Preliminary Results on the Daily and Seasonal Rhythms of Cuttlefish Sepia officinalis (Linnaeus, 1758) Locomotor Activity in Captivity
Abstract
:1. Introduction
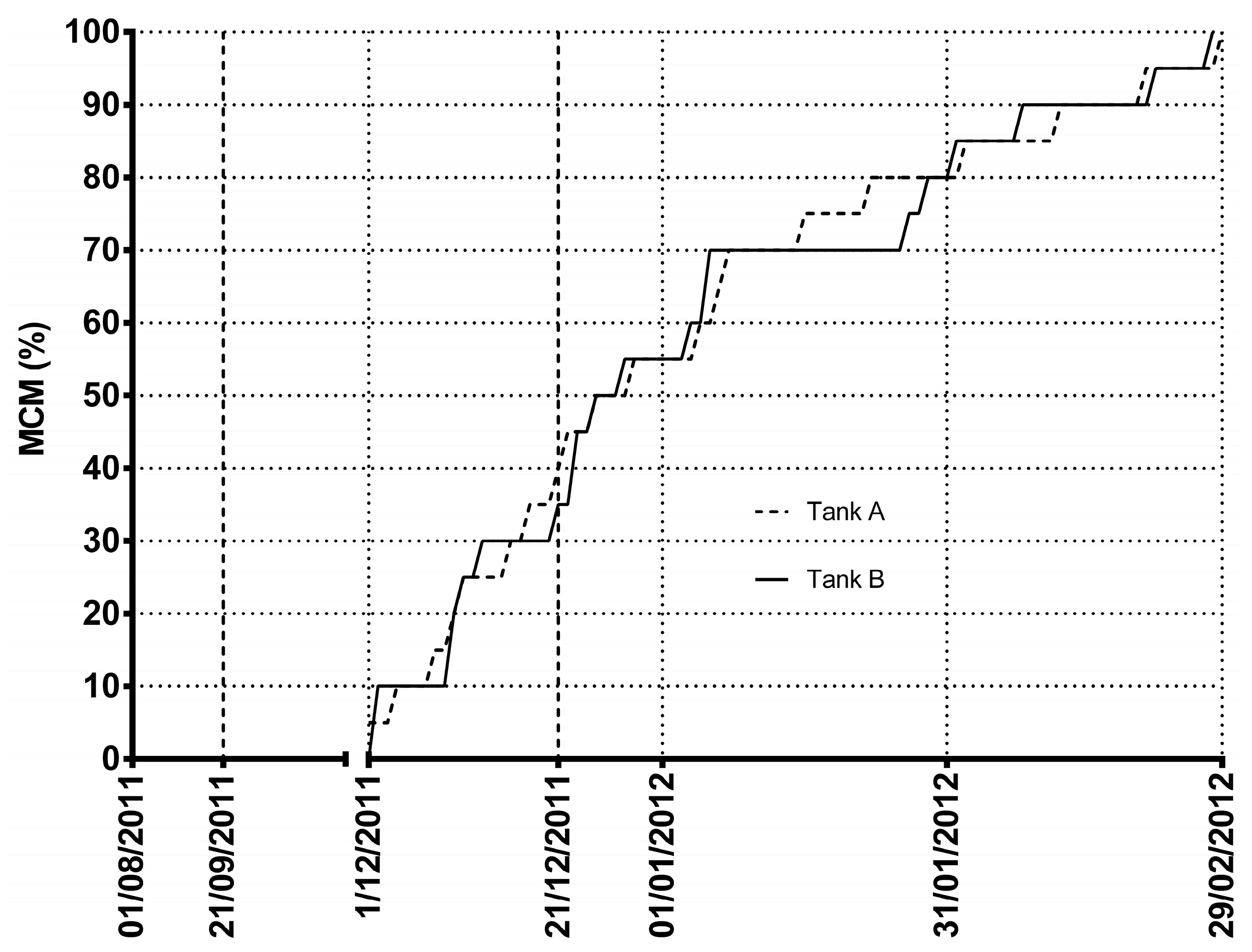
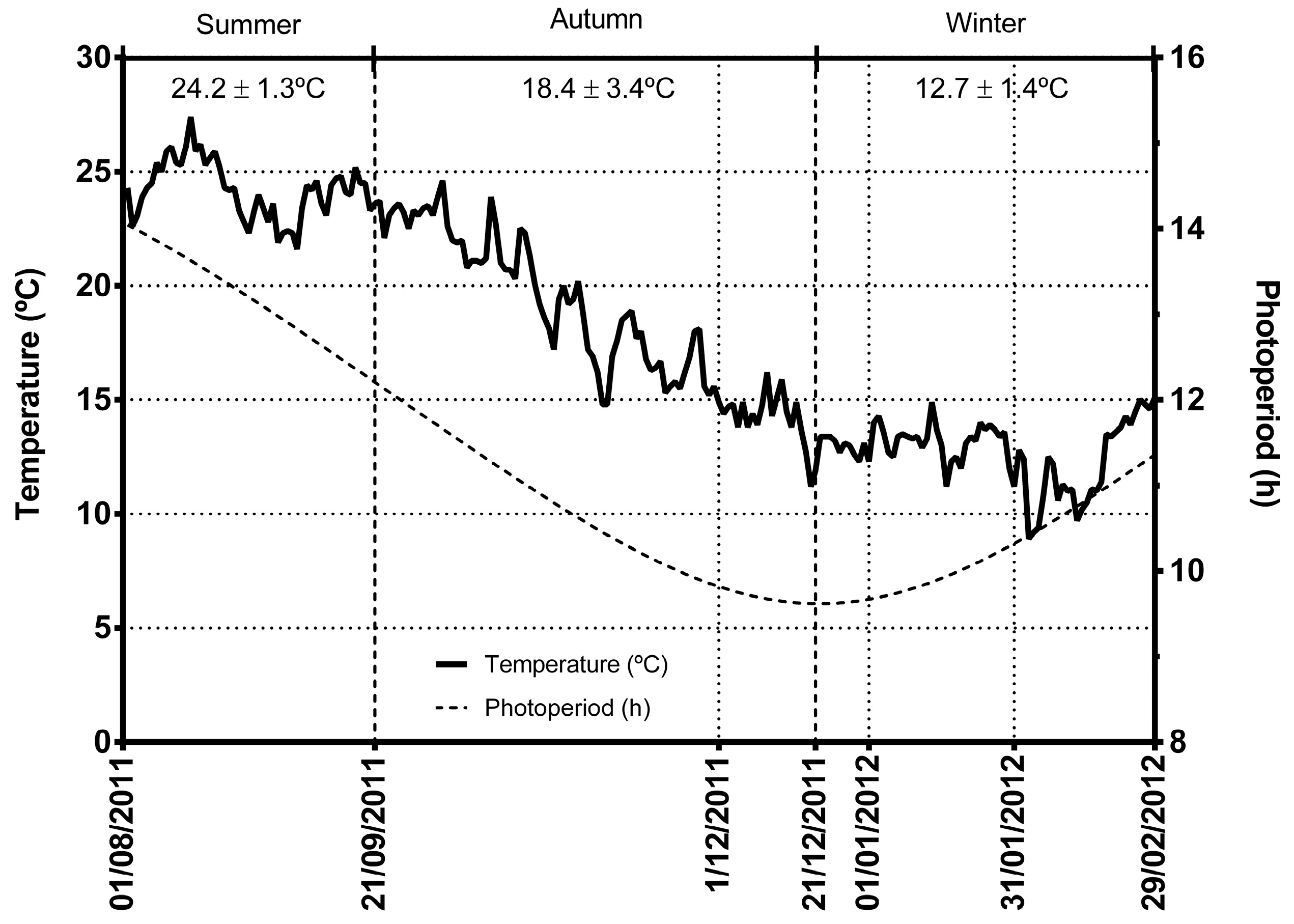
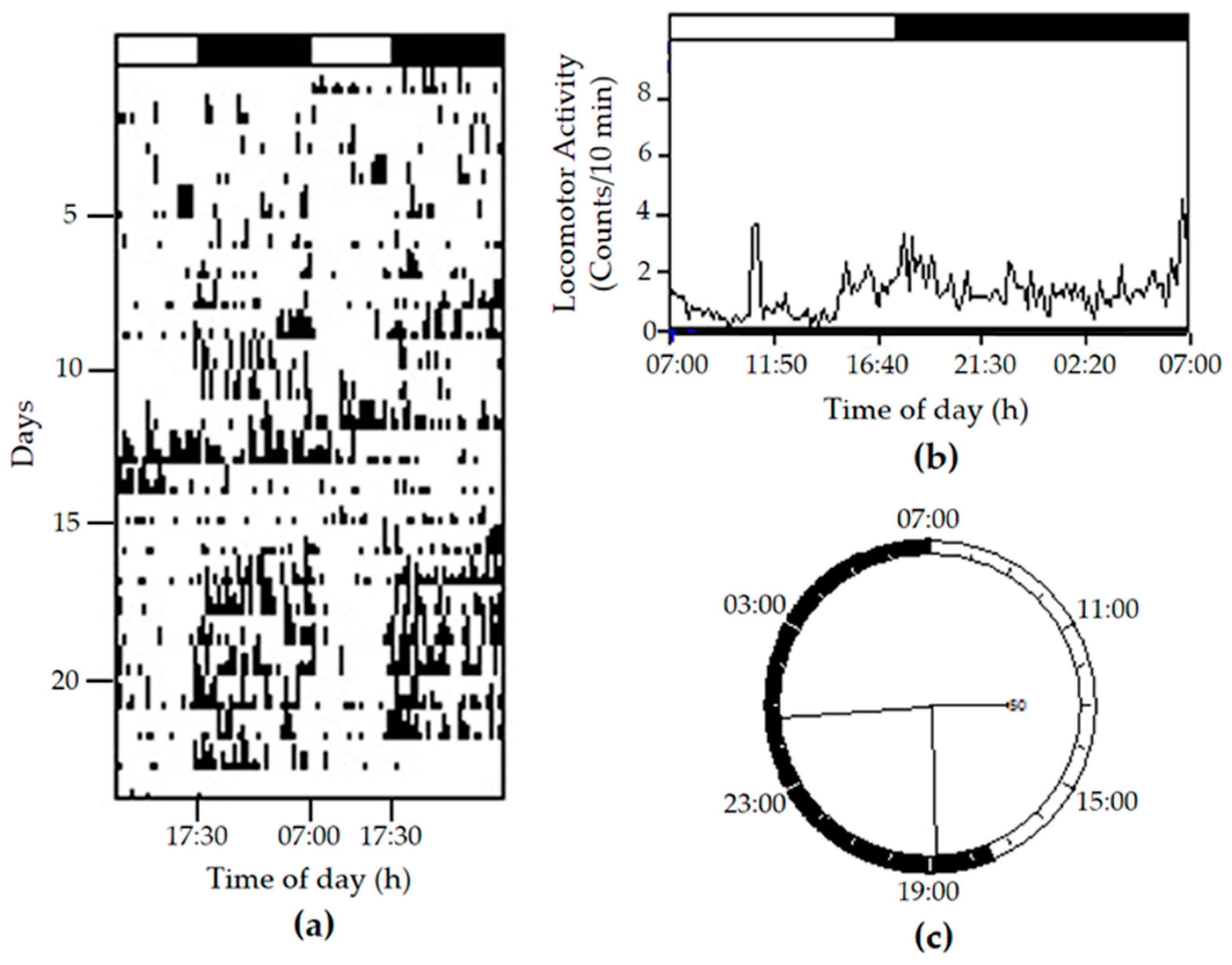
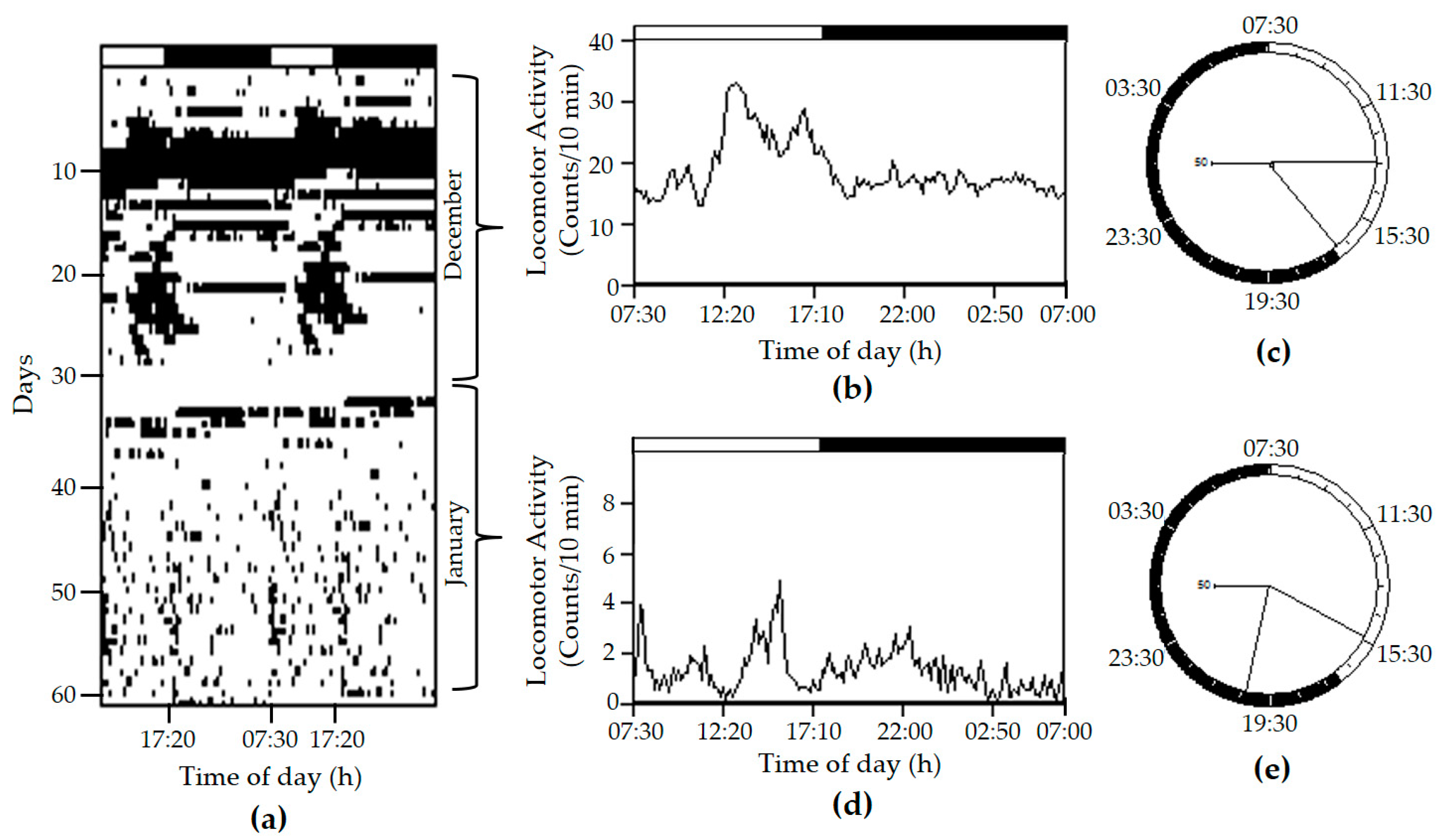
2. Results and Discussion
3. Materials and Methods
3.1. Ethical Statement
3.2. Experimental Design
3.3. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barnabé, G. Bases Biologiques et Écologiques de L’aquaculture; Editorial Acribia, S.A.: Zaragoza, Spain, 1996; p. 519. [Google Scholar]
- Iglesias, J.; Fuentes, L.; Villanueva, R. Cephalopod Culture; Springer: Dordrecht, The Netherlands, 2014; p. 494. [Google Scholar]
- Villanueva, R.; Sykes, A.V.; Vidal, É.A.G.; Rosas, C.; Nabhitabhata, J.; Fuentes, L.; Iglesias, J. Current status and future challenges in cephalopod culture. In Cephalopod Culture; Iglesias, J., Fuentes, L., Villanueva, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 479–489. [Google Scholar]
- Sykes, A.V.; Koueta, N.; Rosas, C. Historical review of cephalopods culture. In Cephalopod Culture; Iglesias, J., Fuentes, L., Villanueva, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 59–75. [Google Scholar]
- Fiorito, G.; Affuso, A.; Anderson, D.B.; Basil, J.; Bonnaud, L.; Botta, G.; Cole, A.; D’Angelo, L.; De Girolamo, P.; Dennison, N.; et al. Cephalopods in neuroscience: Regulations, research and the 3rs. Invertebr. Neurosci. 2014, 14, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.S.; Pope, S.K.; Williamson, R. Circadian rhythms to light-dark cycles in the lesser octopus, Eledone cirrhosa. Mar. Freshw. Behav. Physiol. 1995, 26, 47–57. [Google Scholar] [CrossRef]
- Cobb, C.S.; Williamson, R.; Pope, S.K. The responses of the epistellar photoreceptors to light and their effecyt on circadian rhythms in the lesser octopus, Eledone cirrhosa. Mar. Freshw. Behav. Physiol. 1995, 26, 59–69. [Google Scholar] [CrossRef]
- Meisel, D.V.; Byrne, R.A.; Kuba, M.; Griebel, U.; Mather, J.A. Circadian rhythms in Octopus vulgaris. Berliner Paläobiol. Abh. 2003, 3, 171–177. [Google Scholar]
- Meisel, D.V.; Byrne, R.A.; Kuba, M.; Mather, J.; Ploberger, W.; Reschenhofer, E. Contrasting activity patterns of two related octopus species, Octopus macropus and Octopus vulgaris. J. Comp. Psychol. 2006, 120, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, G.; Affuso, A.; Basil, J.; Cole, A.; de Girolamo, P.; D’Angelo, L.; Dickel, L.; Gestal, C.; Grasso, F.; Kuba, M.; et al. Guidelines for the care and welfare of cephalopods in research—A consensus based on an initiative by cephres, felasa and the boyd group. Lab. Anim. 2015, 49, 1–90. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G.; Waldrop, R.H.; Dumoulin, M.; Aton, S.; Boal, J.G. A preliminary analysis of sleep-like states in the cuttlefish sepia officinalis. PLoS ONE 2012, 7, e38125. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, J.C.; Loros, J.J.; DeCoursey, P.J. Chronobiology: Biological Timekeeping; Sinauer Associates Inc.: Sunderland, MA, USA, 2004. [Google Scholar]
- Bernier, N.J.; Van Der Kraak, G.; Farrell, A.P.; Brauner, C.J. Fish Neuroendocrinology, 1st ed.; Academic Press: London, UK, 2009; Volume 28. [Google Scholar]
- Cermakian, N.; Sassone-Corsi, P. Environmental stimulus perception and control of circadian clocks. Curr. Opin. Neurobiol. 2002, 12, 359–365. [Google Scholar] [CrossRef]
- Oliveira, C.; Sánchez-Vázquez, F.J. Reproduction rhythms in fish. In Biological Clock in Fish; Kulczykowska, E., Popek, W., Kapoor, B., Eds.; Science Publishers: Enfield, NH, USA, 2010; pp. 185–215. [Google Scholar]
- López-Olmeda, J.F.; Sánchez-Vázquez, F.J. Feeding rhythms in fish: From behavioural to molecular approach. In Biological Clock in Fish; Kulczykowska, E., Popek, W., Kapoor, B., Eds.; Science Publishers: Enfield, NH, USA, 2010; pp. 155–184. [Google Scholar]
- Pardini, L.; Kaeffer, B. Feeding and circadian clocks. Reprod. Nutr. Dev. 2006, 46, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vázquez, F.J.; Madrid, J.A.; Zamora, S.; Tabata, M. Feeding entrainment of locomotor activity rhythms in the goldfish is mediated by a feeding-entrainable circadian oscillator. J. Comp. Physiol. A 1997, 181, 121–132. [Google Scholar] [CrossRef]
- Reebs, S.G. Plasticity of diel and circadian activity rhythms in fishes. Rev. Fish Biol. Fish. 2002, 12, 349–371. [Google Scholar] [CrossRef]
- López-Olmeda, J.F. Nonphotic entrainment in fish. Comp. Biochem. Physiol. A 2017, 203, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, R.T.; Messenger, J.B. Cephalopod Behavior; Cambridge University Press: New York, NY, USA, 1996; p. 232. [Google Scholar]
- Denton, E.J.; Gilpin-Brown, J.B. The effect of light on the buoyancy of the cuttlefish. J. Mar. Biol. Assoc. UK 1961, 41, 343–350. [Google Scholar] [CrossRef]
- Quintela, J.; Andrade, J.P. Diel feeding rhythms, daily ration and gastric evacuation rates of Sepia officinalis in the Ria Formosa Lagoon (south Portugal). Bull. Mar. Sci. 2002, 71, 665–680. [Google Scholar]
- Shashar, N.; Rutledge, P.S.; Cronin, T.W. Polarization vision in cuttlefish—A concealed communication channel? J. Exp. Biol. 1996, 199, 2077–2084. [Google Scholar] [PubMed]
- Shashar, N.; Hanlon, R.T.; Petz, A.D.M. Polarization vision helps detect transparent prey. Nature 1998, 393, 222–223. [Google Scholar] [CrossRef]
- Shashar, N.; Hagan, R.; Boal, J.G.; Hanlon, R.T. Cuttlefish use polarization sensitivity in predation on silvery fish. Vis. Res. 2000, 40, 71–75. [Google Scholar] [CrossRef]
- Jäckel, G.; Mark, F.; Gutowska, M.; Claireaux, G.; Ellington, C.; Pörtner, H. Analysis of Diurnal Activity Patterns and Related Changes in Metabolism in the Cephalopod Sepia officinalis. In Annual Main Meeting of the Society for Experimental Biology; Comparative Biochemistry and Physiology: Glasgow, Scotland, 2007; Volume 146A, p. S83. [Google Scholar]
- Shashar, N.; Harosi, F.I.; Banaszak, A.T.; Hanlon, R.T. UV radiation blocking compounds in the eye of the cuttlefish Sepia officinalis. Biol. Bull. 1998, 195, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Shashar, N.; Cronin, T.W. Polarization contrast vision in Octopus. J. Exp. Biol. 1996, 199, 999–1004. [Google Scholar] [PubMed]
- Shashar, N.; Hanlon, R.T. Squids (Loligo pealei and Euprymna scolopes) can exhibit polarized light patterns produced by their skin. Biol. Bull. 1997, 193, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Robin, J.P.; Sykes, A.V.; Koutsoubas, D.; Jereb, P.; Lefkaditou, E.; Koueta, N.; Allcock, A. Sepia officinalis. In Cephalopod Biology and Fisheries in Europe; Jereb, P., Allcock, A., Lefkaditou, E., Piatkowski, U., Hastie, L.C., Pierce, G.J., Eds.; ICES Cooperative Research Report 325; International Council for the Exploration of the Sea: Copenhagen, Denmark, 2015; Volume II, Species Accounts; pp. 53–73. [Google Scholar]
- Zielinski, S.; Portner, H.O. Oxidative stress and antioxidative defense in cephalopods: A function of metabolic rate or age? Comp. Biochem. Physiol. B 2000, 125, 147–160. [Google Scholar] [CrossRef]
- Eriksson, L. Nocturnalism versus diurnalism: Dualism within fish individuals. In Rhythmic Activity of Fishes; Thorpe, J.E., Ed.; Academic Press: New York, NY, USA, 1978; pp. 69–89. [Google Scholar]
- Mat, A.M.; Massabuau, J.-C.; Ciret, P.; Tran, D. Evidence for a plastic dual circadian rhythm in the oyster Crassostrea gigas. Chronobiol. Int. 2012, 29, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Anras, M.-L.B. Demand-feeding behaviour of sea bass kept in ponds: Diel and seasonal patterns, and influences of environmental factors. Aquac. Int. 1995, 3, 186–195. [Google Scholar] [CrossRef]
- Sanchez-Vázquez, F.J.; Madrid, J.A.; Zamora, S. Circadian rhythms of feeding activity in sea bass, Dicentrarchus labrax L.: Dual phasing capacity of diel demand-feeding pattern. J. Biol. Rhythms 1995, 10, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Aranda, A.; Sánchez-Vázquez, F.J.; Madrid, J.A. Influence of water temperature on demand-feeding rhythms in sea bass. J. Fish Biol. 1999, 55, 1029–1039. [Google Scholar] [CrossRef]
- Sanchez-Vazquez, F.J.; Azzaydi, M.; Martinez, F.J.; Zamora, S.; Madrid, J.A. Annual rhythms of demand-feeding activity in sea bass: Evidence of a seasonal phase inversion of the diel feeding pattern. Chronobiol. Int. 1998, 15, 607–622. [Google Scholar] [CrossRef] [PubMed]
- Eurropean Union (EU). Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes, EN ed.; Union, E., Ed.; Official Journal of European Union: Brussels, Belgium, 2010; Volume L276, pp. 33–79. [Google Scholar]
- Portaluppi, F.; Smolensky, M.H.; Touitou, Y. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol. Int. 2010, 27, 1911–1929. [Google Scholar] [CrossRef] [PubMed]
- Sykes, A.V.; Domingues, P.; Andrade, J.P. Sepia officinalis. In Cephalopod Culture; Iglesias, J., Fuentes, L., Villanueva, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 175–204. [Google Scholar]
- Díez-Noguera, A. Methods for serial analysis of long time series in the study of biological rhythms. J. Circadian Rhythms 2013, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice-Hall Inc.: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Vera, L.M.; Cairns, L.; Sánchez-Vázquez, F.J.; Migaud, H. Circadian rhythms of locomotor activity in the nile tilapia Oreochromis niloticus. Chronobiol. Int. 2009, 26, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Díez, A. Representación gráfica y análisis de datos en cronobiología. In Cronobiología Básica y Clínica; Madrid, J.A., Rol de Lama, M.A., Eds.; Editec@red S.L.: Madrid, Spain, 2007; pp. 84–121. [Google Scholar]
- Cornelissen, G. Cosinor-based rhythmometry. Theor. Biol. Med. Model. 2014, 11, 16. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, C.C.V.; Grano-Maldonado, M.I.; Gonçalves, R.A.; Frias, P.A.; Sykes, A.V. Preliminary Results on the Daily and Seasonal Rhythms of Cuttlefish Sepia officinalis (Linnaeus, 1758) Locomotor Activity in Captivity. Fishes 2017, 2, 9. https://doi.org/10.3390/fishes2030009
Oliveira CCV, Grano-Maldonado MI, Gonçalves RA, Frias PA, Sykes AV. Preliminary Results on the Daily and Seasonal Rhythms of Cuttlefish Sepia officinalis (Linnaeus, 1758) Locomotor Activity in Captivity. Fishes. 2017; 2(3):9. https://doi.org/10.3390/fishes2030009
Chicago/Turabian StyleOliveira, Catarina C.V., Mayra I. Grano-Maldonado, Rui A. Gonçalves, Paulo A. Frias, and António V. Sykes. 2017. "Preliminary Results on the Daily and Seasonal Rhythms of Cuttlefish Sepia officinalis (Linnaeus, 1758) Locomotor Activity in Captivity" Fishes 2, no. 3: 9. https://doi.org/10.3390/fishes2030009




