group should be re-suspended in an equal volume of serum-free DMEM and stored in small aliquots for CAM experiments.)
3.4. Application of the Substances to Study the Angiogenesis. Time for Completion: 120–124 h
- Circular discs can be created from filter papers (thickness 1 mm, 6.30 ± 0.04 mm in diameter) using a hole-puncher. The discs should be autoclaved and dried completely before using them as carriers for the test substances to the CAM.
- These circular discs should be soaked with equal volumes of the conditioned media from all experimental groups used for the angiogenic studies. (Note: The dilutions may vary according to the experiments.)
- After keeping the ex-ovo culture back for 96 h (from step 7 of Section 3.2), the cultures should be removed from the incubator and placed into the laminar flow hood. The cling wrap should be removed temporarily.
- The circular discs should be dropped over the CAM with the help of sterilized forceps. (Note: The discs should be carefully dropped over the region where two major blood vessels bifurcated, for a more efficient observation of the effects of the test substances on the angiogenesis.)
- Images of the embryo along with the discs should be taken for the end-point analysis of the CAM assay.
- The cultures should be re-covered with the same cling wrap and maintained in the incubator—in conditions mentioned in step 7 of Section 3.2, for the next 120 h.
3.5. End-point Imaging and Image Analysis. Time for Completion: 6–8 h
- The cups containing the embryos should be taken out of the incubator carefully after 120 h of incubation and then placed under a stereomicroscope/CCD (Charge-coupled Device) camera (in-situ imaging).
- The image of the region surrounding the disc should be captured using a stereomicroscope (8× magnification) and the image of the entire embryo using a CCD digital camera. (Note: The images from the stereomicroscope helped to estimate the microvessel quantity around the implanted disc while the images from the CCD digital camera aided identification of the microvessels arising centrifugally from the disc by portraying the origin of each microvessel.)
- The images obtained from the stereomicroscope can be processed for background corrections using imaging software (Fiji ImageJ, Aphelion Dev, etc.) and the manual counting of the microvessels arising centrifugally from the disc can be done. (Note: The manual counting of the vessels should be done by two unbiased observers, independent from the experimenter to obtain authenticated data. We decided if substantial differences between the counting of different unbiased observers were found, it was better to exclude that experimental sample from analysis. To have better power of study begin the experimentation with a higher number of replicates (N = 10) in each experimental group.)
- The number of micro vessels obtained from each group can be compared using the unpaired t-test—plotted with the help of GraphPad Prism.
4. Expected Results
4.1. Low Efficacy of Previously Reported Methods
4.2. High Efficacy of Improvised Cup-CAM Method
5. Discussion
6. Conclusions
7. Reagent Setup
- We thawed DMEM, mixed with amphotericin and filtered through 0.22 um filter and kept the mixture at 4 °C for up to 2 weeks.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Auerbach, R.; Lewis, R.; Shinners, B.; Kubai, L.; Akhtar, N. Angiogenesis assays: A critical overview. Clin. Chem. 2003, 49, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. Critical factors in the biology of human cancer metastasis: Twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990, 50, 6130–6138. [Google Scholar] [PubMed]
- Hasan, J.; Shnyder, S.D.; Bibby, M.; Double, J.A.; Bicknel, R.; Jayson, G.C. Quantitative angiogenesis assays in vivo—A review. Angiogenesis 2004, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Howell, M.G.; Grandis, J.R. Molecular mediators of metastasis in head and neck squamous cell carcinoma. Head Neck 2005, 27, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, S.; Presta, M. The zebrafish/tumor xenograft angiogenesis assay. Nat. Protoc. 2007, 2, 2918–2923. [Google Scholar] [CrossRef] [PubMed]
- Norrby, K. In vivo models of angiogenesis. J. Cell. Mol. Med. 2006, 10, 588–612. [Google Scholar] [CrossRef] [PubMed]
- Passaniti, A.; Taylor, R.M.; Pili, R.; Guo, Y.; Long, P.V.; Haney, J.A.; Pauly, R.R.; Grant, D.S.; Martin, G.R. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab. Investig. 1992, 67, 519–528. [Google Scholar] [PubMed]
- Ribatti, D.; Nico, B.; Vacca, A.; Presta, M. The gelatin sponge-chorioallantoic membrane assay. Nat. Protoc. 2006, 1, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.; Singh, G. Observations on the use of the avian chorioallantoic membrane (CAM) model in investigations into angiogenesis. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2003, 3, 155–185. [Google Scholar] [CrossRef] [PubMed]
- Romanoff, A.L. The Avian Embryo: Structural and Functional Development; Macmillan: New York, NY, USA, 1960. [Google Scholar]




| CAM Assay Methods | Attributes | |||
|---|---|---|---|---|
| No of Eggs Experimented | Accessibility | Embryo Viability | Overall Efficiency | |
| In-ovo | 30 | Low | 85–95% | Low * |
| Ex-ovo(Petri Dishes and Paper Boats) | 15 (Petri Dishes) 15 (Paper Boats) | High | 15–25% | Low * |
| Ex-ovo(Glasses) | 30 | High | 45–55% | Medium * |
| Cup-CAM | 462 | High | 85–95% | High * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naik, M.; Brahma, P.; Dixit, M. A Cost-Effective and Efficient Chick Ex-Ovo CAM Assay Protocol to Assess Angiogenesis. Methods Protoc. 2018, 1, 19. https://doi.org/10.3390/mps1020019
Naik M, Brahma P, Dixit M. A Cost-Effective and Efficient Chick Ex-Ovo CAM Assay Protocol to Assess Angiogenesis. Methods and Protocols. 2018; 1(2):19. https://doi.org/10.3390/mps1020019
Chicago/Turabian StyleNaik, Monali, Pratush Brahma, and Manjusha Dixit. 2018. "A Cost-Effective and Efficient Chick Ex-Ovo CAM Assay Protocol to Assess Angiogenesis" Methods and Protocols 1, no. 2: 19. https://doi.org/10.3390/mps1020019