1. Introduction
The Spanish microanatomist and Nobel laureate Santiago Ramón y Cajal (1852–1934), the primary architect of the neuron doctrine and the law of dynamic polarization, is considered to be the founder of modern neuroscience [
1,
2]. According to the neuron doctrine, the nerve cell is the anatomical, physiological, and developmental unit of the nervous system [
3,
4]. To a first approximation, the law of dynamic polarization states that nerve impulses are exactly polarized in the neuron; in functional terms, the dendrites and the cell body work as a reception device, the axon works as a conduction device, and the terminal arborizations of the axon work as an application device. The two papers in which Cajal presented this law, namely, “Significación fisiológica de las expansiones protoplasmáticas y nerviosas de las células de la sustancia gris” [
5], and “Leyes de la morfología y dinamismo de las células nerviosas” [
6], must be seen as milestones in the historical development of functional neuroscience [
7,
8]. When taken collectively, Cajal’s contributions to modern biological knowledge are comparable to those of Vesalius, Harvey, Schwann, and Darwin.
At the same time, many philosophers, historians, and neuroscientists agree that modern neuroscience embodies a mechanistic perspective on the explanation of the nervous system [
9,
10,
11]. In modern, mechanistic neuroscience, scientific explanations must describe mechanisms, span several levels, and integrate multiple fields [
10]. To correctly describe a mechanism, a scientific model must first identify the phenomenon the mechanism is responsible for [
12]. The mechanistic phenomenon is, in general, a combination of a target system and some behavior in which the target system is involved [
13]. Second, the model must represent the composing entities, the activities, and the organizational properties of the target system that constitute the mechanism underlying the phenomenon of interest [
14,
15]. The composing entities, and the activities those entities are involved in, typically span several levels of organization in nature, from the atomic and the molecular to the higher levels of complex brain networks and whole organisms. For this reason, the search for mechanisms and their components requires the integration of experimental methods, empirical results, and conceptual resources from different scientific fields: for example, molecular and cellular biology, neurophysiology, computational neuroscience, experimental psychology, and the cognitive sciences. Paradigmatic exemplars of mechanistic explanation are the explanation of the action potential [
10,
16], and the explanation of neurotransmitter release [
17,
18].
Mechanistic philosophers rarely introduce the scientific work of Cajal as a case study of modern (mechanistic) neuroscience. Churchland [
19] (p. 29) holds that Cajal was “mechanistic,” as opposed to “vitalistic,” in that he had thought that electrical induction (instead of “mystical forces and substances”) could explain all communication between neurons. However, the nineteenth-century sense of “mechanism” is only vaguely related to the new mechanistic philosophy [
20].
1. Malanowski and Craver [
22] review Cajal’s [
3] pioneering judgment that dendritic spines are real entities and not artifacts of the Golgi’s staining technique. According to them, Cajal’s result prompted a transparently teleological question: “why do neurons have spines?” that contributed to the search for neural mechanisms. To my knowledge, Stinson and Sullivan [
23] provide the most complete and revealing study of Cajal’s work from the new mechanistic perspective. They suggest that Cajal’s law of dynamic polarization delivers, at best, a how-possible model of the mechanism of nerve impulse, or a re-description of the dynamic polarization phenomenon, at worst. In this paper, I review the extant mechanistic interpretation of Cajal’s contribution to modern neuroscience. Then, I argue that the extant mechanistic interpretation fails to capture the explanatory import of Cajal’s law of dynamic polarization. My claim is not that Cajal’s mechanistic conjecture was true or even partially true. In fact, most synapses involve communication by neurotransmitters rather than direct electrical induction. My claim is that the definitive formulation of Cajal’s law of dynamic polarization, despite its mechanistic inaccuracies, embodies a non-mechanistic pattern of reasoning (i.e., design explanation) that is an integral component of modern neuroscience. While mechanistic explanations account for how an explanandum phenomenon is brought about by identifying the parts, activities, and organization of the underlying mechanism, design explanations account for why the target system exhibit some morphological features by identifying the functional utility or the survival value of those features. Thus, Cajal’s law of dynamic polarization provides something else than a shallow description of the dynamic polarization phenomenon and a false model of the mechanism underpinning that phenomenon; it gives us the first glimpse into the general design principles that govern the morphology of the nervous system.
2. Cajal under the Mechanistic Lens
In some passages of their paper, Stinson and Sullivan [
23] provide a concise analysis of the discovery strategies and markers of progress of Cajal’s contributions to neuroscience, through the lens of the new mechanistic philosophy. As already mentioned, a proper mechanistic explanation must identify the explanandum phenomenon; typically, a target system and some behavior of that system. Stinson and Sullivan focus in the search for the neural mechanisms of learning and memory. Thus, the lives of Descartes, Pavlov, Cajal, Hebb, and Kandel are woven together into a single narrative of discovery, to the extent that they contributed to the mechanistic explanation of learning and memory. These scientists (including Cajal) were not aiming to discover “general laws” nor “large-scale scientific theories” [
23] (p. 381). They were trying to account for a local phenomenon—with local starting points and termination conditions—by identifying the composing entities, activities, and organizational features of the underlying mechanism.
From the mechanistic lens, Cajal’s work is praised as “a prime example of the decomposition strategy at work” [
23] (p. 382). These authors emphasize the explanatory power of Cajal’s early anatomical findings, mainly obtained with Golgi’s silver staining technique, and artfully represented in his drawings. His early work in the microanatomy of the nervous system of vertebrates helped to convince most anatomists that the brain is made up of anatomically discrete cellular units, or neurons, and showed how different kinds of neurons build different organized patterns in the nervous system. Furthermore, Cajal also contributed to the discovery of many anatomical features of the parts of the neuron, for example, dendritic spines, growth cones, and axon collaterals, among other entities.
However, from the same mechanistic lens, Cajal’s contributions to neurophysiology seems to be relatively disappointing. Stinson and Sullivan [
23] (p. 378) introduce the law of dynamic polarization as the hypothesis that “conduction of impulses travel in one direction only, from dendrite to cell body to axon.” Cajal’s histological methods “did not lend themselves well” to discovering how nerve impulses are communicated between neurons [
23] (p. 379). The discovery of the composing entities and activities of the mechanism that mediates the connection between adjacent neurons were “black boxes” for Cajal [
23] (p. 382). Even worse, like many of his contemporaries, Cajal seemed to believe (falsely) that neurofibrils contained in nerve cells take part in the mechanism of neuronal impulse transmission, “harkening back to Descartes’s account” of the nerve impulse [
23] (p. 379). The silver staining technique could not reveal by itself the functional relevance of Cajal’s anatomical findings. Physiological methods were required to discover the mechanisms that mediate neuronal communication. The mechanistic explanation of the directionality of nerve impulses took off only when the English physiologist Charles Scott Sherrington suggested that there was an intercellular barrier, or synapse, that acted as a valve [
17].
A similar attitude against Cajal’s physiological inductions is endorsed by Berlucchi [
24], who states that Cajal wrongly held that the law of dynamic polarization derives from the structural differences between dendritic arborizations and axons because of not paying due attention to existing neurophysiological studies. In particular, Cajal completely disregarded William James’ [
25] proposal of the law of forward direction, and Sherrington’s [
26] experimental proof that axons in the CNS of mammals can conduct impulses both away from and towards the parent cell body. According to Berlucchi, Cajal’s attitude was detrimental to the progress of neuroscience. The history of modern neuroscience reveals that no single experimental technique, however powerful, can by itself afford the complete explanation of how the nervous system works. In this sense, the integration of multiple scientific fields (or “multidisciplinarity,” in Berlucchi’s terms) is inevitably called for to develop a satisfactory explanation of any phenomenon of interest in the neurosciences. Cajal’s scientific style is, in this respect, an example not to follow.
It would be hard to overestimate the enduring contribution of Cajal’s anatomical findings, not only to the mechanistic explanation of learning and memory but also to almost every research program in the history of modern neuroscience. However, I am afraid that the discovery strategies and the marks of progress of Cajal’s most treasured physiological hypothesis, the law of dynamic polarization, are not evident from the mechanistic point of view. The mechanistic perspective reveals that Cajal’s law either delivers a non-explanatory, phenomenological description of the dynamical polarization phenomenon or provides only a how-possibly explanation, that is, a loosely constrained conjecture about the mechanism that produces the dynamic polarization of the neuron [
27]. The first is true if the law merely describes the behavior of the target system, that is, the direction of the current in many centers of the nervous system, without speculating about the mechanism for that phenomenon. The second is true if Cajal provided an unconstrained conjecture about the relevant mechanism, namely the transmission by contact between the terminal arborizations of a given neuron and the protoplasmic expansions or the soma of adjacent neurons. In any case, the histological approach to the nervous system preferred by Cajal could not enable him to intervene appropriately in the activities of the nerve cell and its proper parts to reveal the productive continuity of the mechanism of impulse transmission [
9]. Thus, in due course, his approach was respectfully left behind.
3. The History of the Law of Dynamic Polarization I: The Search for Generality
I contend that the output of Cajal’s lifetime investigations of the law of dynamic polarization is something other than a few scratches on the surface of the phenomenon of the nerve impulse. My argument can be summarized as follows: the mechanistic construal of Cajal’s law does not take into account the evolution of Cajal’s thought on the subject. In particular, Stinson and Sullivan [
23] (p. 378) state Cajal’s law as follows: “conduction of impulses travel in one direction only, from dendrite to cell body to axon.” This formulation corresponds to the original hypothesis supported by Cajal [
5]. Let’s call it the “LDP-1891”. According to the LDP-1891, the dendritic expansions and the soma of the nerve cell have
cellulipetal conduction, while the axis-cylinder and its terminal arborizations have
cellulifugal conduction. However, Cajal was aware that the LDP-1891 faced several empirical anomalies. Consequently, in ref [
6], Cajal replaced it with a new law of
axipetal polarization, according to which the conduction in dendritic processes and the cell body is axipetal (i.e., towards the axon); whereas the conduction in the axon is
dendrifugal and
somatofugal (i.e., it comes from the dendrites and the cell body). Let’s call this new formulation the “LAP-1897”. In the following years, Cajal’s investigations deepened further into the utilitarian significance of the axipetal polarization phenomenon. Finally, in ref [
28], Cajal arrived at a formulation of the law that clearly emphasized its role as a principle of neural design. According to this definitive formulation, “impulses are exactly polarized in the neuron, and take the
minimum path between their point of entry and the origin of the conductor that distributes them.” Let’s call it the “LAP-1899”. My claim is that the LAP-1899 is more than a surface description of the dynamic polarization of the neuron. It also embodies an optimization principle that governs the design of morphological configurations in the nervous system. As such, the LAP-1899 represents a seminal contribution to the wiring optimization approach in modern neuroscience [
29]. To motivate this reinterpretation of Cajal’s physiological contributions, I need to remove some significant epistemological obstacles.
The first obstacle is something of an elephant in the room. In many ways, Cajal was intensely involved in the search for general, physiological laws and the development of large-scale physiological theories in neurobiology. Of course, he was a “fervent adept of the religion of facts” and used to say that “facts remain while theories pass away” [
30] (p. 307). In particular, he claimed that first-hand, anatomical facts remained fixed and stable while their physiological interpretation was subjected to debate and speculation [
30] (p. 309). Furthermore, he was well aware of the perils of generalization by extrapolation of results, be it from one experimental technique to another, or from one animal species to another, or from one nerve cell to another of the same kind but in a different developmental stage. However, Cajal thought that the definitive formulation of the law of dynamic polarization was “applicable to all cases without exception”: to vertebrates and invertebrates, to the adult and the embryo. Indeed, “thanks to its complete generality, it constitutes a valuable key to the interpretation of the courses of the currents in neurons of the nerve centers” [
30] (p. 199). Today, we know that the law of dynamic polarization is a mechanistically fragile generalization and that there are plenty of exceptions to the dynamic polarization rule. In many neurons, nerve impulses can travel backward from the axon and soma regions into the dendrites [
31]. However, it is undeniable that Cajal was seeking generality when he ventured into the physiology of the neuronal forest.
A second obstacle is the slightly anachronistic tendency to assess the importance of Cajal’s law from the standpoint of Sherrington’s theory of the synapse. Considered as a general physiological principle, Cajal’s law had a tremendous impact when it was first formulated [
8]. The law defined the dynamic schema in which any future investigation would have to be situated (it restricted “the space of possible mechanisms,” in Craver’s terms). Since the law depended on the structural autonomy of the neuron, it complemented the neuron doctrine by establishing the functional unity of the neuron. It had a broad heuristic scope. It ruled out Golgi’s assumption of the trophic function of the dendrites. It was coherent with the available knowledge about the embryogenesis and the evolution of the nervous system. In a similar vein, my view is that the law of dynamic polarization, correctly formulated, constituted a general principle of neural design, one that enabled Cajal to envision design explanations of the organization of neuronal elements in many regions of the nervous system. The explanatory role of Cajal’s law that I emphasize here is not directly related, then, to the mechanistic progress in the search for the underlying mechanism of the nerve impulse.
The third obstacle is to take for granted the content of the law of dynamic polarization. The mechanistic interpretation has focused exclusively on the LDP-1891. However, as I mentioned before, the law was “forged little by little, after many attempts and rectifications” [
30] (p. 197). The content of the LDP-1891 is partially different from the content of the LAP-1897. In the latter, the cell body of the nerve cell has lost its centrality as an intermediate station of the nerve impulse. Likewise, the definitive formulation of the LAP-1899 differs from the formulation of the LAP-1897, in that the former accentuates the generality of the law as a principle of neural design. The explanatory deficiency of the original formulation is uncontestable: the LDP-1891 is plainly false, as Cajal himself admits. Thus it can hardly explain anything. Fictions might have some explaining role to play in the sciences [
32], but it is not necessary to take such a bold move to vindicate the explanatory power of Cajal’s approach. In this paper I develop a different strategy.
In the ref [
5] paper: “Significación fisiológica de las expansiones protoplasmáticas y nerviosas de las células de la sustancia gris,” Cajal starts by praising Golgi’s discovery that the protoplasmic expansions (or dendrites) of the nerve cells do not anastomose to each other but end freely within the grey matter. Regarding the axis-cylinders or axons, despite Gerlach’s and Golgi’s doctrine that axons fuse with one another forming a diffuse reticulum within the grey matter, Cajal contends that direct observation never shows such a network of nerve fibers. He studied the texture of the nervous systems of embryos, where one can follow the course of the nerve fibers to their termination points. He observed that axis-cylinders and axon collaterals terminate in free arborizations around many cells of the cerebellum, the spinal cord, the retina, the olfactory bulb, the optic lobe of birds, and so on. After such a revolutionary change in anatomical ideas, scientists expected a similar change in the physiological schema of nerve transmission through the nervous system. Some contact or induction must realize the communication of action from one cell to another. So the questions Cajal is interested in are the following: Which is the direction of the currents that run through the neuron? Do the currents run from the axis-cylinder to the cell and the protoplasmic expansions, or do they move backward, from the dendrites and through the axon to the terminal arborizations of the axon?
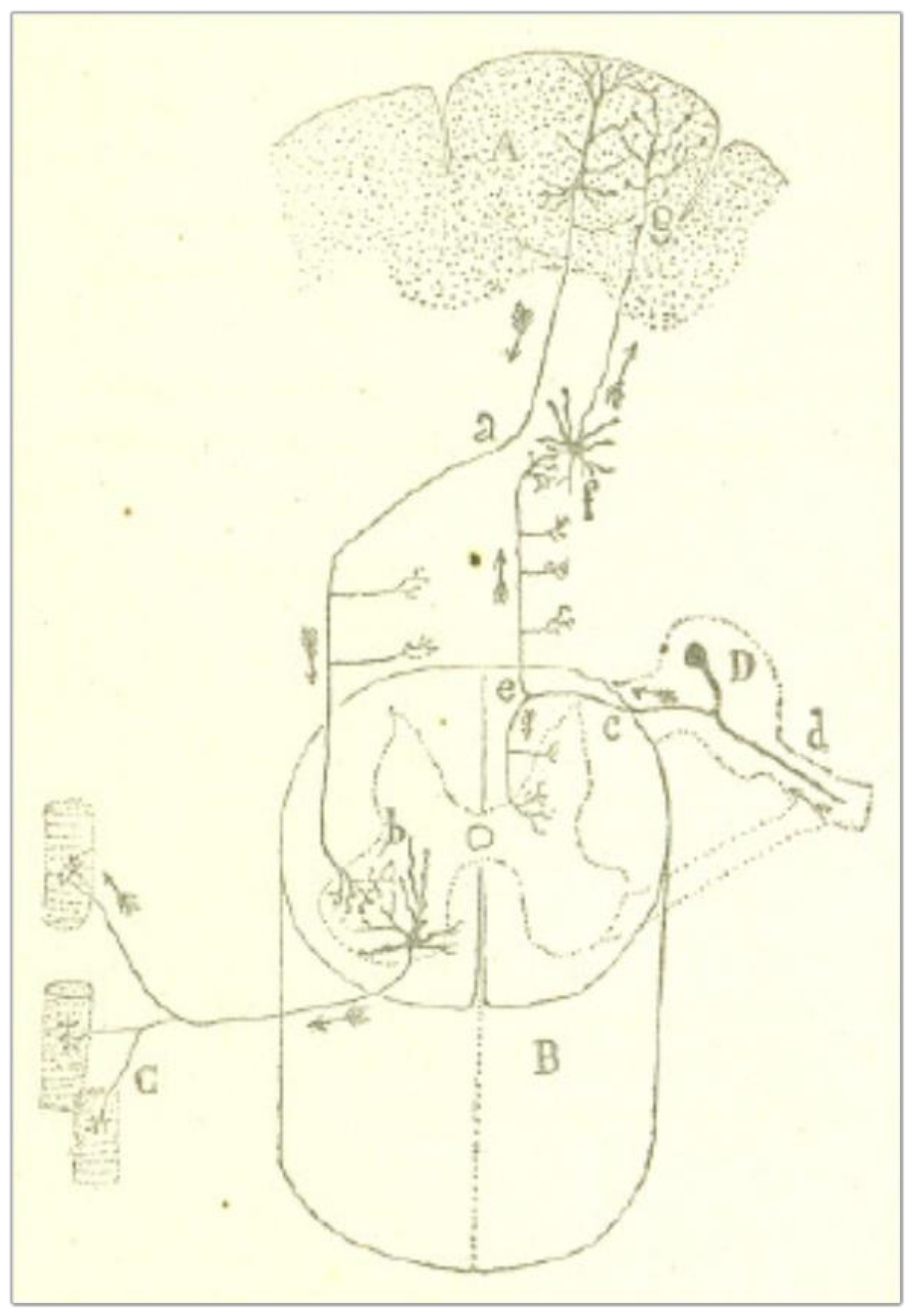
Cajal’s strategy to solve the problem is to imagine, in the first place, the direction of the current on those nerve organs, such as the retina, the olfactory bulb, and the central motor pathways, in which the starting point of the nerve impulse is well-known. Let us consider the olfactory mucosa and the olfactory bulb (
Figure 1). The nerve impulse begins from the protoplasmic expansions of the bipolar cells (a) and goes through a very thin axon to the corresponding glomerulus (b). From there, the excitation is received by the protoplasmic branches of the large pyramidal cells within the bulb (c), which transmit the impulse through thick axons towards the olfactory bulb (d). It is evident that dendritic arborizations always receive the nerve impulse and send it by nerve branches, all the way into the brain.
One can observe the same functional polarization in centrifugal pathways, the main example being the central motor pathway in the brain and the spinal cord (
Figure 2). According to Cajal [
6] (p. 6), the “volitional act” (that is, the current that voluntary movement must produce) is transmitted to the pyramidal cells of the psychomotor region of the cerebral cortex (A); from here it goes down along the pyramidal tract (a) to the anterior horn of the spinal cord (b). The downward movement is then picked up by the protoplasmic expansions of the motor neurons of the spinal cord and transferred to the muscle fibers (C), where the terminal arborizations of the axons are located.
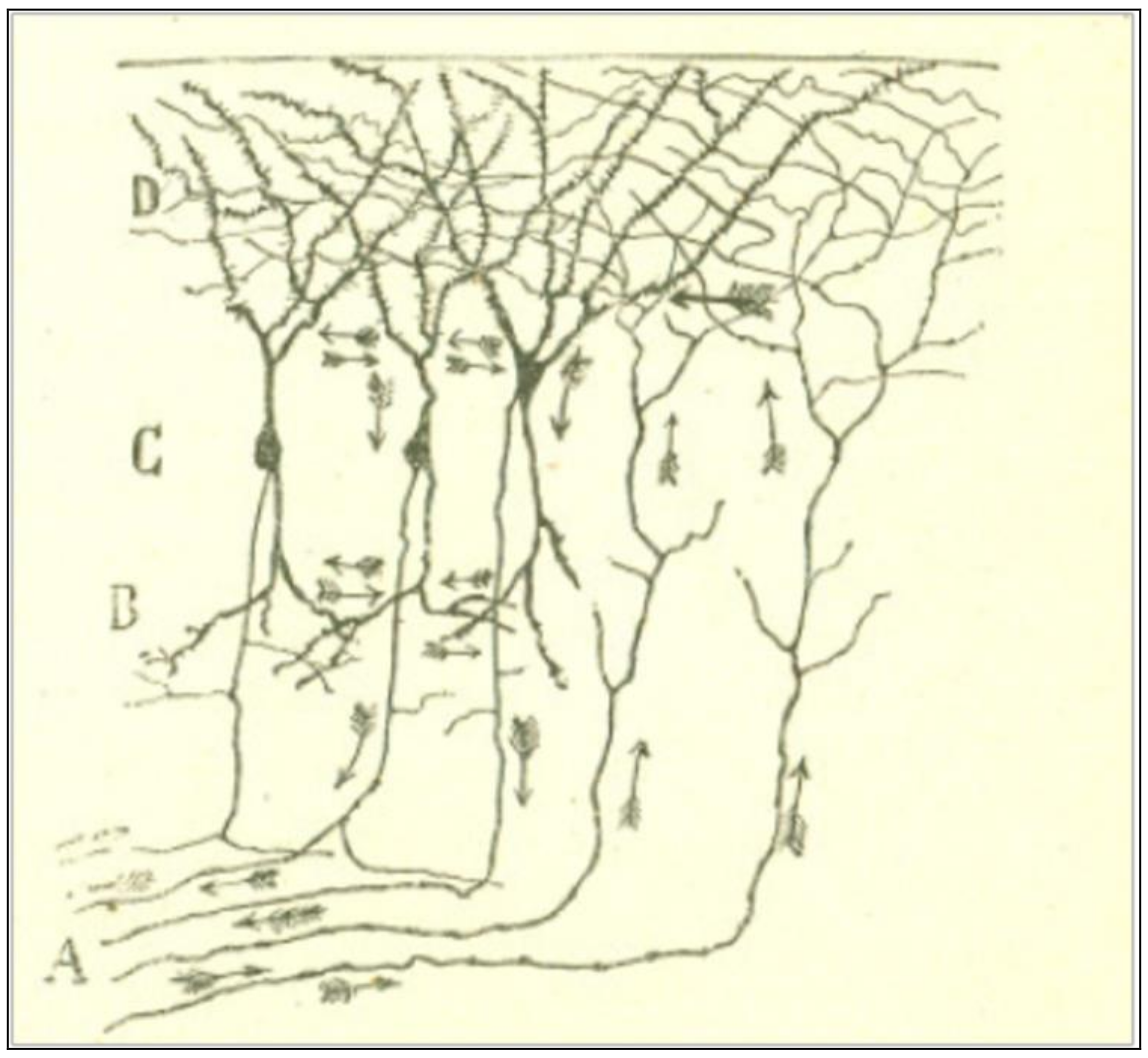
By analogical reasoning, Cajal [
5] holds that the same functional polarization is observed in central pathways of the nervous system, for example, in the cerebral cortex of lizards (
Figure 3). It is known that the pyramidal cells of the cerebral cortex send a protoplasmic stem to the molecular layer of the grey matter, which ramifies into a plexus of thick, tiny branches. Many other nerve fibers, be it from the same layer, or from cells from deeper layers, or even from sensitive tubes from the white matter, also ramify in the same region. Thus, it is easy to conceive that the centrifuge movement starts in the dendrites of the pyramidal cells and goes next through their axonal expansions down the pyramidal pathway.
Every cluster of nerve cells within the grey matter seems to confirm van Gehuchten’s [
33] conjecture (inspired by Cajal’s findings) about the direction of the nerve impulse. The general fact is that dendrites drive the impulse somatopetally, towards the cell body, while the axis-cylinder drives the impulse somatofugally, away from the cell body. This audacious conjecture, empirically supported by Cajal’s anatomical discoveries, constitutes the content of the LDP-1891.
Cajal [
5] acknowledges that there are several objections against the empirical generality of the LDP-1891. The first anomaly is related to the centripetal conduction of the peripheral branch of unipolar spinal cells (
Figure 4D). Cajal finds it to be evident that the current in the peripheral branch of the axonal expansion must run towards the cell body and, from there (or from the common stem), towards the central fiber. The cellulipetal conduction of the axon violates the LDP-1891 since it implies that the stem admits both a cellulifugal and a cellulipetal movement. Cajal deals with this anomaly in a very peculiar way, by a rational interpretation of the ganglion cell morphology from the viewpoint of its ontogenetic and phylogenetic evolution. The peripheral expansion of monopolar sensitive cells in adult vertebrates has the anatomical features of an axon. But, if we descend through the animal series and consider the sensitive cell in lower animals, like worms or mollusks, or if we analyze the neuron in its embryonic state, it exhibits a bipolar morphology, with an external expansion that has, according to Cajal, the distinctive features of a dendrite.
The second anomaly is mentioned by Cajal in his argument against Golgi’s physiological doctrine of the purely vegetative function of dendritic arborizations. He comments that, in some crook-shaped cells of the optic lobe of lower vertebrates, the axon is born not from the cell body but from a protoplasmic branch, and far away from the origin of that branch in the cell body (
Figure 5c). Such a disposition implies that there is cellulifugal conduction in the dendritic stem from its origin in the cell body to the point at which the axon sprouts, thus violating the LDP-1891. Cajal does not provide the solution for this anomaly in the ref [
5] (1891) paper.
The definitive formulation of the LDP-1891 is as follows: the transmission of nerve movement runs from the protoplasmic branches and the cell body towards the nerve expansion. In functional terms, every nerve cell has a reception device (cell body and protoplasmic arborizations), a conduction device (the axis-cylinder), and an application device (the terminal arborizations of the axis-cylinder). The intended scope of this functional law includes every nerve cell of every gray matter center in the nervous system.
4. The History of the Law of Dynamic Polarization II: Cajal’s Glimpse into Neural Design
From the very beginning of the ref [
6] (1897) paper: “Leyes de la morfología y dinamismo de las células nerviosas,” Cajal admits that the ref [
5] formula of the LDP-1891 is not correct. It applies only to sensitive bipolar cells, and to all other neurons whose dendritic expansions sprout from the cell body. The generalization is false whenever the soma of the neuron is dislocated and placed away from the axis-cylinder, as in ganglion cells, or whenever the axon sprouts from a protoplasmic branch, as in the crook-shaped cells of the optic lobe. Encouragingly, Cajal provides a rectification of the law, which applies without exceptions across the animal series. The law of axipetal polarization, or LAP-1897, states that the conduction in dendritic processes and the cell body is axipetal (i.e., towards the axon); whereas the conduction in the axon is dendrifugal and somatofugal (i.e., it comes from the dendrites and the cell body). Note that, according to the LAP-1897, impulses collected by dendritic expansions do not need to pass always through the cell body of the neuron, but can go directly to the axon, where they follow their march towards adjacent neurons. While the LDP-1891 hypothesized a cellulipetal direction of the current in the dendrites and an axipetal course in the soma, the LAP-1897 assigns the same functionality both to the dendrites and the cell body. In this way, the dendritic expansions and the cell body represent a system of convergent currents, the axis-cylinder represents a channel of parallel currents, and the terminal arborizations of the nerve constitute a bundle of divergent currents. The new formulation appears to be entirely general, that is, applicable to all cases without exception. Crucially, it solves the anomalies of the LDP-1891.
Consider the fusiform, crook-shaped neuron of the optic lobe of birds (
Figure 5) again. The nerve expansion sprouts from the upper end of a protoplasmic branch, to descend next to the deep layer of nerve fibers. If we assume that the dendrites transmit the impulse only centripetally, then the cellulifugal current of the dendritic stem (from the cell body to the axon) would violate the LDP-1891. However, if we assume the LAP-1897, the puzzling character of the morphology of the neuron disappears, and its structure fits the general dynamical plan. The nerve impulse does not need to go across the cell body. Indeed, the placement of the soma may vary from neuron to neuron, following two different determinants: the need to connect specific neurons, on the one side, and the need to save space in the system, by settling the nucleus in the place where there are fewer terminal arborizations.
Regarding the ganglion cells of vertebrates (
Figure 4D), the LDP-1891 required us to suppose that the main stem admits both a cellulipetal movement from the peripheral expansion and a cellulifugal current from the cell body towards the central axonal expansion. We can avoid this puzzling result if we consider the arrangement as an instance of the LAP-1897. The sensitive excitation received by the peripheral expansion (D) would go directly to the medulla (M), without going through the cell body, and the conduction of the soma and the main stem would be equally axipetal. Cajal provides a rational interpretation of the morphology of the cell again, suggesting that the cell body seems to have moved away from the bifurcation fiber to satisfy the need to save conduction time and material. To motivate this rational interpretation, he recalls the paradoxical phenomenon exhibited by ganglion cells (
Figure 6).
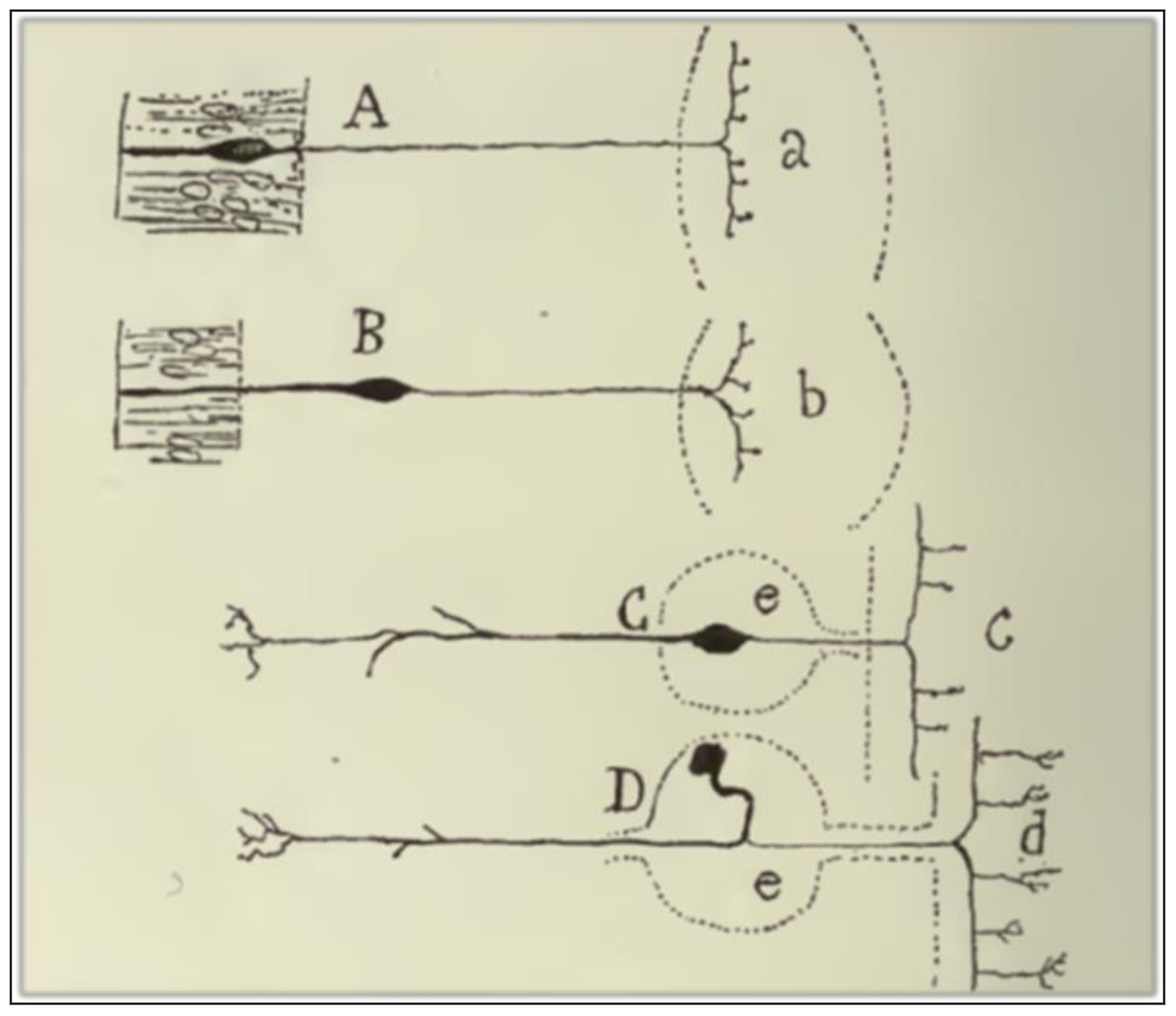
In both ontogenetic and phylogenetic evolution, sensitive ganglion cells shifted from a bipolar shape to a monopolar configuration, that is, from a relatively complex morphology to a relatively simple form. The comparison between the schemes in
Figure 6 reveals the differences in wiring length and, thus, the differences in conduction time, between the ganglion cells of fish and those of higher vertebrates. The bipolar form of the neurons in scheme 6A requires the nerve expansions and the peripheral expansions of the cells to follow a winding path across the cell bodies, lengthening the itinerary of the excitation. In
Figure 6B, of a mammal, cell bodies have taken shelter in the periphery, moving away from the central region of the ganglion, where the sensitive conduits are arranged in rectilinear bundles. Such an arrangement is, to Cajal’s eyes, the most complete and ingenious application of the law of economy of conduction time. In an exercise of Darwinian morphology, Cajal argues that such a disposition is advantageous for the animal, given the patent benefit that high speed in the conduction of tactile and painful impressions must have brought in higher vertebrates in their struggle for existence.
Cajal [
6] highlights that a good indicator of the correctness of a theory is that it contributes to the discovery of new (even more fundamental) principles of explanation applicable to new phenomena. That seems to be the case with the law of dynamic polarization, once we purged it from the mistaken idea that the cell body must necessarily participate in the conduction of incoming currents from the dendritic arborizations. Revealingly, Cajal [
28] asks himself why he could not come up with the LAP-1897 from the start. Why did he maintain the cellulipetal theory for so long? Why did he consider the cell body to be the indispensable center for neuronal conduction? Surprisingly, the answer is that the main epistemological obstacle in the way to the LAP-1897 was the cell theory; in particular, the idea that the cell body is the cell itself, that is, the entire cell, despite the discovery of the omnipresence of enormous dendrites in grey matter. Van Gehuchten noted that, by removing the soma from an obligatory role in nerve conduction, “Cajal abandoned what one refers to as the physiological unity of the neuron” (quoted by [
2] (p. 14)).
The LAP-1897 accounts for the anatomical positioning of the dendrites and the terminal arborizations of the axon; everything else about the anatomy of the nerve cells, including the positioning of the soma, appears to be variable and accommodative. Cajal [
28] (p. 102) raises the following question: “Are these variations merely whims of Nature, arrangements without importance, or have they some physiologic significance?” He answers that every empirical evidence suggests that the anatomical variations in question are of actual use to the dynamics of the system that exhibits them, that is, that they respond to some physiologic design, and are thus the output of evolutionary (maybe Darwinian) mechanisms. The observed displacements are, thus, “morphologic adaptations ruled by laws of economy of time, space and matter” [
28] (p. 102).
The laws of economy of time, space, and matter represent Cajal’s ultimate “inductive effort” to glimpse into the “utilitarian principles” that seems to govern the infinite varieties of form, size, position, and direction of neurons and nerve fibers [
30] (p. 314). As utilitarian principles, these laws might provide a design explanation of all those seemingly capricious variants of the point of emergence of the axon (crook-shaped cells), as well as a design explanation of the dislocation or migration of the cell body during evolution and development (sensitive ganglion cells). They also explain phenomena like the concentration of sensitive and motor neurons in ganglia, the bifurcations in the form of a Y (instead of a T) of the nerve fibers upon their arrival at the posterior cord, and the point of origin of the axon in the dendritic arborizations of the cerebellum grains [
6].
In the final evolution of his thought, Cajal [
28] adds that the laws of economy not only represent constraints in the design of the microanatomy of the nervous system but also are intermingled with the phenomenon of axipetal polarization itself. From a teleological point of view, these principles postulate the construction of the shortest path among connected neuronal territories, with the corresponding time saving as a dynamical consequence, as well as the avoidance of useless holes in grey matter. Cajal characterizes the polarization of the neuron as the general fact that, under normal conditions, “only impulses of the same direction can travel through its appendages” [
28] (p. 116). It becomes evident that, by ruling out impulses of opposite direction travelling through the same appendages, nature wanted to avoid all the interference of impulses with each other. Cell polarization is a constant phenomenon in the nervous system of living, healthy organisms because it represents the answer to the need to avoid transmission interference in organisms that exhibit a differentiation of a surface of reception (skin and senses) and a surface of emission (muscles and glands).
Cajal [
28] (p. 88) synthesizes the content of the law of axipetal polarization in a way that emphasizes the tradeoffs between different design principles and the teleological aspects of the cell polarization phenomenon. Specifically, he states the law of axipetal polarization as an optimization principle. An optimization principle is a general constraint on the space of possible solutions to an optimization problem. An optimization problem requires designing a structure able to carry out a given task within a given operating context, with a minimum cost. According to Cajal [
28], the optimization problem imposed on the organism seems to be the following: to build, with the minimal amount of matter, within the minimum containing space, the most richly differentiated nervous machinery of the most rapid, robust, and efficient reactions. Thus, the definitive formulation of the law of axipetal polarization is as follows [
28] (p. 88):
The LAP-1899: “Impulses are exactly polarized in the neuron, and take the minimum path between their point of entry and the origin of the conductor that distributes them.”
In the living, healthy animal, Cajal asserts, impulses are collected either for dendrites or the cell body; then, they are conducted through these protoplasmic portions by the shortest path to the axon, which distributes them using its multiple arborizations. There is no need to pass through the cell body if it is not in the most direct route from the dendrites to the axon.
5. The Structure of Design Explanation
To fully understand the explanatory import of the LAP-1899 one needs to answer some of the following questions: How do design principles explain in neuroscience? What are the norms of design explanation? And, especially: How do optimization principles explain? A rigorous and complete answer to these questions would require another paper. In the meantime, I hold that Wouters’s [
34,
35] philosophical analysis of design explanation in functional biology gets us on the right track.
Design explanations are usually brought up in answer to explanatory demands in functional biology, that is, that part of biology that is concerned with the way individual organisms are built (e.g., anatomy, morphology), the way they work (e.g., physiology), and the way they behave (e.g., ethology). The basic idea is that design explanations purport to explain why specific organisms have certain traits “by showing that their actual design is better than contrasting designs” [
35]. The hallmark of design explanations is their concern with the utility of a particular trait, often in comparison with merely possible alternatives.
Concerning the explanandum of a design explanation, Wouters [
34] claims that design explanations are answers to questions of the following form: (Q) “Why do
s-organisms have t
1 rather than t
2, t
3, …, t
n?”, where
s is a set of organisms (that might be taxonomically heterogeneous), t
1 is the trait in question (i.e., the presence or character of a specific item or behavior) of
s-organisms, and t
2, t
3, …, t
n are the alternative traits. Design explanations are explicitly or implicitly contrastive: they compare real organisms to hypothetical organisms that may not exist. Questions like (Q) are not questions that ask about causes at the level of individual organisms. Neither are they questions that ask about evolutionary causes at the level of the population. The hypothetical alternatives to the actual trait may have never existed. Questions like (Q) ask about the utility of a character regarding what is needed or useful to stay alive, that is, to maintain the organism, to grow, to develop, and to produce offspring.
Regarding the explanans of a design explanation, the core of an answer to a (Q)-question has the following structure: (C)
s-organisms live in condition
c; and (U) In condition
c the trait t
1 is more useful than traits t
2, t
3, …, t
n, where
c is a conjunction of one or more conditions of organisms and/or environments in which organisms live. Statements like (C) specify conditions that apply to the relevant organisms, and statements like (U) claim that, due to those conditions, the trait in question is more useful to the s-organisms than the alternative traits [
34].
Claims about utilities may vary in strength. Simple design explanations can be classified either as optimization or as requirement explanations, depending on whether the utility claim is an optimization claim or a requirement claim [
34]. A requirement claim has the following form: “In condition
c, trait t
1 is the only useful one among the following traits: t
1, t
2, t
3, …, t
n.” Requirement claims assert that the trait in question is the only one in the reference class that works. Many design explanations derive requirements directly from the laws of physics and chemistry. In contrast, an optimization claim is relatively weaker and has the following form: “In condition
c, trait t
1 is more useful than each of the following traits: t
2, t
3, …, t
n”. Optimization claims assert that the trait in question is the best one in the reference class. Optimization explanations explain why the optimization claim holds, that is, they indicate the disadvantages that the organisms in question would have in the conditions in which they live if an alternative replaced the trait in question.
The train of thought of design explanation is present in Cajal’s physiological reasoning. Why do sensitive ganglion cells in adult mammals exhibit a monopolar morphology (rather than a bipolar or multipolar morphology)? Because there is, in normal conditions, a need to save material, space and time conduction, and moving the cell bodies to the periphery of the ganglion while arranging the sensitive conduits in rectilinear bundles in the central region represents the most advantageous disposition to satisfy that need. Why don’t the nerve impulses pass through the cell body in the sensitive ganglion cells of mammals? Because there is, in normal conditions, a need to save time conduction, and the direct path from the terminal arborizations to the medulla is the minimum path between the point of entry of the impulse and the origin of the conductor that distributes it. Why do sensitive and motor neurons concentrate in ganglia in invertebrates, as Spencer noticed in 1896? Because there is a need to economize protoplasm and space, and the successive concentration of initially separated neurons economizes conductors and allows one fiber to communicate the excitation to a high number of neurons. Why do the nerve fibers bifurcate in the form of a Y (instead of a T) upon their arrival at the posterior cord? Because there is, in normal conditions, a need to save conduction time, and the Y-shaped bifurcation represents the shortest path between the entry point and the exit point of the excitation.
Note that design explanation is not a kind of mechanistic explanation. Mechanistic explanations aim to account for how a specific capacity of a target system is brought about by identifying the composing entities, activities, and organization of the underlying mechanism. In contrast, design explanations aim to account for why parts exhibit specific morphological features, why biological roles are performed the way they are, and why the anatomical elements are organized as they are rather than in some other conceivable way.
6. Concluding Remarks
Cajal did not regard the LAP-1899 as the schematic description of the mechanism that underlies neuronal communication, nor as a phenomenological model of the dynamical polarization phenomenon itself, but as a thread in a web of mutually constraining principles of neural design.
Modern neuroscientists know that the wiring cost of connections in the brain has multiple origins: it arises from volume, metabolic requirements, signal delay and attenuation, and guidance defects in development, among other factors [
29,
36]. Since long-range connections in the brain are a limited resource, it is expectable that, other things being equal, nature would prefer the layout with minimum wiring cost. From this point of view, we can interpret the different organizations of neural components within the brain as solutions to specific wiring minimization problems constrained by a given neuronal connectivity. This principle of neural design is known as the wiring optimization principle [
37]. In general, the wiring optimization principle states that, given a fixed connectivity pattern between neural components within the brain, the wiring cost determines neuronal architecture.
Neuroscientists have used the wiring optimization principle to generate a whole family of optimization models that account for many features of brain organization, to wit: why there are separate visual cortical areas [
38,
39], why the neocortex folds in a characteristic species-specific pattern, why neurons in the mammalian visual cortex are organized into multiple maps [
36], why ocular dominance patterns exist, why we found orientation preference patterns in the visual cortex, why axonal and dendritic arbors have particular branching angles [
40], and why axons and dendrites occupy a 3/5 fraction of gray matter [
41], among many other phenomena. Some modern neuroscientists have argued that the reduction of path lengths appears to be at least as necessary as the minimization of wire length. Long distance connections are metabolically expensive, but they have the benefit of reducing the number of intermediate transmission steps in neural pathways [
37]. The benefits of processing efficiency obtained by adding long-distance projections might outweigh the wiring costs of establishing those additional connections.
In any case, Cajal’s foundational inquiries into the phenomenon of dynamic polarization and the laws of economy of space, time, and matter constitute the starting point of a scientific tradition aimed at discovering what the principles of neural design are, and how they relate to each other, to explain the anatomy and morphology of the nervous system at different levels of organization.