Introducing a Regulatory Policy Framework of Bait Fishing in European Coastal Lagoons: The Case of Ria de Aveiro in Portugal
Abstract
:1. Introduction
2. Results
2.1. Descriptive Statistics
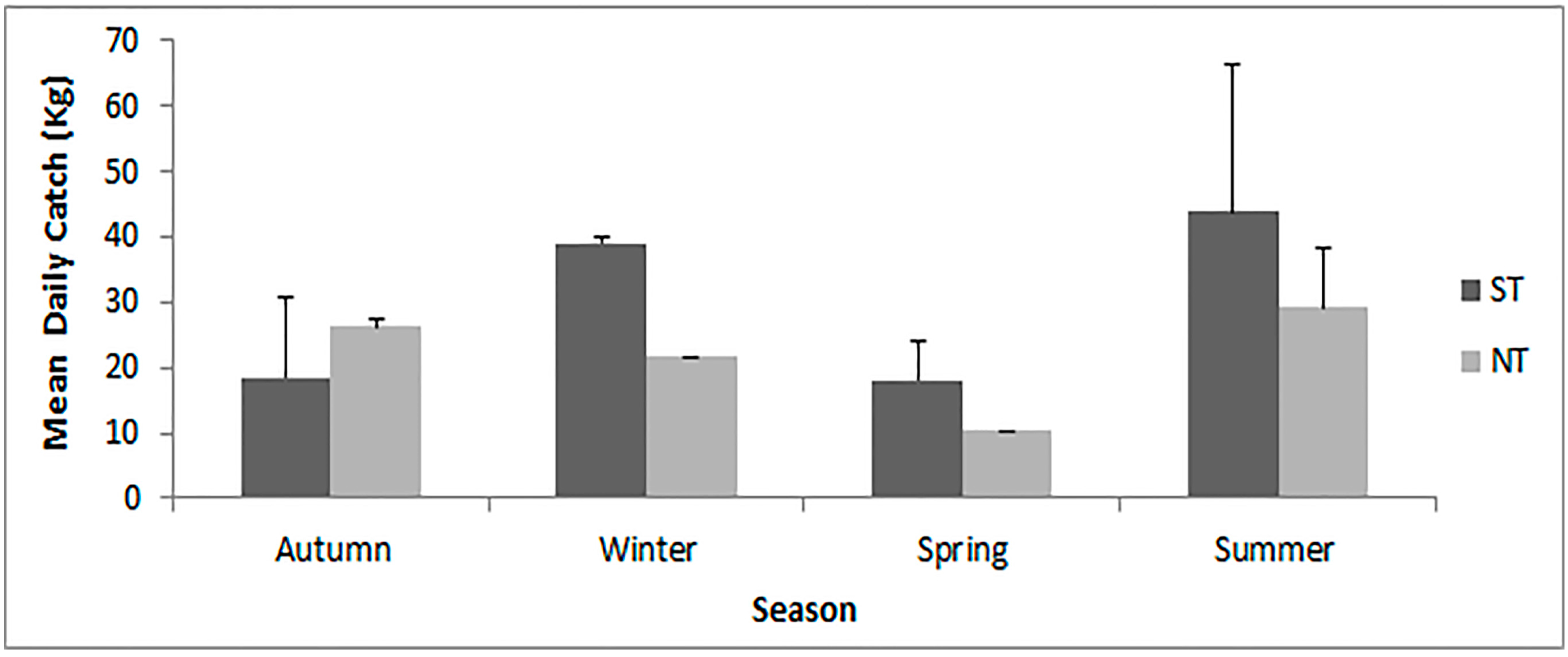
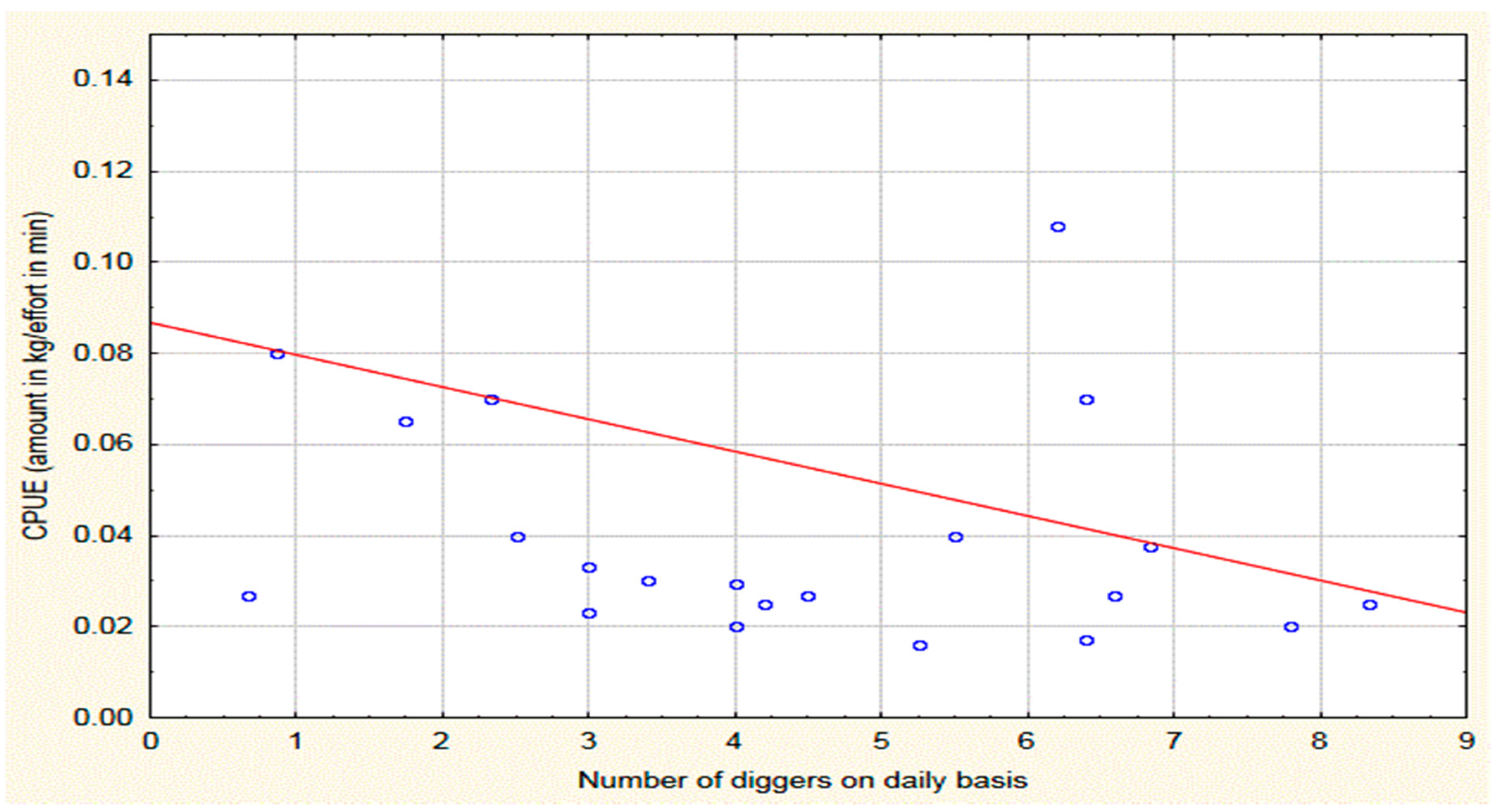
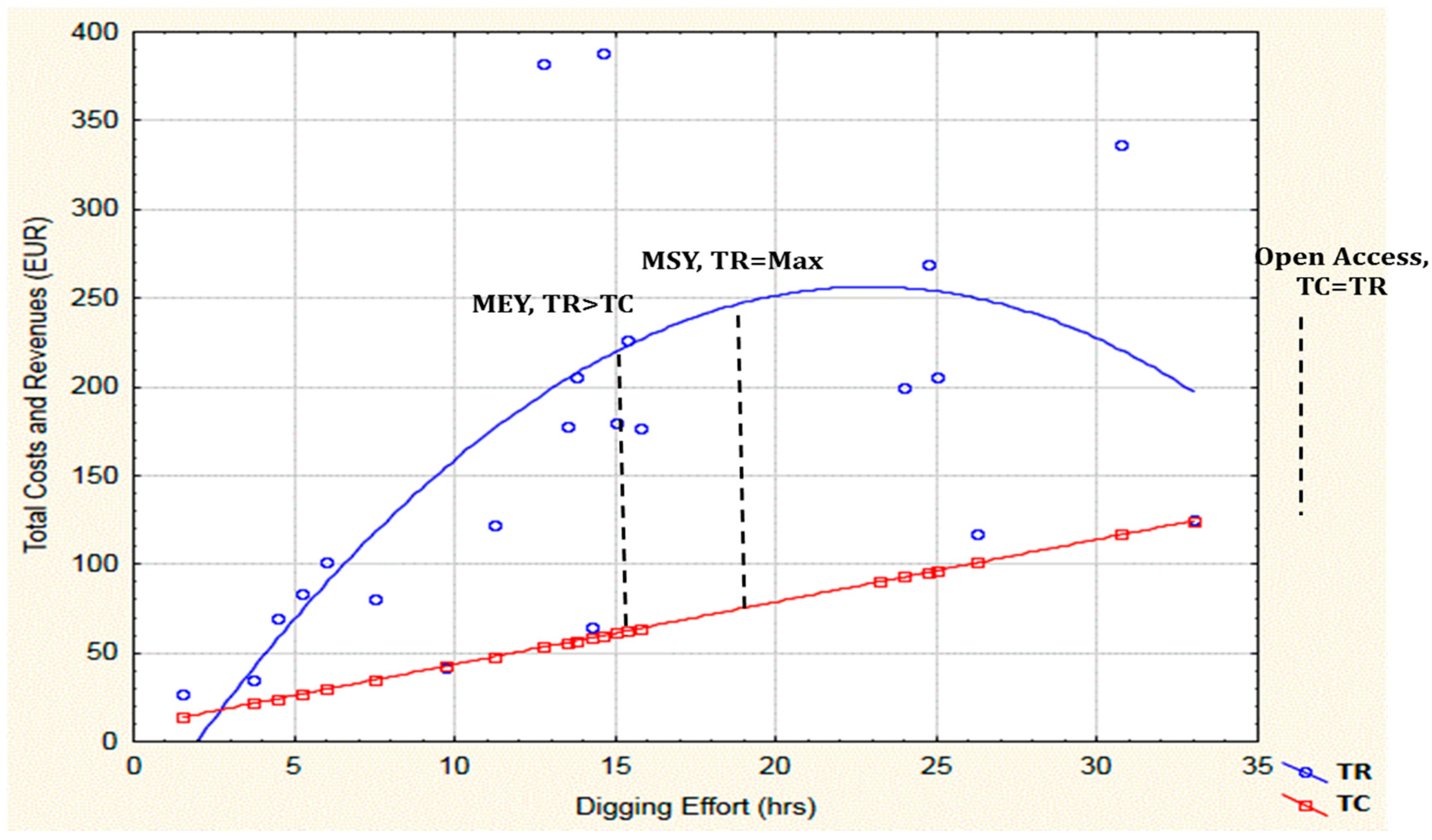
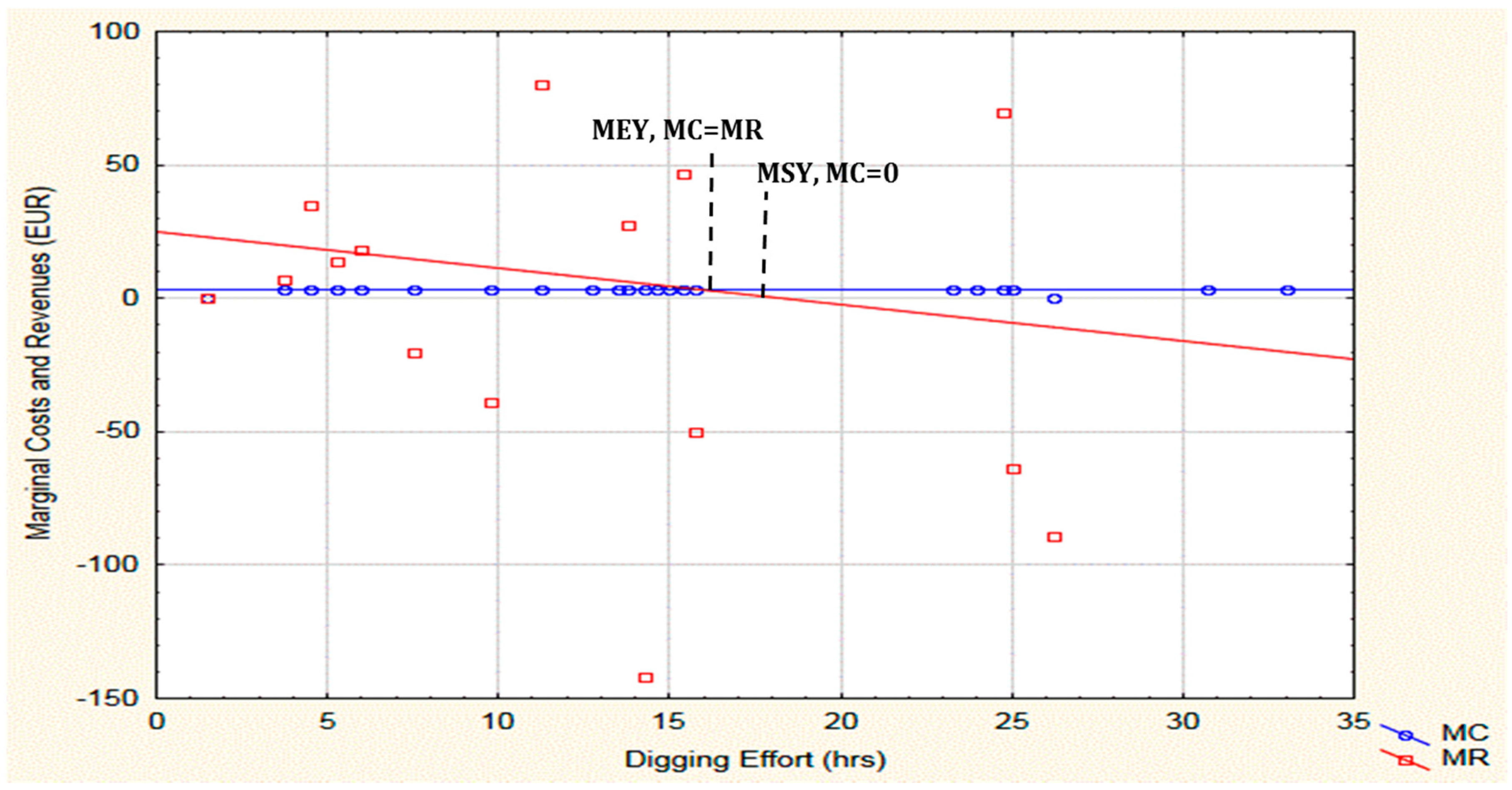
2.2. Catch, CPUE and Bioeconomic Results
3. Discussion
3.1. Can Remote Monitoring of the Bait Digging Effort Be Used as an Effective Assessment Tool?
3.2. How Are Catches Affected by Digging Effort?
3.3. How Does the Open-Access Scenario Reflect the Effect of Tidal Trends and Seasonal Variations in Digging Effort and Catch?
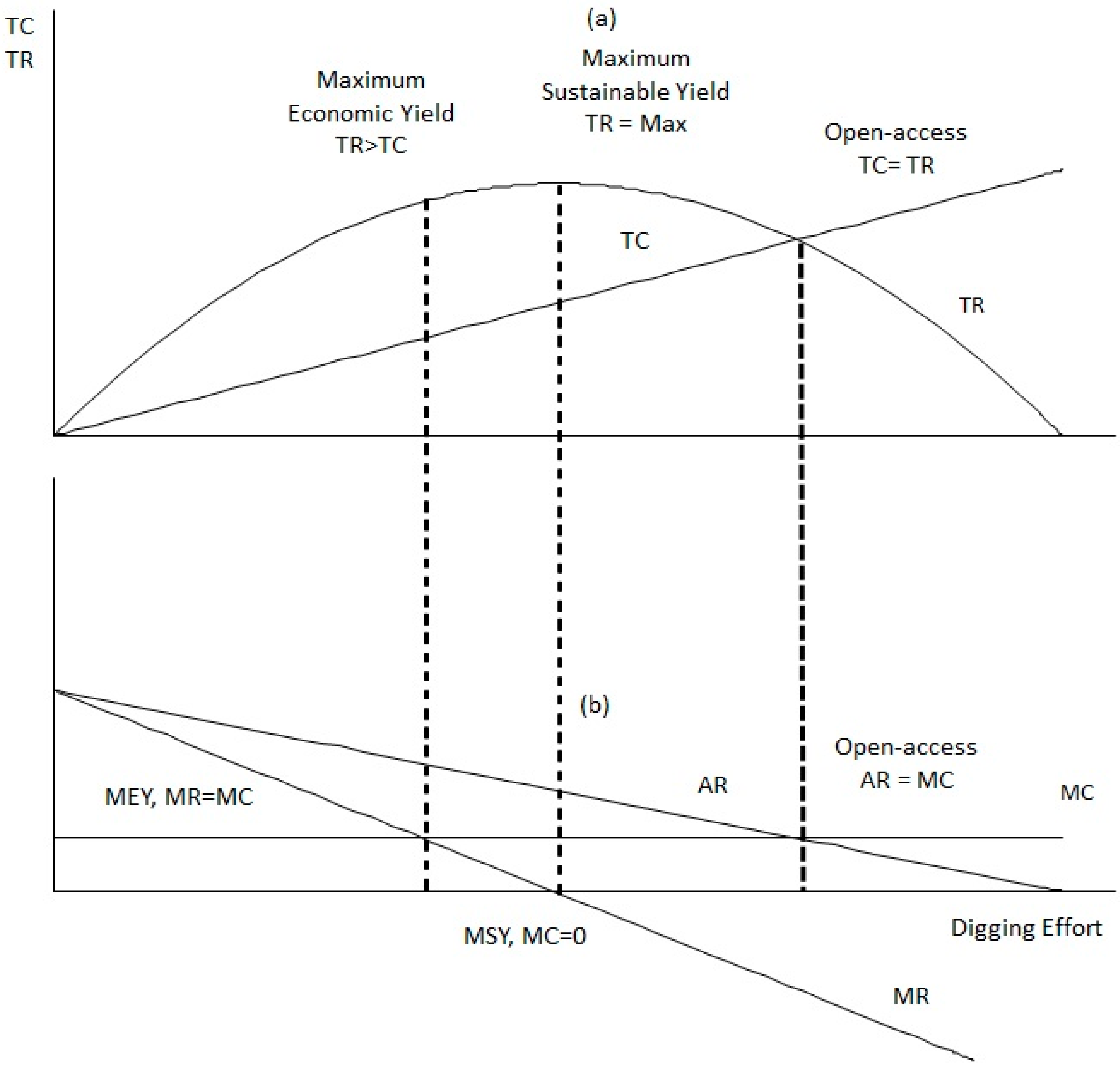
3.4. Can MEY Scenario Explore the Potential of Exceeding or Falling below the Daily Catch Limitation?
3.5. How Does MEY Scenario Matches MSY?
3.6. Does the Static Bioeconomic Analysis Support the Quota Introduced by the Ordinance?
4. Materials and Methods
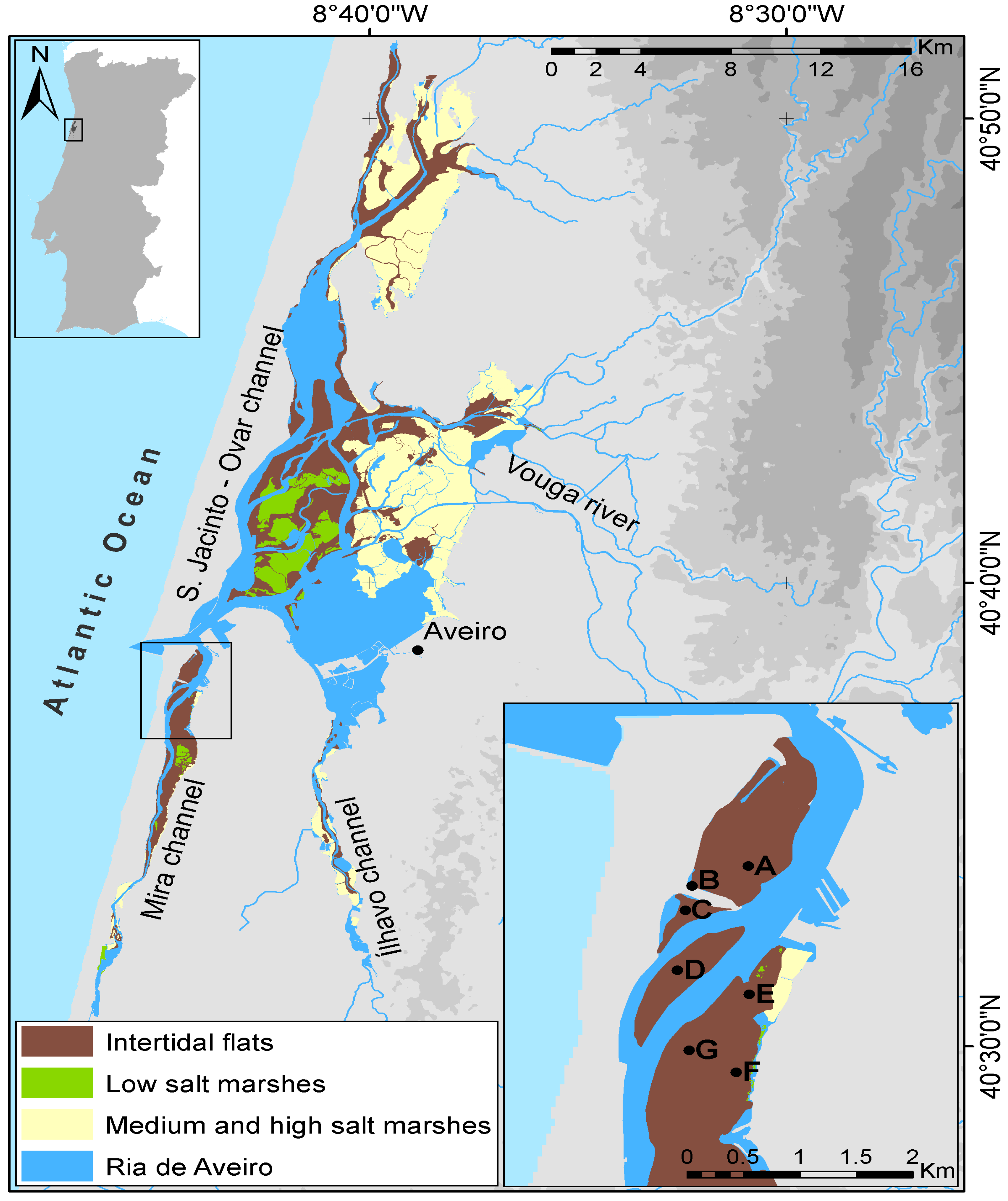
4.1. Study Site Description and Survey Design
4.2. Fishing Effort and Maximum Sustainable Yield Estimations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. Survey on Casulo Fishing in Aveiro Lagoon 2012–2013

| 1.1 Gender: | |
| 1.2 Age: | |
| 1.3 Area of Residence: | |
| 1.4 Primary professional occupation: | |
| 1.5 Degree of Studies: | |
| 1.6 Bait Fishing Experience (in years): |
| 2.1 Frequency of digging activity: | |
| 2.2 Duration of the digging session: | |
| 2.3 Weight of total capture: | |
| 2.4 Number of individuals captured: | |
| 2.5 Destination of captures (domestic market/export): | |
| 2.6 Expected value of first sale: |
References
- Alós, J.; Arlinghaus, R.; Palmer, M.; March, D.; Álvarez, I. The influence of type of natural bait on fish catches and hooking location in a mixed-species marine recreational fishery, with implications for management. Fish. Res. 2009, 97, 270–277. [Google Scholar] [CrossRef]
- Leguerrier, D.; Niquil, N.; Petiau, A.; Bodoy, A. Modelling the impact of oyster culture on a mudflat flood web in Marennes Ol’eron Bay (France). Mar. Ecol. Prog. Ser. 2004, 273, 147–162. [Google Scholar] [CrossRef]
- Rossi, F.; Forster, R.M.; Montserrat, F.; Ponti, M.; Terlizzi, A.; Ysebaert, T.; Middelburg, J.J. Human trampling as short-term disturbance on intertidal mudflats: Effects on macrofauna biodiversity and population dynamics of bivalves. Mar. Biol. 2007, 151, 2077–2090. [Google Scholar] [CrossRef]
- Cunha, T.; Hall, A.; Queiroga, H. Estimation of the Diopatra neapolitana annual harvest resulting from digging activity in Canal de Mira, Ria de Aveiro. Fish. Res. 2005, 76, 56–66. [Google Scholar] [CrossRef]
- Freitas, F.; Cunha, T.; Hall, A.; Queiroga, H. Diopatra neapolitana, Importância Sócio-económica e Sustentabilidade das Capturas, no Canal de Mira, Ria de Aveiro. In Proceedings of the Aveiro Jornadas da Ria de Aveiro, Aveiro, Portugal, 13 October 2011. [Google Scholar]
- Choe, S. On the life history of the Polychaete worm, Diopatra neapolitana. Bull. Jpn. Soc. Sci. Fish. 1960, 26, 430–437. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Pires, P.; Mendo, S.; Quintino, V. Diopatra neapolitana and Diopatra marocensis from the Portuguese coast: Morphological and genetic comparison. Estuar. Coast. Shelf Sci. 2009, 85, 609–617. [Google Scholar] [CrossRef]
- Murray, S.N.; Denis, T.G.; Kido, J.S.; Smith, J.R. Human Visitation and the Frequency and Potential Effects of Collecting on Rocky Intertidal Populations in Southern California Marine Reserves. California Cooperative Oceanic Fisheries Investigations (CalCOFI): CA, USA, 1999; Volume 40, pp. 100–106. Available online: http://calcofi.org/publications/calcofireports/v40/Vol_40_Murray_etal.pdf (accessed on 4 September 2017).
- Watson, G.J.; Murray, J.M.; Schaefer, M.; Bonner, A. Successful local marine conservation requires appropriate educational methods and adequate enforcement. Mar. Policy 2015, 52, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Code of Federal Regulations (CFR), Parks, Forest and Public Policy; U.S. Superintendent of Documents, Office of the Federal Register (OFR) and the Government Publishing Office: Washington, DC, USA, 2005.
- Rogers, S.I. A Review of Closed Areas in the United Kingdom Exclusive Economic Zone; Science Series Technical Report No. 106; CEFAS-Centre for Environment, Fisheries and Aquaculture Science: Lowestoft, UK, 1997; Available online: https://www.cefas.co.uk/publications/techrep/tech106.pdf (accessed on 17 September 2017).
- Lancaster, D.; Dearden, P.; Bann, N.C. Drivers of recreational fisher compliance in temperate marine conservation areas: A study of Rockfish Conservation Areas in British Columbia, Canada. Glob. Ecol. Conserv. 2015, 4, 645–657. [Google Scholar] [CrossRef]
- Lancaster, D.; Dearden, P.; Haggarty, D.R.; Volpe, J.P.; Ban, N.C. Effectiveness of shore-based remote camera monitoring for quantifying recreational fisher compliance in marine conservation areas. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 804–813. [Google Scholar] [CrossRef]
- Teles, F.; Santinha, G.; Marques, J.L.; Pinho, L.; Cunha, C.R.; Wolf, J.-H.; Borges, M. Estratégia Integrada de Desenvolvimento Territorial 2014–2020: Quadro Comum de Investimentos da Região de Aveiro; Revisão v.3; Comunidade Intermunicipal da Região de Aveiro: Aveiro, Portugal, 2014. [Google Scholar]
- Rudd, M.A.; Tupper, M.H.; Folmer, H.; Van Kooten, G.C. Policy analysis for tropical marine reserves: Challenges and directions. Fish Fish. 2003, 4, 65–85. [Google Scholar] [CrossRef]
- Ferse, S.C.; Costa, M.M.; Manez, K.S.; Adhuri, D.S.; Glaser, M. Allies, not aliens—Increasing the role of local communities in marine protected area implementation. Environ. Conserv. 2010, 37, 23–34. [Google Scholar] [CrossRef]
- Beukema, J.J. Long-term effects of mechanical harvesting of lugworms Arenicola marina on the zoobenthic community of a tidal flat in the Wadden Sea. Neth. J. Sea Res. 1995, 33, 219–227. [Google Scholar] [CrossRef]
- Brown, B.; Wilson, W.H. The role of commercial digging of mudflats as an agent for change of in faunal intertidal populations. J. Exp. Mar. Biol. Ecol. 1997, 218, 49–61. [Google Scholar] [CrossRef]
- Ferns, P.N.; Rostron, D.M.; Siman, S.H.Y. Effects of mechanical cockle harvesting on intertidal communities. J. Appl. Ecol. 2000, 37, 464–474. [Google Scholar] [CrossRef]
- Watson, G.J.; Farrell, P.; Stanton, S.; Skidmore, L.C. Effects of bait collection on Nereis virens populations and macrofaunal communities in the Solent, UK. J. Mar. Biol. Assoc. 2007, 87, 703–716. [Google Scholar] [CrossRef]
- Navon, M.; Dauvin, J.C. The immediate impact of intertidal pebble fork harvesting on the warty venus Venus verrucosa benthic community. Cah. Biol. Mar. 2013, 54, 385–392. [Google Scholar]
- Birchenough, S. Impact of Bait Collecting in Poole Harbour and Other Estuaries within the Southern IFCA District; Southern Inshore Fisheries and Conservation Authority: Dorset, UK, 2013. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/312998/fcf-baitcollecting.pdf (accessed on 13 September 2017).
- Neves de Carvalho, A.; Lino Vaz, A.S.; Boto Sérgio, T.I.; Talhadas dos Santos, P.J. Sustainability of bait fishing harvesting in estuarine ecosystems—Case study in the Local Natural Reserve of Douro Estuary, Portugal. J. Integr. Coast. Zone Manag. 2013, 13, 157–168. [Google Scholar]
- Watson, G.J.; Murray, J.M.; Schaefer, M.; Bonner, A. Bait worms: A valuable and important fishery with implications for fisheries and conservation management. Fish Fish. 2016. [Google Scholar] [CrossRef]
- Thrush, S.F.; Dayton, P.K. Disturbance to Marine Benthic Habitats by Trawling and Dredging: Implications for Marine Biodiversity. Annu. Rev. Ecol. Syst. 2002, 33, 449–473. [Google Scholar] [CrossRef]
- Walters, C.J.; Martell, S.D. Fisheries Ecology and Management; Princeton University Press: Princeton, NJ, USA, 2004. [Google Scholar]
- INE—Statistics Portugal. Censos 2011 Resultados Definitivos—Portugal. Census 2011 Final Results—Portugal; Instituto Nacional de Estatística, I.P Editor Publ.: Lisbon, Portugal, 2011. [Google Scholar]
- Aleixo, A. Pesca Lúdica e Apanha do Casulo Na Ria de Aveiro: 2012/2013. Master’s Thesis, University of Aveiro, Aveiro, Portugal, 2013. [Google Scholar]
- Leitao, F.M.; Gaspar, M.B. Immediate effect of intertidal non-mechanised cockle harvesting on macrobenthic communities: A comparative study. Sci. Mar. 2007, 71, 723–733. [Google Scholar] [CrossRef]
- Merino, G.; Quetglas, A.; Maynou, F.; Garau, A.; Arrizabalaga, H.; Murua, H.; Santiago, J.; Barange, M.; Prellezo, R.; García, D.; et al. Improving the performance of a Mediterranean demersal fishery toward economic objectives beyond MSY. Fish. Res. 2015, 161, 131–144. [Google Scholar] [CrossRef]
- Boldina, I.; Beninger, P.G. Fine-scale spatial distribution of the common lugworm Arenicola marina, and effects of intertidal clam fishing, Estuarine. Coast. Shelf Sci. 2014, 143, 32–40. [Google Scholar] [CrossRef]
- Clay, J.W. World Agriculture and the Environment: A Commodity-by-Commodity Guide to Impacts and Practices; Island Press: Washington, DC, USA, 2003. [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD). OECD Review of Fisheries: Policies and Summary Statistics 2015; OECD Publishing: Paris, France, 2015. [Google Scholar]
- Kuparinen, A.; Boit, A.; Valdovinos, F.; Lassaux, H.; Martinez, N. Fishing-induced life-history changes degrade and destabilize harvested ecosystems. Sci. Rep. 2016, 6, 22245. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, J.; Symes, D. Science for sustainable fisheries management: An interdisciplinary approach. Fish. Res. 2013, 139, 61–64. [Google Scholar] [CrossRef]
- Squires, D.; Vestergaard, N. Putting Economics into Maximum Economic Yield. Mar. Resour. Econ. 2016, 31, 101–116. [Google Scholar] [CrossRef]
- Choquenot, D.; Nicol, S.J.; Koehn, J.D. Bioeconomic modeling in the development of invasive fish policy. N. Z. J. Mar. Freshw. Res. 2004, 38, 419–428. [Google Scholar] [CrossRef]
- Pita, A.; Casey, J.; Hawkins, S.J.; Villarreal, M.R.; Gutiérrez, M.J.; Cabral, H.; Carocci, F.; Abaunza, P.; Pascual, S.; Presa, P. Conceptual and practical advances in fish stock delineation. Fish. Res. 2016, 173, 185–193. [Google Scholar] [CrossRef]
- Seijo, J.C.; Caddy, J.F. Uncertainty in bio-economic reference points and indicators of marine fisheries. Mar. Freshw. Res. 2000, 51, 477–483. [Google Scholar] [CrossRef]
- Russo, T.; Parisi, A.; Garofalo, G.; Gristina, M.; Cataudella, S.; Fiorentino, F. SMART: A Spatially Explicit Bio-Economic Model for Assessing and Managing Demersal Fisheries, with an Application to Italian Trawlers in the Strait of Sicily. PLoS ONE 2014, 9, e86222. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.; Freitas, R.; Quintino, V.; Rodrigues, A.M. Can Diopatra neapolitana (Annelida: Onuphidae) regenerate body damage caused by bait digging or predation? Estuar. Coast. Shelf Sci. 2012, 110, 36–42. [Google Scholar] [CrossRef]
- Dias, J.M.; Lopes, J.F.; Dekeyser, I. Tidal propagation in Ria de Aveiro Lagoon, Portugal. Phys. Chem. Earth B 2000, 25, 369–374. [Google Scholar] [CrossRef]
- AMBIECO/PLRA. Estudo da Caracterização da Qualidade Ecoligica da Ria de Aveiro; Ria de Aveiro POLIS LITORAL, AMBIECO: Aveiro, Portugal, 2011. [Google Scholar]
- Hoenig, J.M.; Robson, D.S.; Jones, C.M.; Pollock, K.H. Scheduling Counts in the Instantaneous and Progressive Count Methods for Estimating Sport fishing Effort. N. Am. J. Fish. Manag. 1993, 13, 723–736. [Google Scholar] [CrossRef]
- Pollock, K.H.; Hoenig, J.M.; Jones, C.M.; Robson, D.S.; Greene, C.J. Catch rate estimation for roving and access point surveys. N. Am. J. Fish. Manag. 1997, 17, 11–19. [Google Scholar] [CrossRef]
- Cochrane, K.L.; Garcia, S.M. A Fishery Manager’s Guidebook; The Food and Agriculture Organization of the United Nations: Rome, Italy; Wiley-Blackwell: Oxford, UK, 2009. [Google Scholar]
- Yamaguchi, M. Estimating growth parameters from growth rate data: Problems with marine sedentary invertebrates. Oecologia 1975, 20, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Fujita, R.; Thornhill, D.J.; Karr, K.; Cooper, C.H.; Dee, L.E. Assessing and managing data-limited ornamental fisheries in coral reefs. Fish Fish. 2012, 15, 661–675. [Google Scholar] [CrossRef]
- Dagli, E.; Ergen, Z.; Cinar, M.E. One year observation on the population structure of, D. neapolitann Delle Chiaje (Polychaeta: Onuphidae) in Izmir Bay (Aegean Sea, eastern Mediterranean). Mar. Ecol. 2006, 26, 265–272. [Google Scholar] [CrossRef]
- Begon, M.; Townsed, C.R.; Harper, J.L. Ecology: Individuals, Populations and Communities, 3rd ed.; Wiley-Blackwell: Oxford, UK, 1996. [Google Scholar]
- Begon, M.; Townsed, C.R.; Harper, J.L. Ecology: From Individuals to Ecosystems, 4th ed.; Wiley-Blackwell: Oxford, UK, 2006. [Google Scholar]
- Caddy, J.F.; Defeo, O. Enhancing or Restoring the Productivity of Natural Populations of Shellfish and Other Marine Invertebrate Resources; Technical Paper No. 448; FAO Fisheries: Rome, Italy, 2003. [Google Scholar]
- Flaaten, O. Fisheries Economics and Management, 1st ed.; Bookboon Publ.: Copenhagen, Denmark, 2016; ISBN 978-87-403-1193-8. Available online: http://bookboon.com/en/fisheries-economics-and-management-ebook (accessed on 12 August 2017).
- Clark, C.W. The Worldwide Crisis in Fisheries: Economic Models and Human Behavior; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Pascoe, S.; Vieira, S.; Thebaud, O. Allocating repairs and maintenance costs to fixed or variable costs in fisheries bioeconomic models. Appl. Econ. Lett. 2015, 22, 127–131. [Google Scholar] [CrossRef]
- Macher, C.; Boncoeur, J. Optimal Selectivity and Effort Cost: A Simple Bioeconomic Model with an Application to the Bay of Biscay Nephrops Fishery. Mar. Resour. Econ. 2010, 25, 213–232. [Google Scholar] [CrossRef]
- Gordon, S. The Economic Theory of a Common-Property Resource: The Fishery. J. Political Econ. 1954, 62, 124–142. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xenarios, S.; Queiroga, H.; Lillebø, A.I.; Aleixo, A. Introducing a Regulatory Policy Framework of Bait Fishing in European Coastal Lagoons: The Case of Ria de Aveiro in Portugal. Fishes 2018, 3, 2. https://doi.org/10.3390/fishes3010002
Xenarios S, Queiroga H, Lillebø AI, Aleixo A. Introducing a Regulatory Policy Framework of Bait Fishing in European Coastal Lagoons: The Case of Ria de Aveiro in Portugal. Fishes. 2018; 3(1):2. https://doi.org/10.3390/fishes3010002
Chicago/Turabian StyleXenarios, Stefanos, Henrique Queiroga, Ana I. Lillebø, and Ana Aleixo. 2018. "Introducing a Regulatory Policy Framework of Bait Fishing in European Coastal Lagoons: The Case of Ria de Aveiro in Portugal" Fishes 3, no. 1: 2. https://doi.org/10.3390/fishes3010002





