2.2.1. Dependencies of Cross Sections on Momentum Transfer
The analysis of neutron data combines the structural models’ application in reciprocal space and rebuilding of the correlation functions to determine more exactly the structures of the original gel and its derivatives. As seen in
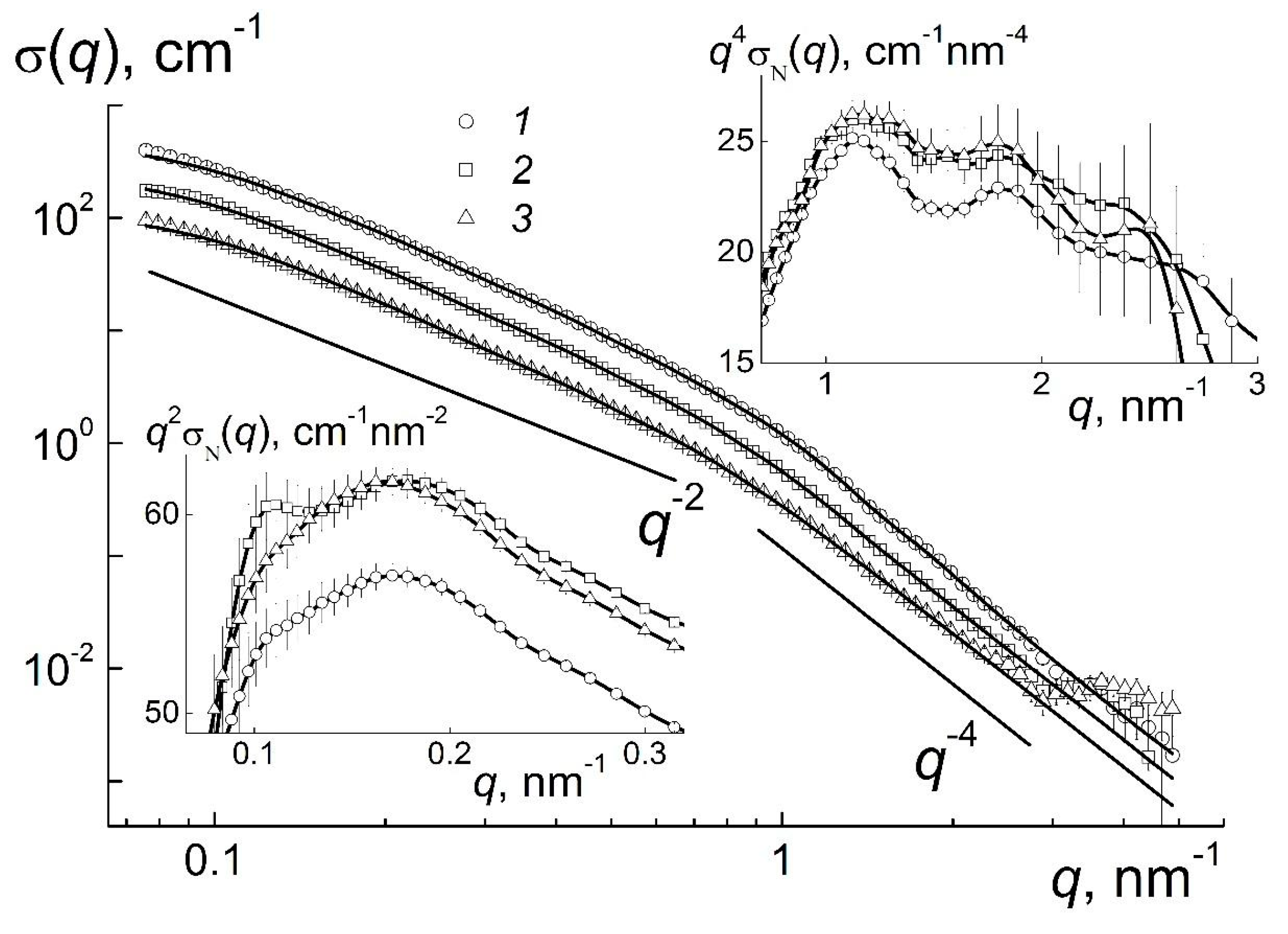
Figure 1, the scattering cross sections σ(
q) for pristine gel and diluted systems grow by four orders in magnitude at low momentum transfers, that indicates an assembly of particles at the scales of
R ~ π/
qε ≥ 10
1 nm. At the same time, the dilution leads to the decrease of cross sections σ(
q) according to the decrement of concentration, while at first glance, the behavior of σ(
q) does not change. Hence, the gel’s ordering achieved above the critical point (
С1 >
C* ~ 4.2 wt %) is rather stable and exists after two- and four-fold dilution.
The retention of stable local structures below the critical point
C* can be treated as a disintegration of the original gel network into large domains in water. Their size exceeds the value ~2π/
qmin ~ 10
2 nm that is defined by the minimal momentum transfer
qmin. Such a micro-gel exists in the range of concentrations from
C*/4 ≤
С ≤
С*. It is remarkable that all the curves of σ(
q) demonstrate a kink at the momentum transfer
q ~
q* ~ 1 nm
−1 (
Figure 1). Obviously, at
q ≥
q* the scattering on single particles dominate, whereas at
q ≤
q* the interference is observed in scattering at the distances 2π/
q ≥
ds exceeding the particles’ diameter
ds ~ 2π/
q* ~ 6 nm. In these regions the cross sections show the exponential behaviors, σ(
q) ~ 1/
qD, with the parameters
D =
D1 ~ 4 and
D =
D2 ~ 2 where the first one corresponds to Porod’s law observed for the particles with sharp borders and the second one indicates chain-like aggregates of particles (
Figure 1).
The existence of low-dimensional structures, i.e., linear (chain-like) aggregates is clearly visible from the q-dependence of the cross section, σ(
q) ~ 1/
q2, since this scattering law is established from the statistics of flexible chains (polymers) [
16]. At the same time, except for linear fragments, the observed structures may include some branched (e.g., dendrimer-like) fragments characterized by the exponent
D2 > 2 [
16,
17].
The logarithmic presentation (
Figure 1) has revealed the basic motives of gel structuring. The subtle features of the systems are visible by a compensation of the exponential dependencies when we used the Kratky- and Porod-plots at
q ≥
q* ~ 1 nm
−1 and
q <
q* for the cross sections σ
N(
q) = σ(
q)/
C normalized onto the diamonds’ concentrations, given in
Figure 1 (inserts).
The right inset in
Figure 1 illustrates the spacing (
dint) between the centers of neighboring particles coupled in network structure of gel. The spacing
dint defines the position of the peak,
qint ≈ 1.1 nm
−1 =
X/
dint where the coefficient
X corresponds to the maximum
X =
qintdint ≈ 7.72 of Debye scattering functions
for a pair of particles at the distance
dint. The value of
dint ≈ 7 nm exceeds a characteristic diameter of a diamond particle,
dint −
dS ≈ 1 nm, i.e., the particles in the network structure of gel contact via water shells with a thickness of two molecular layers. The first (
qint) and second maxima (~2
qint) on the curves for different concentrations confirm a short range order of particles in gels.
On the other hand, the data at low momentum transfers (
Figure 1, left insert) testify that the structuring at higher distance
Dw =
X/qw ≈ 40 nm where
qw ≈ 0.18 nm
−1 is the maximum position for a broad peak at
q ~ 0.1–0.3 nm
−1. This spacing should be attributed already to the size of the cells in gels’ multilevel structures, which are discussed below.
This analysis was extended to obtain more detailed information on the quality of the particles’ surface and the features of their association. The approximations of the data (
Figure 1) with the exponential law
have given the coefficients
Ji showing the scattering ability of the observed objects and the parameters
Di which characterize their geometry (
Figure 2).
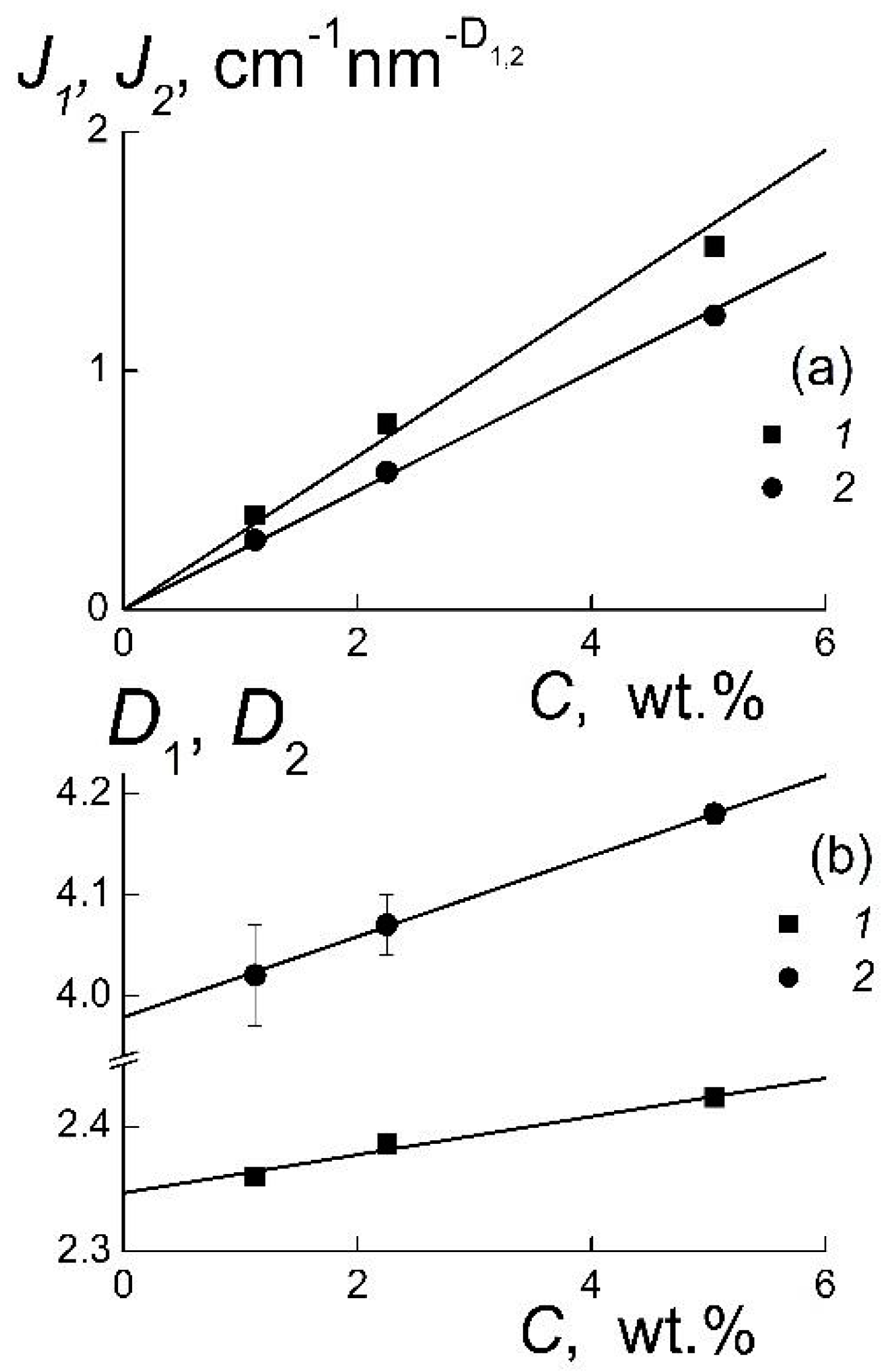
A linear behavior of the coefficients
J1,
J2 indicates a change of the amount of particles’ or aggregates by the variation of concentration, when their geometry does not undergo any substantial transformation (
Figure 2a). Only a weak linear decrease of the geometric parameters
D1,
D2 is observed (
Figure 2b). The
D2 reflects the geometry of particles’ surface. This parameter declines by dilution, and in the limit,
C → 0, the value of
D2 =
D02 = 3.98 ± 0.04 is very close to the exponent in Porod’s law describing the scattering for the particles with sharp borders [
18]. It confirms a perfection of the diamond crystals’ facets that is of principal importance for the configuration of potentials inducing the diamonds’ electrostatic attraction due to different electric charges of the facets [
19,
20]. Meanwhile, even at a moderate concentration (
С3 = 5.05 wt %) the scattering from the particles’ surface is disturbed due to the interference in scattering from neighboring particles that gives
D02 > 4 (
Figure 2b). This must be distinguished from the results [
5,
12] for the suspensions of diamonds in graphene shells, which showed a deviation of scattering from Porod’s law.
The sharp surfaces of diamonds facilitate their association into branched structures evidenced from the values of
D1 ~ 2.3–2.4 > 2 (
Figure 2b). Such a magnitude in the range 2 <
D1 < 3 is inherent in the structures formed via a diffusion limited aggregation (DLA) which creates mass fractals [
17]. For pristine gel the fractal dimension
D1 ~ 2.4 exceeds the parameter for a linear Gaussian polymer chain
DG = 2 [
16], that confirms the existence of branched aggregates forming a network of gel. A dilution of gel retains mostly the exponent
D1 decreasing linearly with concentration (
Figure 2b). In the limit
С → 0, the extrapolated
D1 =
D01 = 2.35 ± 0.01 is substantially greater than the
DG = 2 for linear chains. This shows a good stability of local structure of gel grown at the volume fraction of diamonds
φ1 = 1.44% keeping in its integrity at their low content,
φ3 = 0.22%. It is noticeable that this ordering is realized in the ensembles of particles with relatively broad size-distributions. For a comprehensive analysis of these structures, it is necessary to rebuild the size-distributions of diamonds.
2.2.3. Correlation Functions
The ensembles of diamond particles are characterized by the functions γ(
R) showing the correlations between various scattering centers (atomic nuclei, particles, aggregates). The function γ(
R) = <δ
ρ(
r)·δ
ρ(
r + R)> is the averaged product of the deviations, δ
ρ(
r) =
ρ(
r) − <
ρ>, δ
ρ(
r + R) =
ρ(
r + R) − <
ρ>, in scattering length densities from the mean value <
ρ> in the sample. A pair correlation is considered for two points at the distance
R. The magnitude of <
ρ> = Σ(
biNi) is defined by the contributions of nuclei with scattering lengths
bi and concentrations
Ni. For isotropic samples, the functions γ(
R) being the Fourier-transforms of scattering data [
18] depend on the modulus of vector
R,
The γ(
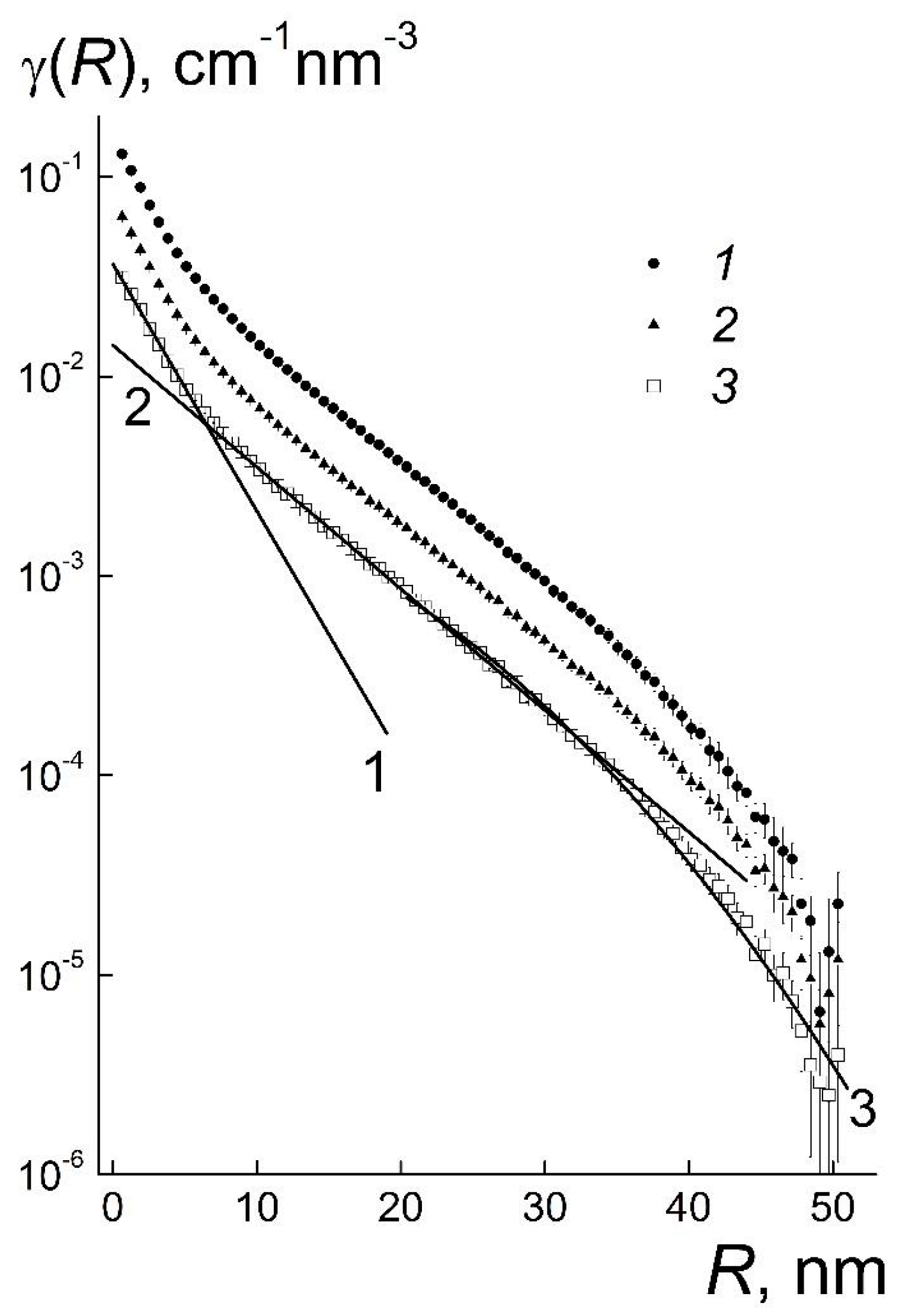
R) for the samples are similar and demonstrate three regions of behavior (
Figure 4).
When the radii, 0 ≤
R ≤ 6 nm, are less than the diameter of the particles, the functions γ(
R) obey the exponential law γ
1(
R) = g
1exp(−
R/
r1). It includes the correlation length
r1 comparable to the radius of particles and the coefficient
g1 proportional to the concentration of diamonds (
Table 1). Since the functions γ
1(
R) describe the atomic correlations inside the particles, the parameter
r1 is the averaged correlation radius of particles
r1 ~
R1m which is slightly larger than their geometric radius (
Figure 3). This follows from the comparison of γ
1(
R) and the correlation function γ
S ~ [1 − (3/4)(
R/
rS) + (1/16)(
R/
rS)
3] of a sphere [
18] with the radius
rS =
R1m.
At low radii,
R ≤
r1, the γ
1(
R) is approximately a linear function, γ
1 ~ [1 −
R/
r1]. At the same time, the linear term γ
S ~ [1 − (3/4)(
R/
rS)] dominates in the correlation function γ
S(
R) at
R ≤
rS. The identity γ
1(
R) ≡ γ
S(
R) is fulfilled if
rS = (3/4)
r1. Thus, in gel the particles with a correlation radius
r1 = 3.40 ± 0.01 nm have the geometric radius
rS = (3/4)
r1 ≈ 2.6 nm ≈
R1m in accordance with experimental data (
Figure 3). In addition, the γ
1(
R) yields the information on the contrast factor of diamonds Δ
ρD relative to the surrounding medium. The Δ
ρD depends on the scattering lengths and the concentrations of chemical elements in the particles and surroundings. This gives the indication of how tightly packed the particles are. In pristine gel with the volume fraction of diamonds
φ = 1.44%, the parameter
g1 = (Δ
ρD)
2φ (
Table 1) affords the estimate Δ
ρD = (10.36 ± 0.01) × 10
10 cm
−2. The Δ
ρD=
ρD − <
ρ> = (1 −
φ)(
ρD −
ρW) is the difference between the scattering length densities
ρD of the carbon material and a similar parameter <
ρ> for the whole volume of the sample <
ρ> =
φρD + (1 −
φ)
ρW, where
ρW = −0.56 × 10
10 cm
−1 is the scattering length density for light water.
The obtained scattering length density of particles ρD = ΔρD/(1 − φ) + ρW = (9.95 ± 0.01) × 1010 cm−2 is 15% lower than a similar parameter, ρDI = 11.7 × 1010 cm−2, for the crystals of diamonds with a characteristic density of 3.5 g/cm3. The deficit can be explained by the presence of volume (surface) defects (vacancies) in diamonds. On the other hand, we neglected the possible influence of the gel’s structure factor, taken as S(q → 0) = 1. Even at a low volume content of particles, their coordination may cause a deviation of this factor from unity, S(q) < 1.
In these systems the structuring is revealed at the radii, 6 ≤
R ≤ 20 nm, comparable to the particles’ diameter (first coordination sphere) that is described by the exponential function, γ
2(
R) =
g2exp(−
R/
r2). The coefficient
g2 is proportional to the particles’ concentration but the correlation radius
r2 ~ 7 nm remains almost constant (
Table 1). This confirms a stability of the local ordering even by a substantial dilution of gel.
For the functions γ1(R), γ2(R) describing the correlations inside the particles and in the first coordination sphere, one can compare the correlation volumes, V1 = 4π∫exp(−R/r1)R2dR = 8πr13 ≈ 990 nm3 and V2 = 8πr23 ≈ 9.3 × 103 nm3. The volume V2 exceeds V1 by an order of magnitude but the first coordination sphere is not completely filled with particles. This is evident from a comparison of the forward cross sections σ2 and σ1 calculated in the limit q → 0 using the functions γ1, γ2.
The cross section σ1 = (ΔρD)2φV1 = g1V1 is defined by the parameters of single particles while the magnitude of σ2 = (ΔρD)2φ(mV1) = g2V2 is proportional to the number of particles (m) within the correlation volume V2. The ratio of cross sections gives the value m = (g2VС)/(g1V1) = 3.6 ± 0.1. Hence, a particle in the gel is connected with two to three neighboring particles. This short linear or branched fragment (aggregation number m) is really stable even in the case of a four-fold dilution of gel where only a slight reduction in the aggregation number is observed (~10%).
The structure of gel outside the first coordination sphere is seen in the γ(
R) behaviors which obey the law γ
3(
R) =
g3exp[−(
R/
r3)
2] at
R ≥ 20 nm. It corresponds to Guinier’s function exp[−(
qRG)
2/3] with the gyration radius
RG = (3/4)
1/2r3 being proportional to the correlation length
r3. In gel and diluted systems, the lengths
r3 ~ 20 nm are the same (
Table 1), as well as the gyration radii
RG = (3/4)
1/2r3 ~ 17 nm. One may imagine the structure of these samples being composed of spherical domains having the diameter of ~2
RG ~ 40 nm.
This approximation is appropriated at
q ≤ 0.1 nm
−1 (
Figure 1) while in the extended interval,
q ≤ 1 nm
−1, these domains are characterized as chain fractal aggregates (
Table 1). The distribution of distances between the particles inside them is described by the function
G(R) = γ
3(R)R2 having the maximum at
R =
r3 as the most probable distance between the units in aggregates. For a Gaussian chain connecting two particles at the distance
r3 ~ 17 nm, the number of units is equal to
n = (
r32/
dS2) + 1 ≈ 16, and the contour length
LC =
ndS ≈ 80 nm. The latter should be compared to the spacing between the chains in the gel that is set by their numerical concentration,
NL =
φ/(
nπ
dS3/6) ≈ 1.2 × 10
16 cm
−3 for the particles’ volume fraction,
φ = 1.44%. For uniform distribution of the chains in the gel, the average distance between them,
Dw ≈
NL−1/3 ≈ 40 nm ~ 2
r3, is approximately half of the contour length. The chains principally overlap and form the interconnected structure of the gel with cells’ diameter ~
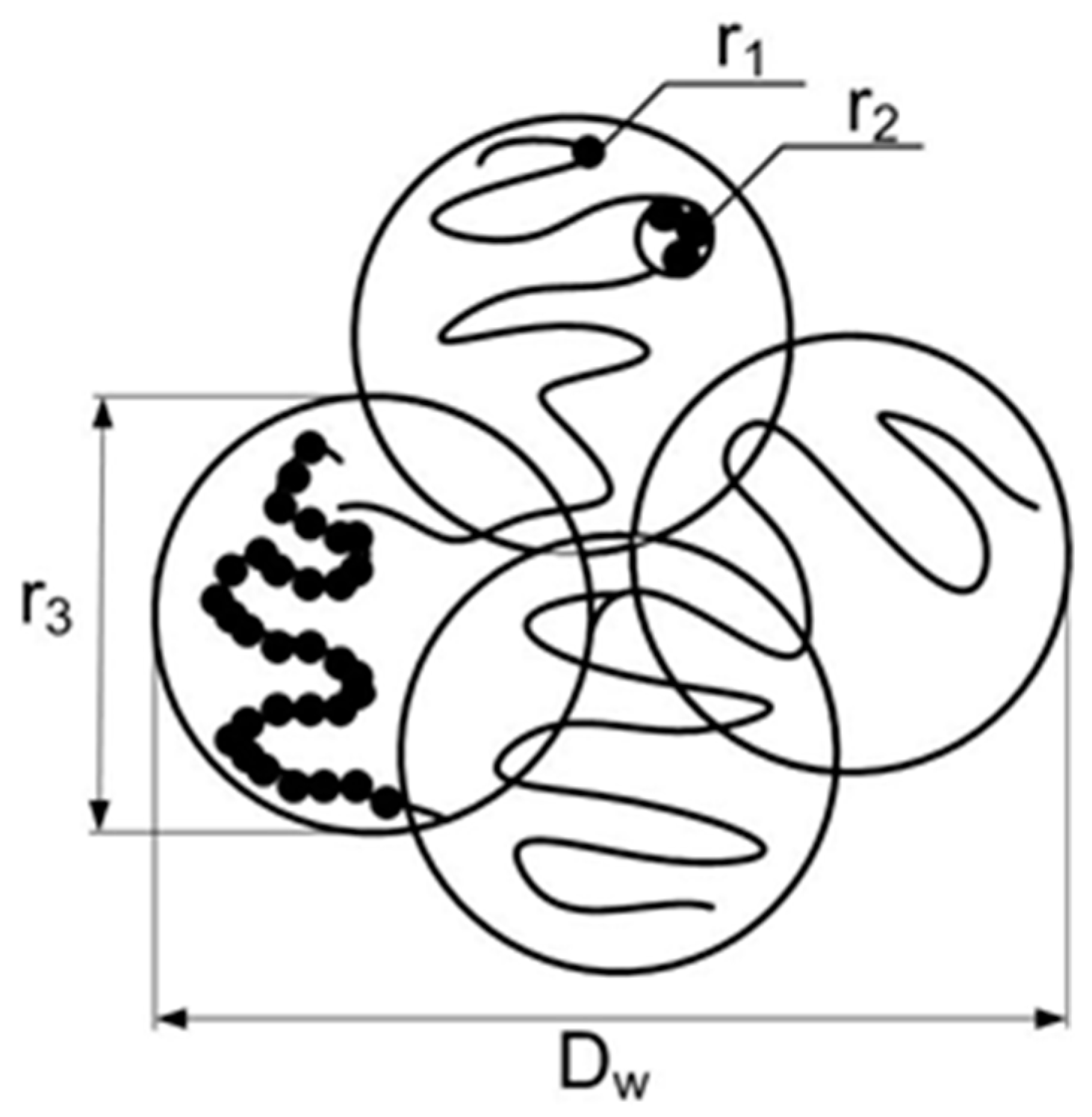
Dw ~ 40 nm. This is illustrated in
Figure 5 where three levels of gel structure are shown. Single particles (size
r1 ~ 3 nm, the first level) create small linear (branched) fragments (correlation radii
r2 ~ 7 nm, the second level) associated into chain structures (size
r3 ~ 20 nm) forming the cells (diameter
Dw ~ 2
r3 ~ 40 nm) which are integrated into a gel network.
In the network, the junctions’ functionality
fC =
mL/
n can be found using the amount of particles (
mL) per a cell. This value is included in the forward cross section σ
3 = (Δ
ρD)
2φ(π
dS3/6)
mLSL =
g3VL which is defined by the parameters of the correlation function (
g3,
r3) where
VL = 4π∫γ
3(
R)
R2dR = π
3/2r33 is the correlation volume and
SL(
q → 0) is the structure factor of the systems of the cells. The model of random contacts of spherical objects in the sample volume [
18] was used to evaluate the
SL = 1 − 8
VL/
V1L, where
V1L =
fC/
NL is the volume per cell that is determined by the numerical concentration of the cells (
NL). The parameter
VL =
fCVn/β includes the volume of the chain’s region,
Vn = π
r33/6, and the coefficient β characterizing the density of the chains’ package in a cell. If the regions of chains fill the total volume, β = 1. If between the regions any cavities exist, β < 1, e.g., in the case of spheres, the most dense packing β = β
S ≈ 0.74. Further we used the average β
a = (1 + β
S)/2 ≈ 0.87 to estimate the S
L = 1 − 8(
φ/
nβ
a)(
r3/
dS)
3 ≈ 0.54. Under these assumptions the amount of particles in a cell and the functionality of the network junctions is determined,
mL= (
g3VL)/[(Δ
ρD)
2φ(π
dS3/6)
SL] ≈ 70 and
fC =
mL/
n ≈ 4.These parameters are attributed to the original and diluted systems, saving almost their initial structural features at different scales (from one to tens of diameter of particle) characterizing the multilevel order detected in gels.