Changes in Intestinal Gene Expression of Zebrafish (Danio rerio) Related to Sterol Uptake and Excretion upon β-Sitosterol Administration
Abstract
:1. Introduction
2. Results
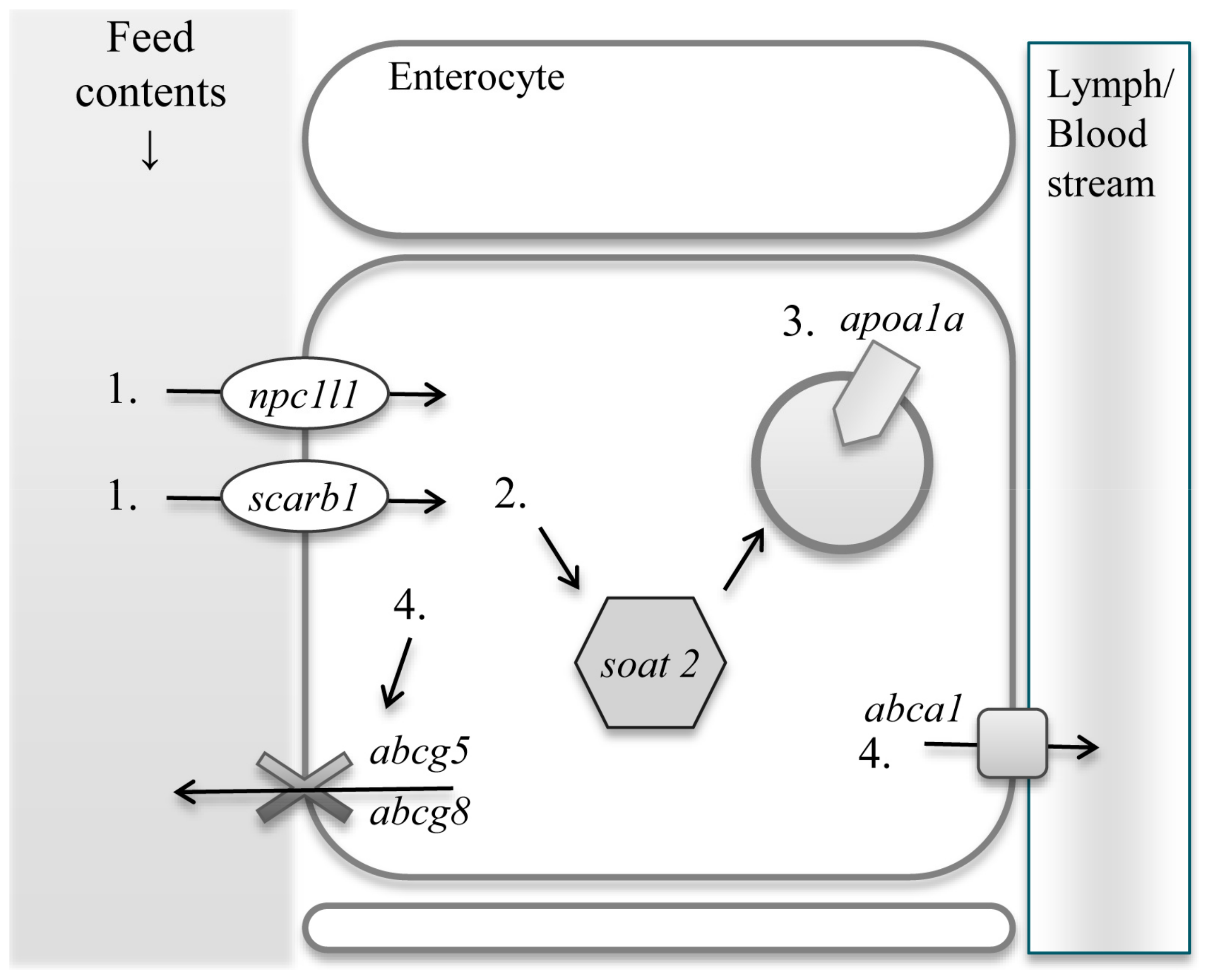
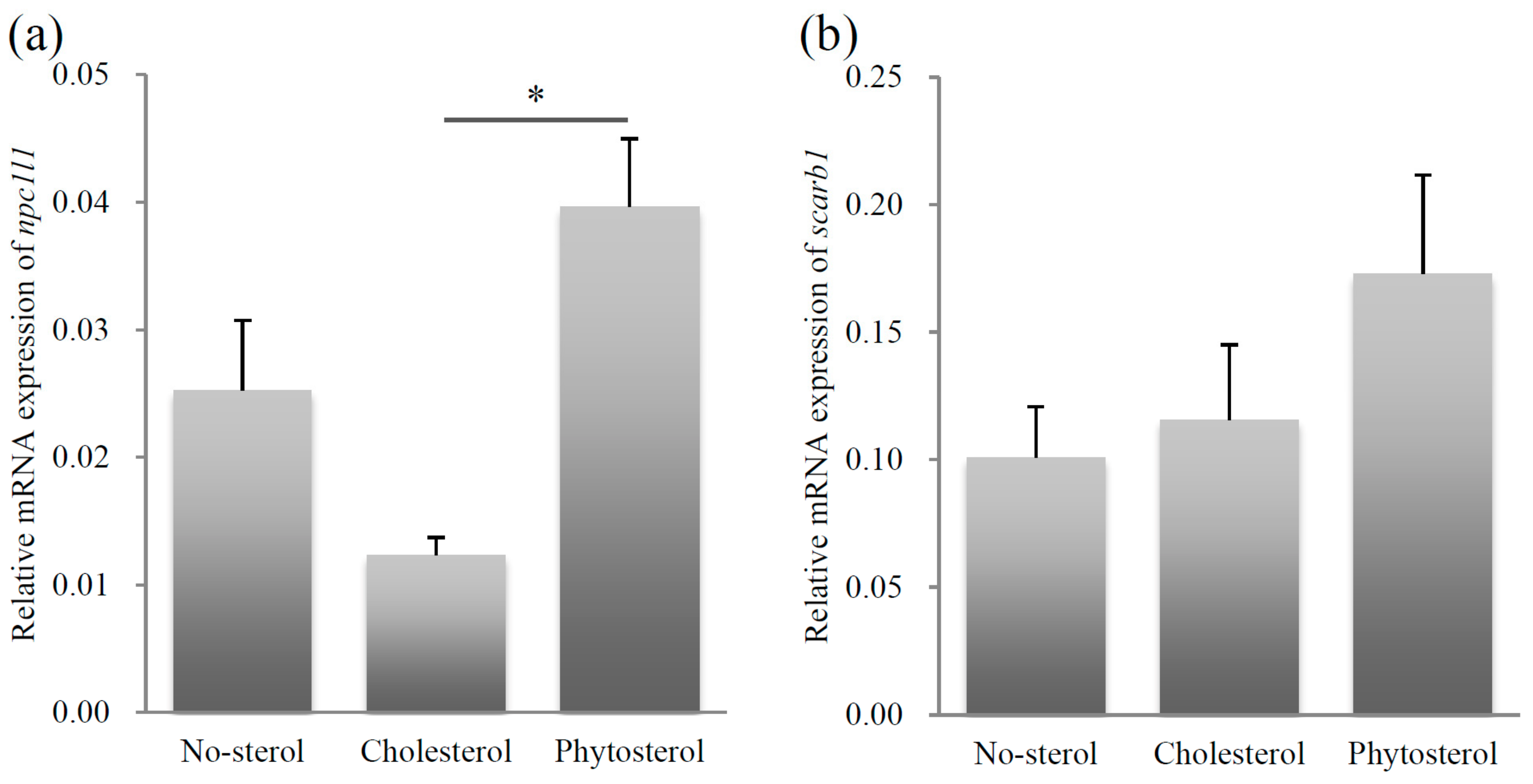
2.1. Relative mRNA Expression Levels of Sterol Uptake Related Genes
2.2. Relative mRNA Expression of Sterol Modification and Transportation Related Genes
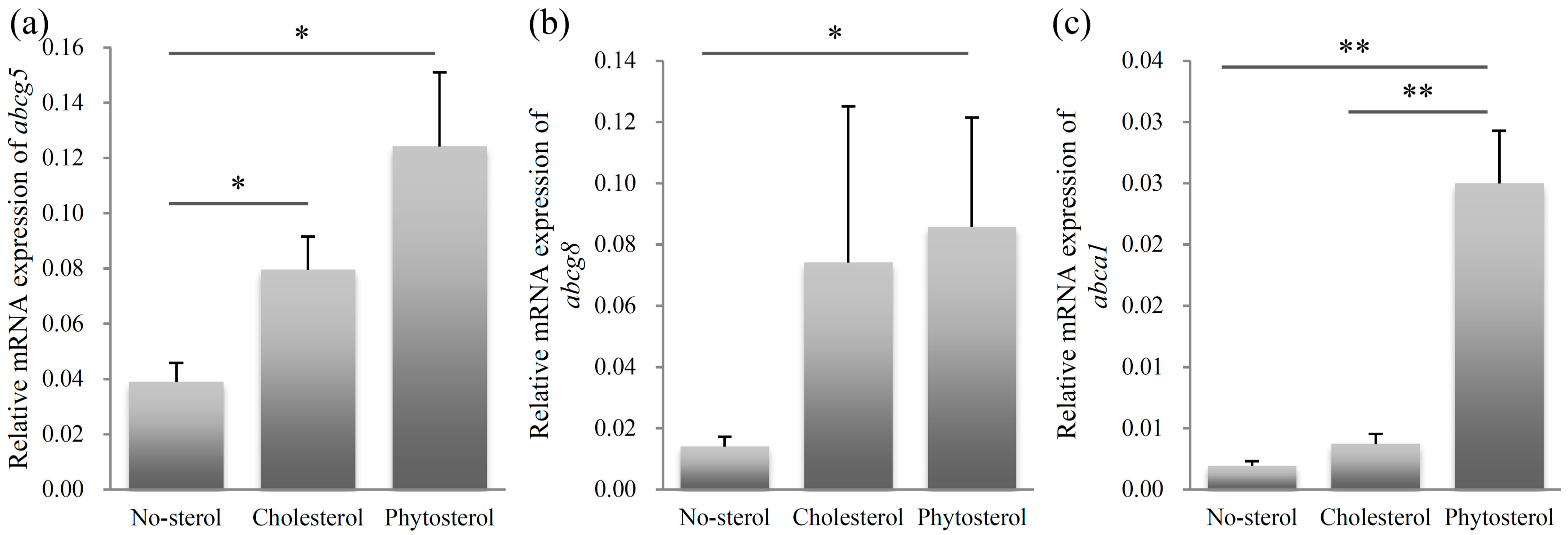
2.3. Relative mRNA Expression Level of Sterol Excretion Genes
3. Discussion
4. Materials and Methods
4.1. Fish
4.2. Feed Production
4.3. Sampling
4.4. Real Time PCR
4.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Olsen, R.L.; Hasan, M.R. A limited supply of fishmeal: Impact on future increases in global aquaculture production. Trends Food Sci. Technol. 2012, 27, 120–128. [Google Scholar] [CrossRef]
- Burel, C.; Boujard, T.; Escaffre, A.M.; Kaushik, S.J.; Boeuf, G.; Mol, K.A.; Van der Geyten, S.; Kühn, E.R. Dietary low-glucosinolate rapeseed meal affects thyroid status and nutrient utilization in rainbow trout (Oncorhynchus mykiss). Br. J. Nutr. 2000, 83, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.; Pratoomyot, J.; Taggart, J.B.; Bron, J.E.; Guy, D.R.; Bell, J.G.; Tocher, D.R. Genotype-specific responses in Atlantic salmon (Salmo salar) subject to dietary fish oil replacement by vegetable oil: A liver transcriptomic analysis. BMC Genom. 2011, 12, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Torrecillas, S.; Robaina, L.; Caballero, M.; Montero, D.; Calandra, G.; Mompel, D.; Karalazos, V.; Kaushik, S.; Izuquierdo, M.S. Combined replacement of fishmeal and fish oil in European sea bass (Dicentrarchus labrax): Production performance, tissue composition and liver morphology. Aquaculture 2017, 474, 101–112. [Google Scholar] [CrossRef]
- Gould, R.G.; Jones, R.J.; LeRoy, G.V.; Wissler, R.W.; Taylor, C.B. Absorbability of beta-sitosterol in humans. Metabolism 1969, 18, 652–662. [Google Scholar] [CrossRef]
- Salen, G.; Ahrens, J.E.; Grundy, S. Metabolism of beta-sitosterol in man. J. Clin. Investig. 1970, 49, 952–967. [Google Scholar] [CrossRef] [PubMed]
- Kritchevsky, D.; Tepper, S.; DiTullo, N.; Holmes, W. The sterols of seafood. J. Food Sci. 1967, 32, 64–66. [Google Scholar] [CrossRef]
- Miller, M.R.; Nichols, P.D.; Carter, C.G. The digestibility and accumulation of dietary phytosterols in Atlantic salmon (Salmo salar L.) smolt fed diets with replacement plant oils. Lipids 2008, 43, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Ballantine, J.; Roberts, J.; Lavis, A. The sterols of some marine teleosts. Comp. Biochem. Physiol. Part B Comp. Biochem. 1982, 73, 481–484. [Google Scholar] [CrossRef]
- Ozyurt, G.; Kuley, E.; Etyemez, M.; Ozoğul, F. Comparative seasonal sterol profiles in edible parts of Mediterranean fish and shellfish species. Int. J. Food Sci. Nutr. 2013, 64, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Krol, E.; Douglas, A.; Tocher, D.R.; Crampton, V.O.; Speakman, J.R.; Secombes, C.J.; Martin, S.A.M. Differential responses of the gut transcriptome to plant protein diets in farmed Atlantic salmon. BMC Genom. 2016, 17, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacchi, L.; Secombes, C.J.; Bickerdike, R.; Adler, M.A.; Venegas, C.; Takle, H.; Martin, S.A.M. Transcriptomic and physiological responses to fishmeal substitution with plant proteins in formulated feed in farmed Atlantic salmon (Salmo salar). BMC Genom. 2012, 13, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altmann, S.W.; Davis, H.R.; Zhu, L.J.; Yao, X.; Hoos, L.M.; Tetzloff, G.; Iyer, S.P.; Maguire, M.; Golovko, A.; Zeng, M.; et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 2004, 303, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Abumrad, N.A.; Davidson, N.O. Role of the gut in lipid homeostasis. Physiol. Rev. 2012, 92, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Bietrix, F.; Yan, D.; Nauze, M.; Rolland, C.; Bertrand-Michel, J.; Comera, C.; Schaak, S.; Barbaras, R.; Groen, A.K.; Perret, B.; et al. Accelerated lipid absorption in mice overexpressing intestinal SR-BI. J. Biol. Chem. 2006, 281, 7214–7219. [Google Scholar] [CrossRef] [PubMed]
- Hauser, H.; Dyer, J.H.; Nandy, A.; Vega, M.A.; Werder, M.; Bieliauskaite, E.; Weber, F.E.; Compassi, S.; Gemperli, A.; Boffelli, D.; et al. Identification of a receptor mediating absorption of dietary cholesterol in the intestine. Biochemistry 1998, 37, 17843–17850. [Google Scholar] [CrossRef] [PubMed]
- Temel, R.E.; Gebre, A.K.; Parks, J.S.; Rudel, L.L. Compared with Acyl-CoA: Cholesterol O-acyltransferase (ACAT) 1 and lecithin: Cholesterol acyltransferase, ACAT2 displays the greatest capacity to differentiate cholesterol from sitosterol. J. Biol. Chem. 2003, 278, 47594–47601. [Google Scholar] [CrossRef] [PubMed]
- Otis, J.P.; Zeituni, E.M.; Thierer, J.H.; Anderson, J.L.; Brown, A.C.; Boehm, E.D.; Cerchione, D.M.; Ceasrine, A.M.; Avraham-Davidi, I.; Tempelhof, H.; et al. Zebrafish as a model for apolipoprotein biology: Comprehensive expression analysis and a role for ApoA-IV in regulating food intake. Dis. Model Mech. 2015, 8, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Tsubakio-Yamamoto, K.; Nishida, M.; Nakagawa-Toyama, Y.; Masuda, D.; Ohama, T.; Yamashita, S. Current therapy for patients with sitosterolemia-effect of ezetimibe on plant sterol metabolism. J. Atheroscler. Thromb. 2010, 17, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Jesch, E.D.; Seo, J.M.; Carr, T.P.; Lee, J.Y. Sitosterol reduces messenger RNA and protein expression levels of Niemann-Pick C1-like 1 in FHs 74 Int cells. Nutr. Res. 2009, 29, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Bura, K.S.; Lord, C.; Marshall, S.; McDaniel, A.; Thomas, G.; Warrier, M.; Zhang, J.; Davis, M.A.; Sawyer, J.K.; Shah, R.; et al. Intestinal SR-BI does not impact cholesterol absorption or transintestinal cholesterol efflux in mice. J. Lipid Res. 2013, 54, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Voshol, P.J.; Schwarz, M.; Rigotti, A.; Krieger, M.; Groen, A.K.; Kuipers, F. Down-regulation of intestinal scavenger receptor class B, type I (SR-BI) expression in rodents under conditions of deficient bile delivery to the intestine. Biochem. J. 2001, 356, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Berge, K.E.; Tian, H.; Graf, G.A.; Yu, L.; Grishin, N.V.; Schultz, J.; Kwiterovich, P.; Shan, B.; Barnes, R.; Hobbs, H.H. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 2000, 290, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Plat, J.; Mensink, R.P. Increased intestinal ABCA1 expression contributes to the decrease in cholesterol absorption after plant stanol consumption. FASEB J. 2002, 16, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A.M. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35, W71–W74. [Google Scholar] [CrossRef] [PubMed]

| Contents | No-Sterol | Cholesterol | Sitosterol |
|---|---|---|---|
| Casein | 69.9 | 67.9 | 67.9 |
| Arginine | 0.4 | 0.4 | 0.4 |
| Cysteine | 0.9 | 0.9 | 0.9 |
| Taurine | 4.4 | 4.3 | 4.3 |
| Oil 1 | 9.6 | 9.3 | 9.3 |
| Cornstarch | 10.1 | 9.8 | 9.8 |
| Vitamin mix | 1.0 | 1.0 | 1.0 |
| Mineral mix | 3.5 | 3.4 | 3.4 |
| Choline Cl | 0.2 | 0.2 | 0.2 |
| Sterol | 0 | 3.0 | 3.0 |
| Total | 100 | 100 | 100 |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Accession |
|---|---|---|---|
| npc1l1 | GGACTGGCGGGATCAT | GCCGAGAGCTGTGATG | XM_009304174.2 |
| scarb1 | TTCCACATCGTCAATC | CCACAGACATGCTCTC | NM_198921.2 |
| soat2 | TGGAACTCCACTTCGT | GTCAAACACTCACCCA | XM_017356504.1 |
| apoa1a | GGACGGAACCGACTAT | GGAGGTGGTCTGGGCA | NM_131128.1 |
| abcg5 | CTGGCAGAGCTGGCTA | AAACACCAGCTCCCT | NM_001128690.1 |
| abcg8 | CATGGCACTGTTTGTG | AAACCAAGACGCCACC | XM_005156538.3 |
| abca1a | CAGTATGGCATCCCTC | TCCATCCGCATTTCTC | NM_001309465.1 |
| rpl8 | AATCCACACCGGCCAG | GCCAACGGGAAGCACA | NM_200713.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takase, M.; Ushio, H. Changes in Intestinal Gene Expression of Zebrafish (Danio rerio) Related to Sterol Uptake and Excretion upon β-Sitosterol Administration. Fishes 2018, 3, 1. https://doi.org/10.3390/fishes3010001
Takase M, Ushio H. Changes in Intestinal Gene Expression of Zebrafish (Danio rerio) Related to Sterol Uptake and Excretion upon β-Sitosterol Administration. Fishes. 2018; 3(1):1. https://doi.org/10.3390/fishes3010001
Chicago/Turabian StyleTakase, Mai, and Hideki Ushio. 2018. "Changes in Intestinal Gene Expression of Zebrafish (Danio rerio) Related to Sterol Uptake and Excretion upon β-Sitosterol Administration" Fishes 3, no. 1: 1. https://doi.org/10.3390/fishes3010001




