Silicene Nanoribbons on Pb-Reconstructed Si(111) Surface †
Abstract
:1. Introduction
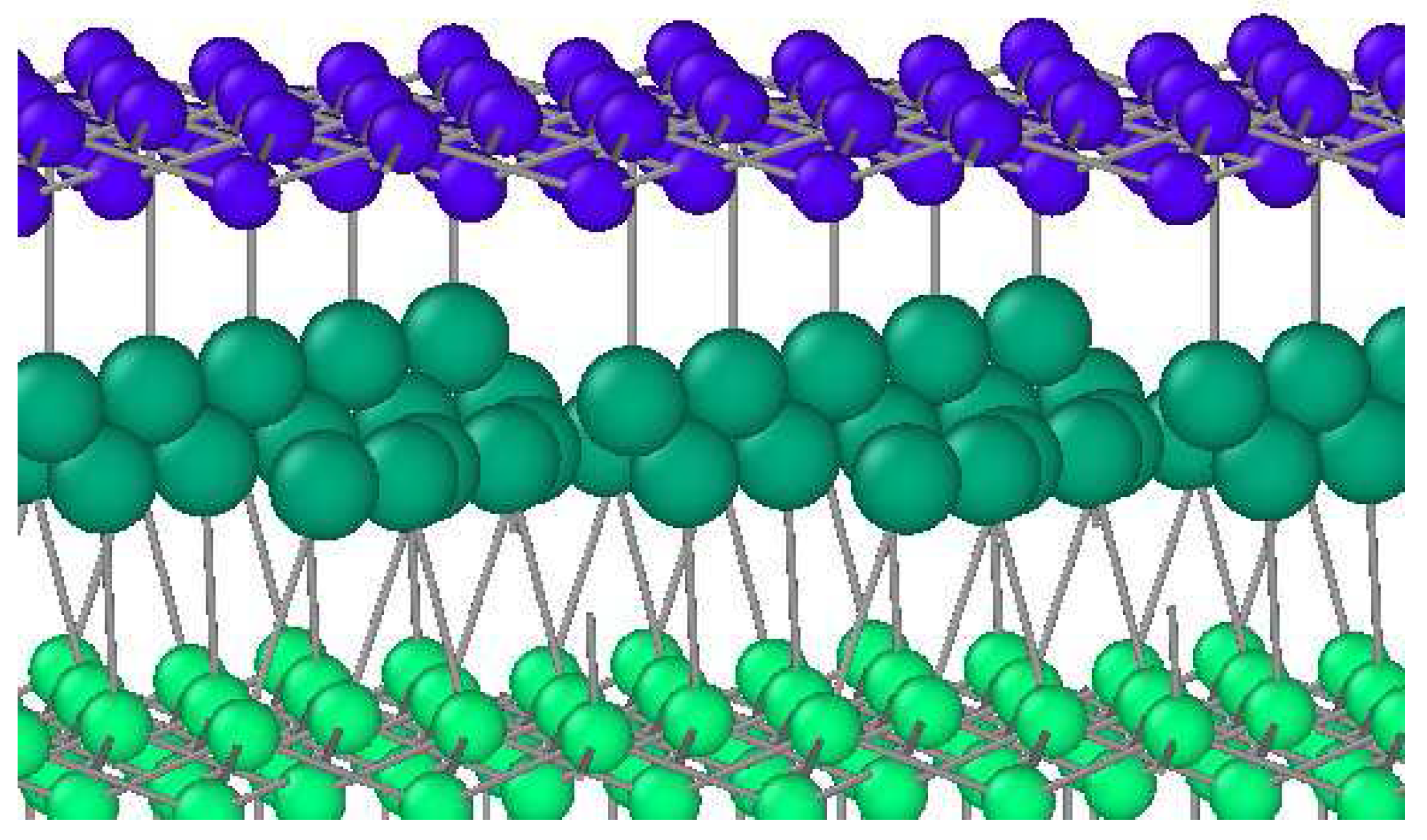
2. Materials and Methods
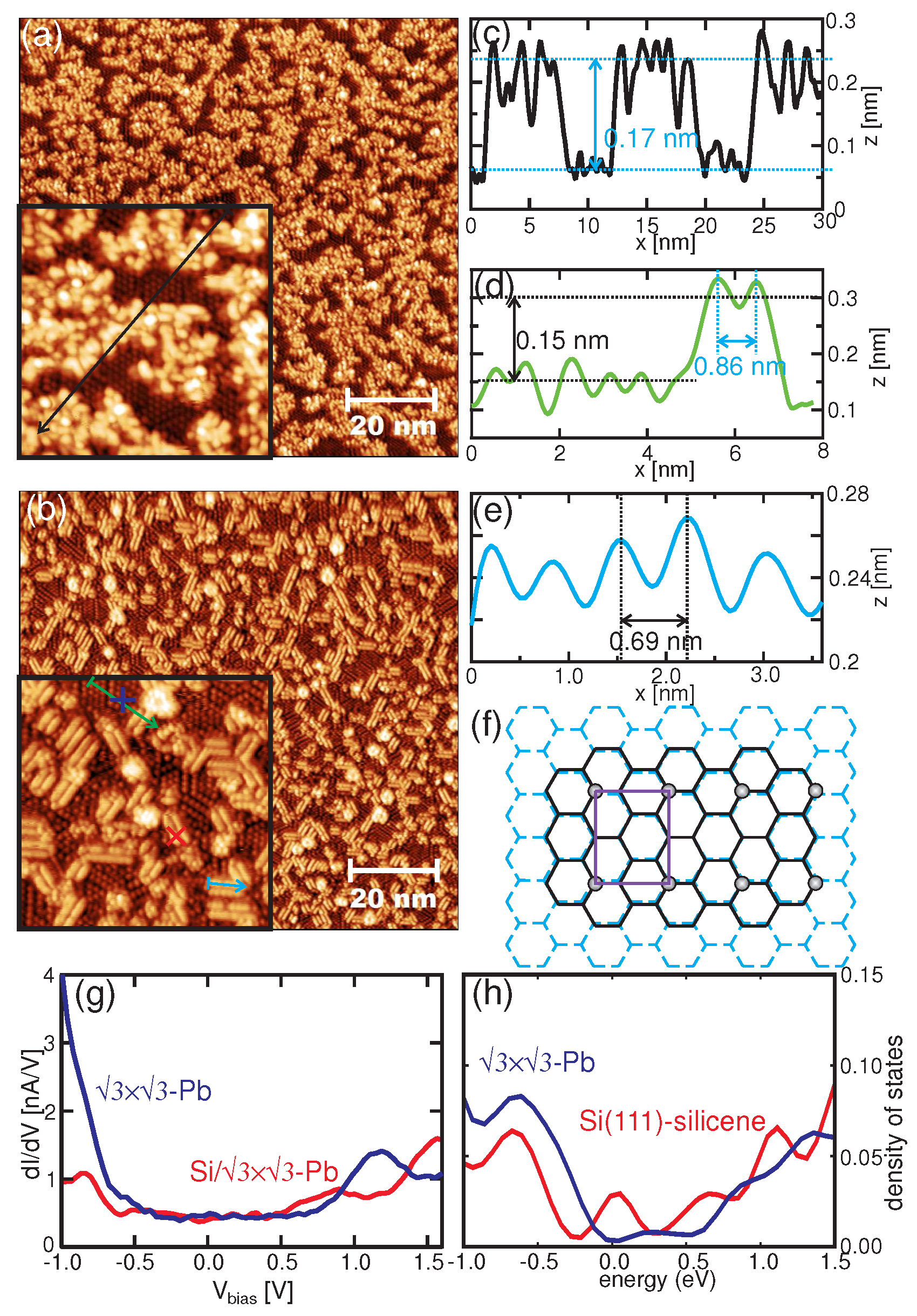
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphen. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhimanapati, G.R.; Lin, Z.; Meunier, V.; Jung, Y.; Cha, J.; Das, S.; Xiao, D.; Son, Y.; Strano, M.S.; Cooper, V.R.; et al. Recent advances in two-dimensional materials beyond graphene. ACS Nano 2015, 12, 11509–11539. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Castro Neto, A.H. 2D materials and van der Waals heterostructures. Science 2016, 353. [Google Scholar] [CrossRef] [PubMed]
- Molle, A. Xenes: A new emerging two-dimensional materials platform for nanoelectronics. ECSP Trans. 2016, 75, 163–173. [Google Scholar] [CrossRef]
- Houssa, M.; Dimoulas, A.; Molle, A. Silicene: A review of recent experimental and theoretical investigations. J. Phys. Condens. Matter 2015, 27, 253002. [Google Scholar] [CrossRef] [PubMed]
- Le Lay, G.; Salomon, E.; Angot, T. Silicene: Silicon conquers the 2D world. Europhys. News 2016, 47, 17–21. [Google Scholar] [CrossRef]
- Grazanetti, C.; Cinquanta, E.; Molle, A. Two-dimensional silicon: The advent of silicene. 2D Mater. 2016, 3, 012001. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, H.; Yu, Z.; Quhe, R.; Zhou, S.; Wang, Y.; Liu, C.C.; Zhong, H.; Han, N.; Lu, J.; et al. Rise of silicene: A competitive 2D material. Prog. Mater. Sci. 2016, 83, 24–151. [Google Scholar] [CrossRef]
- Molle, A.; Grazianetti, C.; Cinquanta, E. Silicene: Silicon at the two dimensional limit and its aplications to nanoelectronics. ECSP Trans. 2016, 75, 703–709. [Google Scholar] [CrossRef]
- Tao, L.; Cinquanta, E.; Chiappe, D.; Grazianetti, C.; Fanciulli, M.; Dubey, M.; Molle, A.; Akinwande, D. Silicene field-effect transistors operating at room temperature. Nat. Nanotechnol. 2015, 10, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Verri, G.G.; Lew Yan Voon, L.C. Electronic structure of silicon-based nanostructures. Phys. Rev. B 2007, 76, 075131. [Google Scholar] [CrossRef]
- Takeda, K.; Shiraishi, K. Theoretical possibility of stage corrugation in Si and Ge analogs of graphite. Phys. Rev. B 1994, 50, 14916. [Google Scholar] [CrossRef]
- Cahangirov, S.; Topsakal, M.; Aktürk, E.; Sahin, H.; Ciraci, S. Two- and One-Dimensional Honeycomb Structures of Silicon and Germanium. Phys. Rev. Lett. 2009, 102, 236804. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Feng, W.; Yao, Y. Quantum spin Hall effect in silicene and two-dimensional germanium. Phys. Rev. Lett. 2011, 107, 076802. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Manchon, A.; Sabeeh, K.; Schwingenschlögl, U. Quantum spin/valley Hall effect and topological insulator phase transitions in silicene. Appl. Phys. Lett. 2013, 102, 162412. [Google Scholar] [CrossRef]
- Wu, S.C.; Shan, G.; Yan, B. Prediction of Near-Room-Temperature Quantum Anomalous Hall Effect on Honeycomb Materials. Phys. Rev. Lett. 2014, 113, 256401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Liu, L.F.; Liu, W.M. Quantum anomalous Hall effect and tunable topological states in 3d transition metals doped silicene. Sci. Rep. 2013, 3, 2908. [Google Scholar] [CrossRef] [PubMed]
- Podsiadły-Paszkowska, A.; Krawiec, M. Spin-polarized gapped Dirac spectrum of unsupported silicene. Appl. Surf. Sci. 2016, 373, 45–50. [Google Scholar] [CrossRef]
- Drummond, N.D.; Zolyomi, V.; Falko, V.I. Electrically tunable band gap in silicene. Phys. Rev. B 2012, 85, 075423. [Google Scholar] [CrossRef]
- Ni, Z.; Liu, Q.; Tang, K.; Zheng, J.; Zhou, J.; Qin, R.; Gao, Z.; Yu, D.; Lu, J. Tunable Bandgap in Silicene and Germanene. Nano Lett. 2012, 12, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.A.; Gao, S.P.; Stein, R.; Coard, G. Tuning the electronic structure of silicene and germanene by biaxial strain and electric field. Phys. Rev. B 2015, 91, 245403. [Google Scholar] [CrossRef]
- Gürel, H.H.; Özcelik, V.O.; Ciraci, S. Effects of charging and perpendicular electric field on the properties of silicene and germanene. J. Phys. Condens. Matter 2013, 25, 305007. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, B.; Houssa, M.; Scalisea, E.; Pourtoisb, G.; Afanas‘ev, V.V.; Stesmans, A. First-principles electronic functionalization of silicene and germanene by adatom chemisorption. Appl. Surf. Sci. 2014, 291, 104–108. [Google Scholar] [CrossRef]
- Podsiadły-Paszkowska, A.; Krawiec, M. Electrical and mechanical controlling of the kinetic and magnetic properties of hydrogen atoms on free-standing silicene. J. Phys. Condens. Matter 2016, 28, 284004. [Google Scholar] [CrossRef] [PubMed]
- Podsiadły-Paszkowska, A.; Krawiec, M. Silicene on metallic quantum wells: an efficient way of tuning silicene-substrate interaction. Phys. Rev. B 2015, 92, 165411. [Google Scholar] [CrossRef]
- Vogt, P.; De Padova, P.; Quaresima, C.; Avila, J.; Frantzeskakis, E.; Asensio, M.C.; Resta, A.; Ealet, B.; Le Lay, G. Silicene: Compelling experimental evidence for graphenelike two-dimensional silicon. Phys. Rev. Lett. 2012, 108, 155501. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Ding, Z.; Meng, S.; Yao, Y.; He, X.; Cheng, P.; Chen, L.; Wu, K. Evidence of Silicene in Honeycomb Structures of Silicon on Ag(111). Nano Lett. 2012, 12, 3507–3511. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Arafune, R.; Kawahara, K.; Tsukahara, N.; Minamitani, E.; Kim, Y.; Takagi, N.; Kawai, M. Structure of silicene grown on Ag(111). Appl. Phys. Express 2012, 5, 045802. [Google Scholar] [CrossRef]
- Jamgotchian, H.; Colington, Y.; Hamazaouri, N.; Ealet, B.; Hoarau, J.; Aufray, B.; Biberian, J.P. Growth of silicene layers on Ag(111): Unexpected effect of the substrate temperature. J. Phys. Condens. Matter 2012, 24, 172001. [Google Scholar] [CrossRef] [PubMed]
- Fleurence, A.; Friedlein, R.; Ozaki, T.; Kawai, H.; Wang, Y.; Yamada-Takamura, Y. Experimental evidence for epitaxial silicene on diboride thin films. Phys. Rev. Lett. 2012, 108, 245501. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wang, Y.; Zhang, L.; Du, S.; Wu, R.; Li, L.; Zhang, Y.; Li, G.; Zhou, H.; Hofer, W.A.; et al. Buckled silicene formation on Ir(111). Nano Lett. 2013, 13, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Morishita, T.; Spencer, M.J.S.; Kawamoto, S.; Snook, I.K. A new surface and structure for silicene: Polygonal silicene formation on the Al(111) surface. J. Phys. Chem. C 2013, 117, 22142. [Google Scholar] [CrossRef]
- Aizawa, T.; Suehara, S.; Otani, S. Silicene on Zirconium Carbide (111). J. Phys. Chem. C 2014, 118, 23049. [Google Scholar] [CrossRef]
- Chiappe, D.; Scalise, E.; Cinquanta, E.; Granzietti, C.; van der Broek, B.; Fanciulli, M.; Houssa, M.; Molle, A. Two-dimensional Si nanosheets with local hexagonal structure on a MoS2 surface. Adv. Mater. 2014, 26, 2096–2101. [Google Scholar] [CrossRef] [PubMed]
- Sahaf, H.; Masson, L.; Leandri, C.; Aufray, B.; Le Lay, G. Formation of a one-dimensional grating at the molecular scale by self-assembly of straight silicon nanowires. Appl. Phys. Lett. 2007, 90, 263110. [Google Scholar] [CrossRef]
- Kara, A.; Leandri, C.; Davila, M.E.; De Padova, P.; Ealet, B.; Oughaddou, H.; Aufray, B.; Le Lay, G. Physics of silicene stripes. J. Supercond. Nov. Magn. 2009, 22, 259–263. [Google Scholar] [CrossRef]
- De Padova, P.; Quaresima, C.; Ottaviani, C.; Sheverdyaeva, P.M.; Moras, P.; Carbone, C.; Topwal, D.; Olivieri, B.; Kara, A.; Oughaddou, H.; et al. Evidence of graphene-like electronic signature in silicene nanoribbons. Appl. Phys. Lett. 2010, 96, 261905. [Google Scholar] [CrossRef]
- De Padova, P.; Quaresima, C.; Olivieri, B.; Perfetti, P.; Le Lay, G. sp2-like hybridization of silicon valence orbitals in silicene nanoribbons. Nano Lett. 2011, 98, 081909. [Google Scholar]
- Feng, B.; Li, H.; Meng, S.; Chen, L.; Wu, K. Structure and quantum well states in silicene nanoribbons on Ag(110). Surf. Sci. 2016, 645, 74–79. [Google Scholar] [CrossRef]
- Tchalala, M.R.; Enriquez, H.; Mayne, A.J.; Kara, A.; Roth, S.; Silly, M.G.; Bendounan, A.; Sirotti, F.; Greber, T.; Aufray, B.; et al. Formation of one-dimensional self-assembled silicon nanoribbons on Au(110)-(2×1). Appl. Phys. Lett. 2013, 102, 083107. [Google Scholar] [CrossRef]
- Leandri, C.; Le Lay, G.; Aufray, B.; Girardeaux, C.; Avila, J.; Dávila, M.E.; Asensio, M.C.; Ottaviani, C.; Cricenti, A. Self-aligned silicon quantum wires on Ag(110). Surf. Sci. 2005, 574, L9–L15. [Google Scholar] [CrossRef]
- De Padova, P.; Vogt, P.; Resta, A.; Avila, J.; Razado-Colambo, I.; Quaresima, C.; Ottaviani, C.; Olivieri, B.; Bruhn, T.; Hirahara, T.; et al. Evidence of Dirac fermions in multilayer silicene. Appl. Phys. Lett. 2013, 102, 163106. [Google Scholar] [CrossRef]
- De Padova, P.; Avila, J.; Resta, A.; Razado-Colambo, I.; Quaresima, C.; Ottaviani, C.; Olivieri, B.; Bruhn, T.; Vogt, P.; Asensio, M.C.; et al. The quasiparticle band dispersion in epitaxial multilayer silicene. Appl. Phys. Lett. 2013, 102, 163106. [Google Scholar] [CrossRef]
- De Padova, P.; Generosi, A.; Paci, B.; Ottaviani, C.; Quaresima, C.; Olivieri, B.; Salomon, E.; Angot, T.; Le Lay, G. Multilayer silicene: Clear evidence. 2D Mater. 2016, 3, 031011. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, H. Absence of a Dirac cone in silicene on Ag(111): First-principles density functional calculations with a modified effective band structure technique. Phys. Rev. B 2013, 87, 245430. [Google Scholar] [CrossRef]
- Guo, Z.; Furuya, S.; Iwata, J.I.; Oshiyama, A. Absence and presence of Dirac electrons in silicene on substrates. Phys. Rev. B 2013, 87, 235435. [Google Scholar] [CrossRef]
- Lin, C.; Arafune, R.; Kawahara, K.; Kanno, M.; Tsukahara, N.; Minamitani, E.; Kim, Y.; Kawai, M.; Takagi, N. Substrate-induced symmetry breaking in silicene. Phys. Rev. Lett. 2013, 110, 076801. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gao, J.; Zhao, J. Silicene on Substrates: A Way To Preserve or Tune Its Electronic Properties. J. Phys. Chem. C 2013, 117, 10353–10359. [Google Scholar] [CrossRef]
- Kanno, M.; Arafune, R.; Lin, C.L.; Minamitani, E.; Kawai, M.; Takagi, N. Electronic decoupling by h-BN layer between silicene and Cu(111): A DFT-based analysis. New J. Phys. 2014, 16, 105019. [Google Scholar] [CrossRef]
- Zhu, J.; Schwingenschlögl, U. Structural and Electronic Properties of Silicene on MgX2 (X = Cl, Br, and I). Appl. Mater. Interfaces 2014, 6, 11675–11681. [Google Scholar] [CrossRef] [PubMed]
- Podsiadły-Paszkowska, A.; Krawiec, M. Dirac fermions in silicene on Pb(111) surface. Phys. Chem. Chem. Phys. 2015, 17, 2246–2251. [Google Scholar] [CrossRef] [PubMed]
- Schwartzentruber, B.S.; Mo, Y.W.; Webb, M.B.; Legally, M.G. Scanning tunneling microscopy studies of structural disorder and steps on Si surfaces. J. Vac. Sci. Technol. A 1989, 7, 2901. [Google Scholar] [CrossRef]
- Ganz, E.; Hwang, I.S.; Xiong, F.; Theiss, S.K.; Golovchenko, J. Growth and morphology of Pb on Si(111). Surf. Sci. 1991, 257, 259–273. [Google Scholar] [CrossRef]
- Horikoshi, K.; Tong, X.; Nagao, T.; Hasegawa, S. Structural phase transitions of Pb-adsorbed Si(111) surfaces at low temperatures. Phys. Rev. B 1999, 60, 13287. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augumented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Hwang, I.S.; Martinez, R.E.; Liu, C.; Golovchenko, J.A. High coverage phases of Pb on the Si(111) surface: Structures and phase transitions. Surf. Sci. 1995, 323, 241–257. [Google Scholar] [CrossRef]
- Hupalo, M.; Tringides, M.C. “Devil staircase” in Pb/Si(111) ordered phases. Phys. Rev. Lett. 2003, 90, 216106. [Google Scholar] [CrossRef] [PubMed]
- Svec, M.; Chab, V.; Tringides, M.C. Resolving the coverage puzzle of the Pb/Si(111)- phase. J. Appl. Phys. 2009, 106, 053501. [Google Scholar] [CrossRef]
- Zhang, T.; Cheng, P.; Li, W.J.; Sun, Y.J.; Wang, G.; Zhu, X.G.; He, K.; Wang, L.; Ma, X.; Chen, X.; et al. Superconductivity in one-atomic-layer metal films grown on Si(111). Nat. Phys. 2010, 6, 104–108. [Google Scholar] [CrossRef]
- Liu, H.; Gao, J.; Zhao, J. Silicene on Substrates: interaction mechanism and growth behavior. J. Phys. Conf. Ser. 2014, 491, 012007. [Google Scholar] [CrossRef]
- Brihuega, I.; Ugeda, M.M.; Gómez-Rodriguez, J.M. Surface diffusion of Pb single adatoms on the Si(111)-R30°-Pb system. Phys. Rev. B 2007, 76, 035422. [Google Scholar] [CrossRef]
- Nita, P.; Jałochowski, M.; Krawiec, M.; Stȩpniak, A. One-dimensional diffusion of Pb atoms on the Si(553)-Au surface. Phys. Rev. Lett. 2011, 107, 026101. [Google Scholar] [CrossRef] [PubMed]
- Nita, P.; Palotás, K.; Jałochowski, M.; Krawiec, M. Surface diffusion of Pb atoms on the Si(553)-Au surface in narrow quasi-one-dimensional channels. Phys. Rev. B 2014, 89, 165426. [Google Scholar] [CrossRef]
- Fan, W.C.; Ignatiev, A.; Huang, H.; Tong, S.Y. Observation and structural determination of ()R30°. Phys. Rev. Lett. 1989, 62, 1516. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, P.; Saintoney, S.; Pirri, C.; Bolmont, D.; Gewinner, G. Surface states and reconstruction of epitaxial R30° Er silicide on Si(111). Phys. Rev. B 1994, 50, 10886. [Google Scholar] [CrossRef]
- Jałochowski, M.; Palotás, K.; Krawiec, M. Spilling of electronic states in Pb quantum wells. Phys. Rev. B 2016, 93, 035437. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, J.; Li, H.; Meng, S. Stacking-dependent electronic structure of bilayer silicene. Appl. Phys. Lett. 2014, 104, 131904. [Google Scholar] [CrossRef]
- Acun, A.; Akaishi, A.; Angot, T.; Araidai, M.; Aristov, V.; Aruga, T.; Avdeev, M.; Banas, K.; Banas, A.; Banhartet, F.; et al. Atomically Controlled Surfaces Interfaces and Nanostructures; Bianconi, A., Marcelli, A., Eds.; Superstripes Press: Rome, Italy, 2016; ISBN 9788866830597. [Google Scholar]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stȩpniak-Dybala, A.; Jałochowski, M.; Krawiec, M. Silicene Nanoribbons on Pb-Reconstructed Si(111) Surface. Condens. Matter 2016, 1, 8. https://doi.org/10.3390/condmat1010008
Stȩpniak-Dybala A, Jałochowski M, Krawiec M. Silicene Nanoribbons on Pb-Reconstructed Si(111) Surface. Condensed Matter. 2016; 1(1):8. https://doi.org/10.3390/condmat1010008
Chicago/Turabian StyleStȩpniak-Dybala, Agnieszka, Mieczysław Jałochowski, and Mariusz Krawiec. 2016. "Silicene Nanoribbons on Pb-Reconstructed Si(111) Surface" Condensed Matter 1, no. 1: 8. https://doi.org/10.3390/condmat1010008





