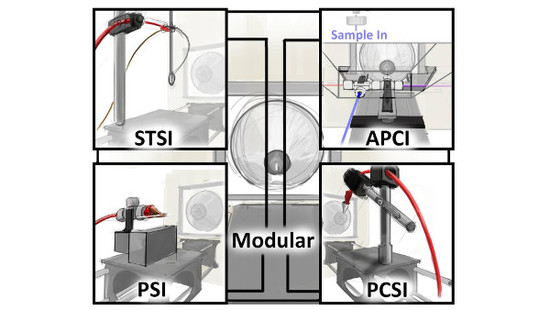
A Low-Cost, Simplified Platform of Interchangeable, Ambient Ionization Sources for Rapid, Forensic Evidence Screening on Portable Mass Spectrometric Instrumentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Portable MS System and Ambient Ionization Sources
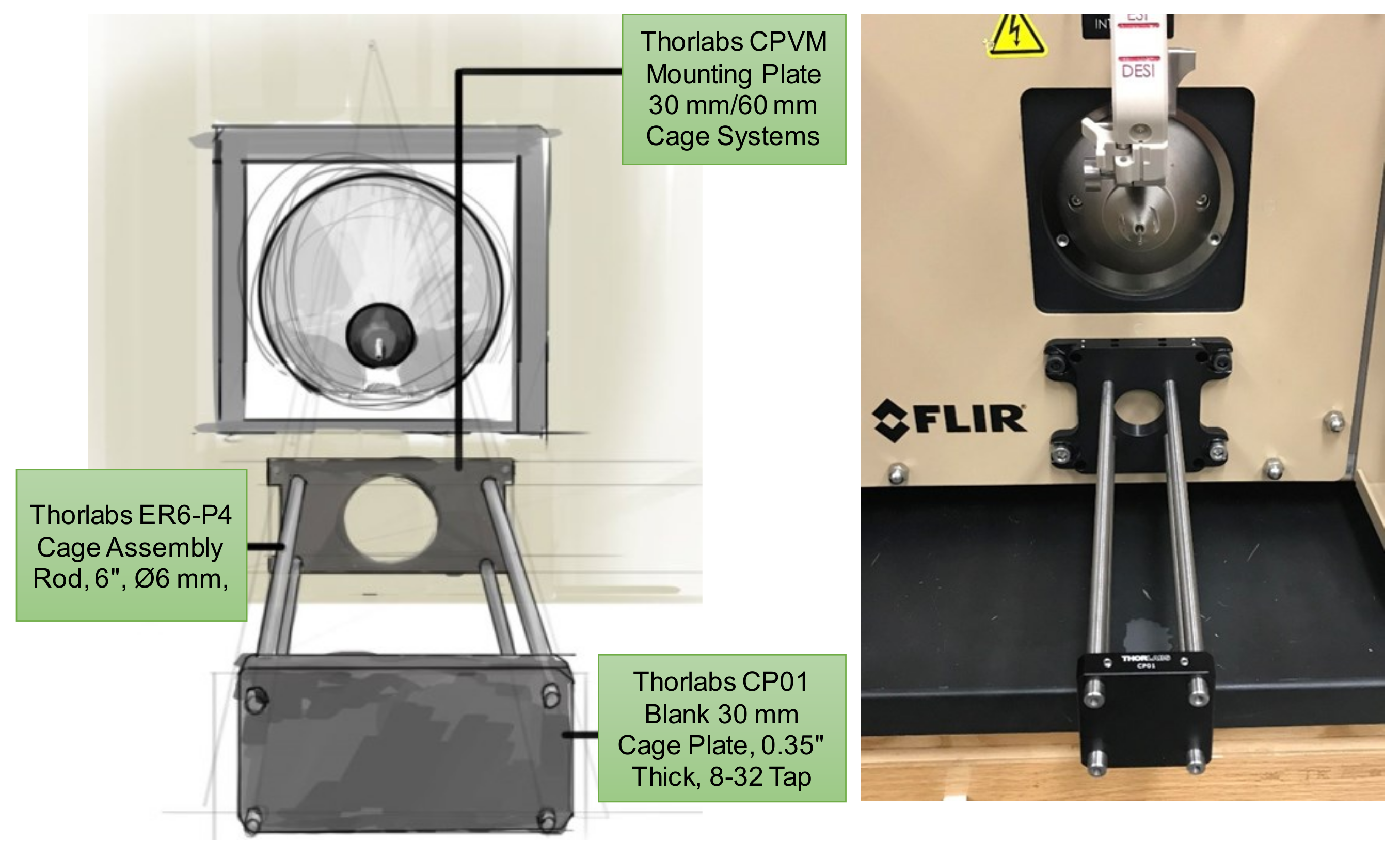
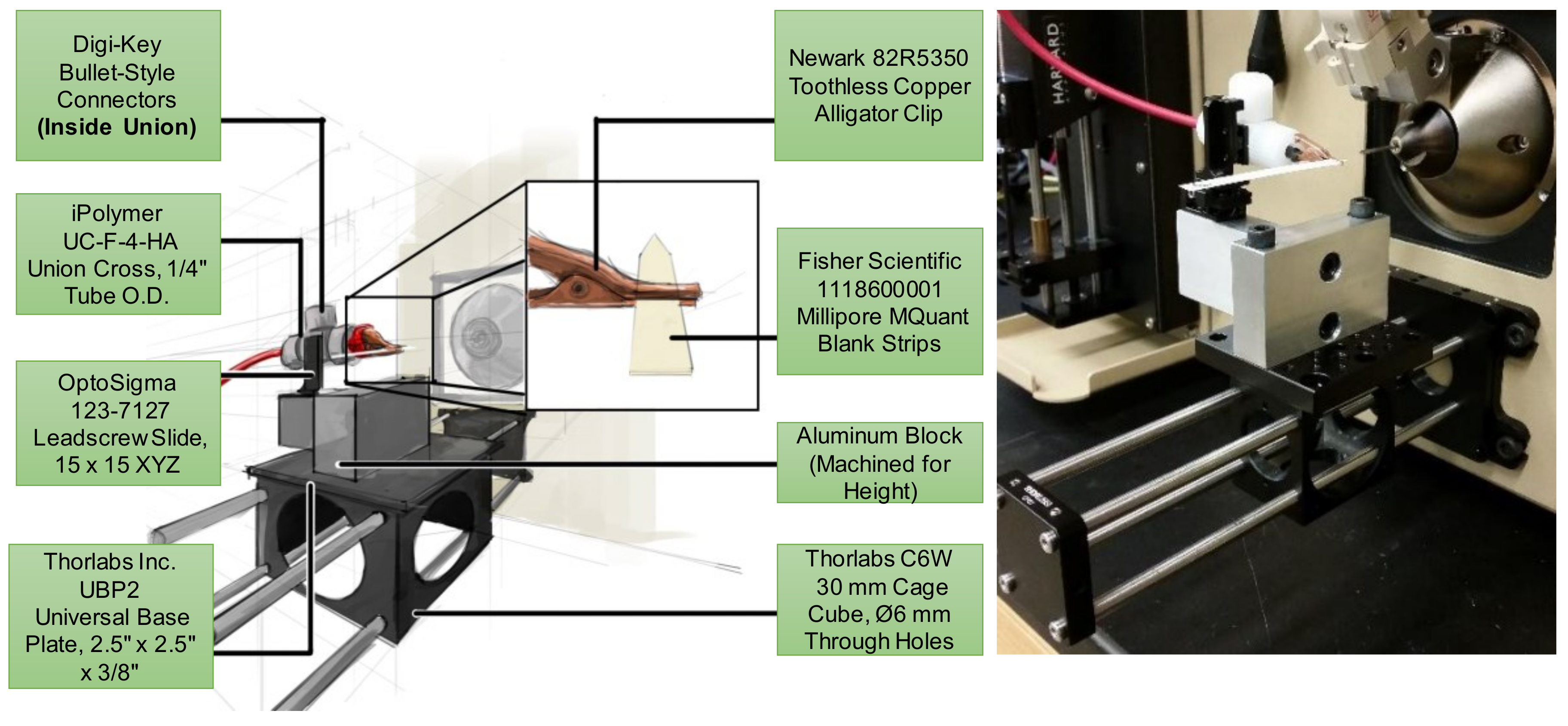
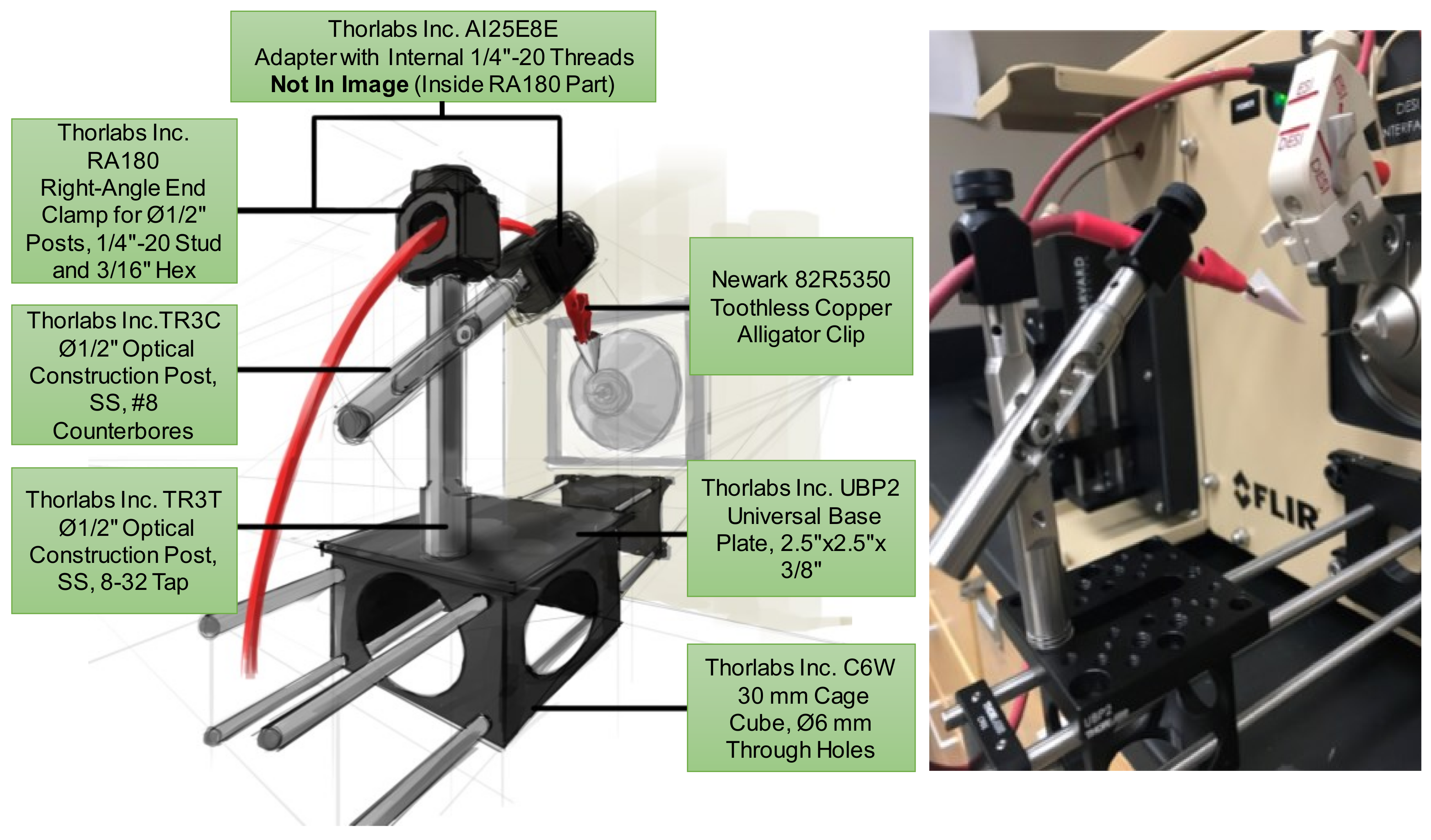
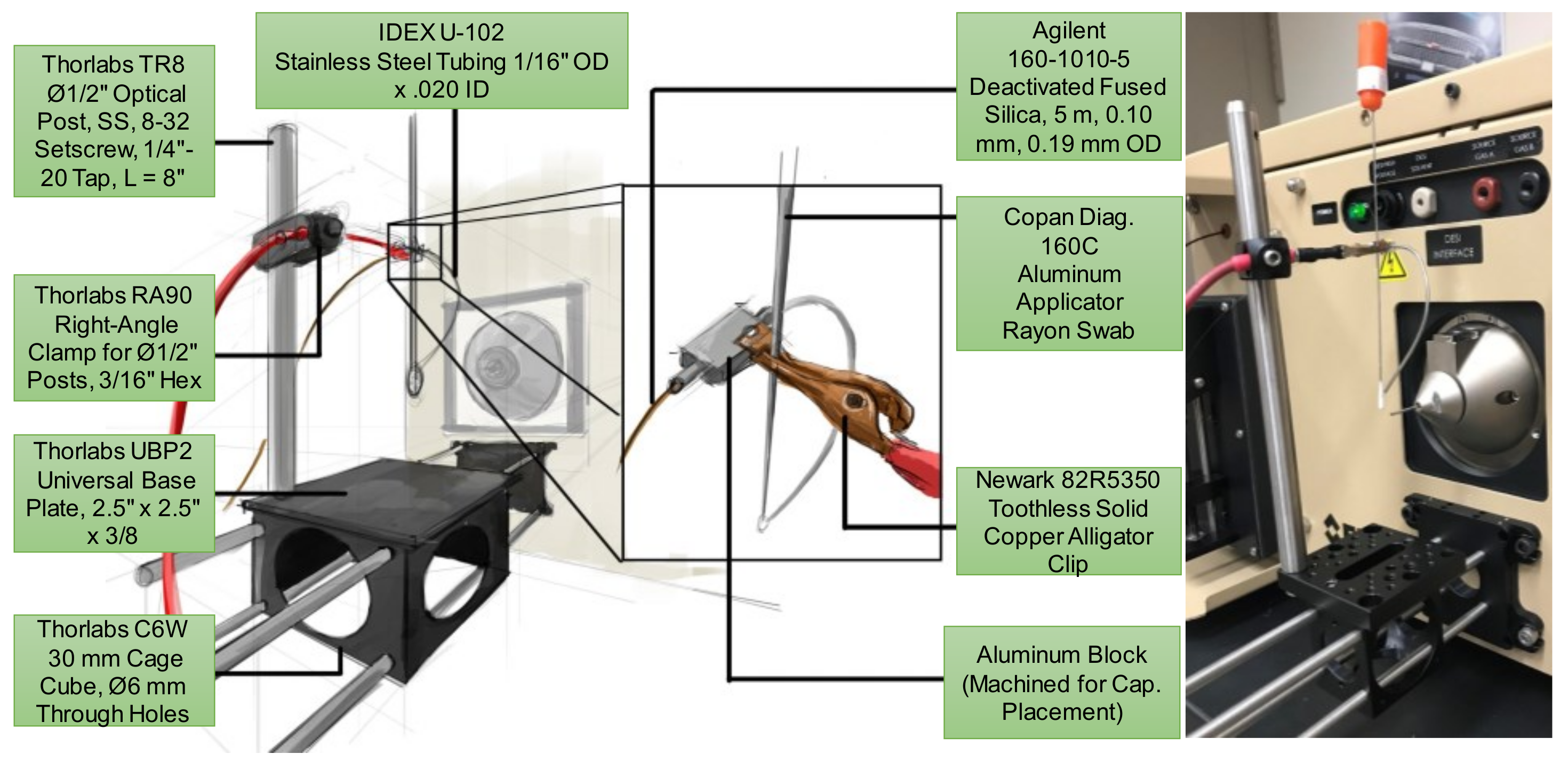
2.2. Centralized Mounting System for Interchangeable Ion Source Modules
3. Results and Discussion
3.1. Paper Spray Ionization (PSI)
3.2. Paper Cone Spray Ionization (PCSI)
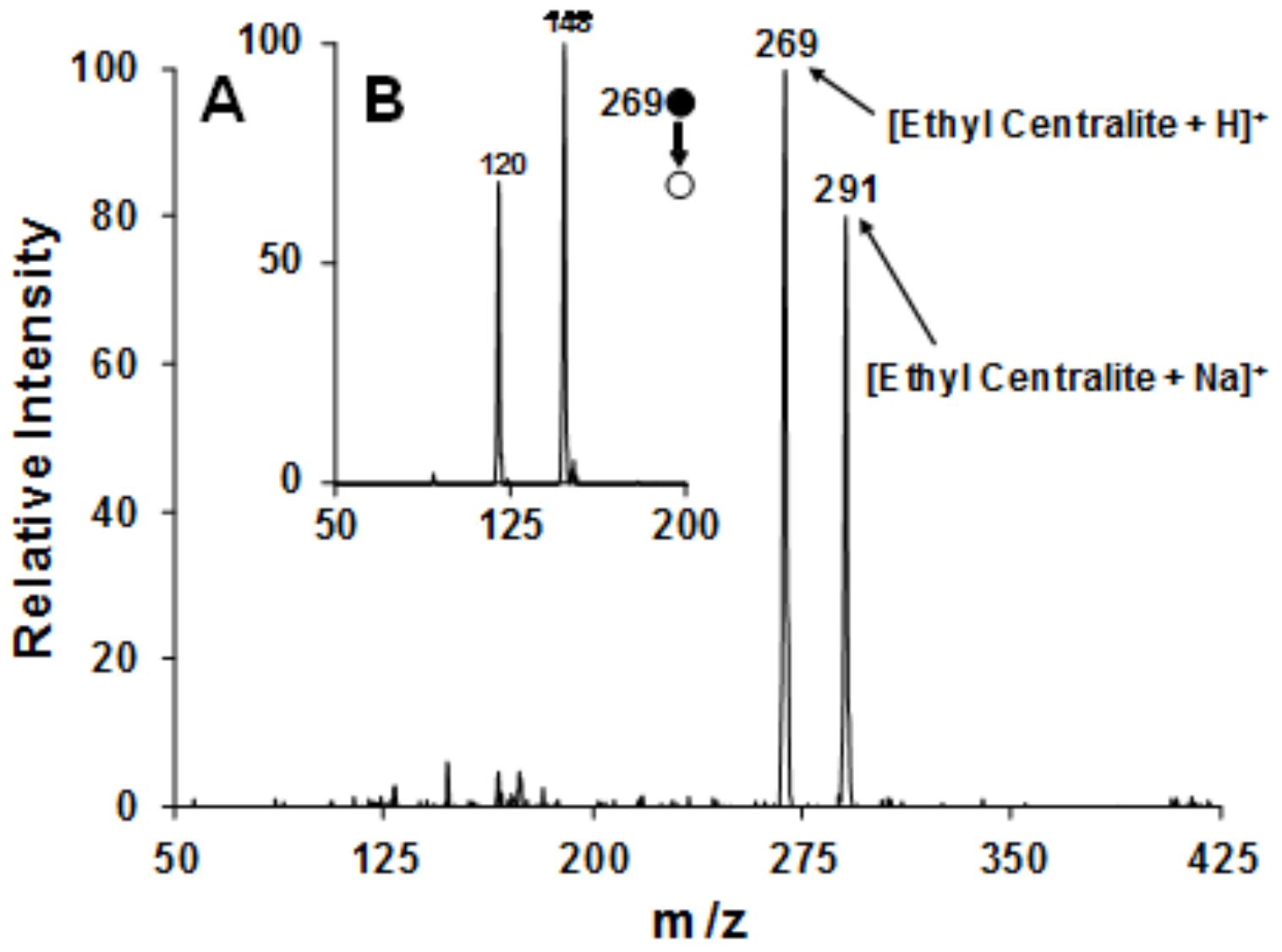
3.3. Swab Touch Spray Ionization (STSI)
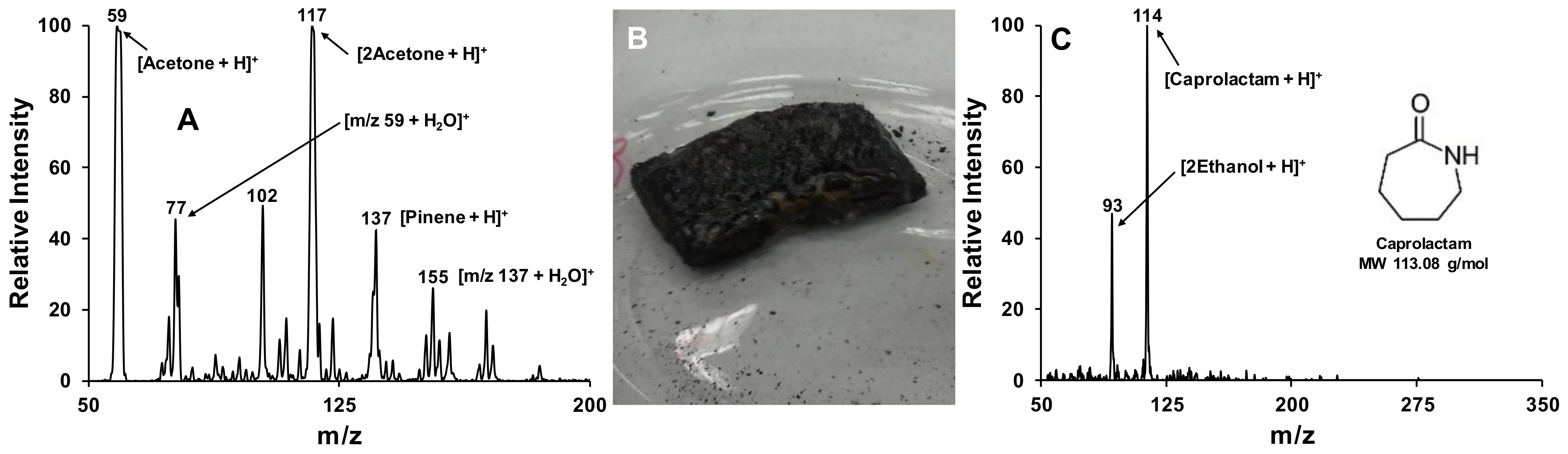
3.4. Atmospheric Pressure Chemical Ionization (APCI)
4. Conclusions
Supplementary Material
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guo, Q.; Gao, L.; Zhai, Y.; Xu, W. Recent developments of miniature ion trap mass spectrometers. Chin. Chem. Lett. 2017. [Google Scholar] [CrossRef]
- Snyder, D.T.; Pulliam, C.J.; Ouyang, Z.; Cooks, R.G. Miniature and fieldable mass spectrometers: Recent advances. Anal. Chem. 2016, 88, 2–29. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, X.; Zhai, Y.; Xu, W. Mini 2000: A robust miniature mass spectrometer with continuous atmospheric pressure interface. Instruments 2018, 2. [Google Scholar] [CrossRef]
- Plocoste, T.; Jacoby-Koaly, S.; Petit, R.-H.; Molinié, J.; Roussas, A. In situ quantification and tracking of volatile organic compounds with a portable mass spectrometer in tropical waste and urban sites. Environ. Technol. 2017, 38, 2280–2294. [Google Scholar] [CrossRef] [PubMed]
- Mach, P.M.; McBride, E.M.; Sasiene, Z.J.; Brigance, K.R.; Kennard, S.K.; Wright, K.C.; Verbeck, G.F. Vehicle-mounted portable mass spectrometry system for the covert detection via spatial analysis of clandestine methamphetamine laboratories. Anal. Chem. 2015, 87, 11501–11508. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.M.; Cleary, S.R.; O’Leary, A.E.; Gizzi, M.C.; Mulligan, C.C. Balancing the utility and legality of implementing portable mass spectrometers coupled with ambient ionization in routine law enforcement activities. Anal. Methods 2017, 9, 5015–5022. [Google Scholar] [CrossRef]
- Brown, H.; Oktem, B.; Windom, A.; Doroshenko, V.; Evans-Nguyen, K. Direct Analysis in Real Time (DART) and a portable mass spectrometer for rapid identification of common and designer drugs on-site. Forensic Chem. 2016, 1, 66–73. [Google Scholar] [CrossRef]
- Lam, R.; Lennard, C.; Kingsland, G.; Johnstone, P.; Symons, A.; Wythes, L.; Fewtrell, J.; O’Brien, D.; Spikmans, V. Person-portable equipment in environmental forensic investigations: Application to fire scenes. Aust. J. Forensic Sci. 2018, 1–10. [Google Scholar] [CrossRef]
- Hall, S.E.; O’Leary, A.E.; Lawton, Z.E.; Bruno, A.M.; Mulligan, C.C. Trace-Level Screening of Chemicals Related to Clandestine Desomorphine Production with Ambient Sampling, Portable Mass Spectrometry. J. Chem. 2017. [Google Scholar] [CrossRef]
- Evans-Nguyen, K.M.; Quinto, A.; Hargraves, T.; Brown, H.; Speer, J.; Glatter, D. Transmission mode desorption electrospray ionization (TM-DESI) for simultaneous analysis of potential inorganic and organic components of radiological dispersion devices (RDDs). Anal. Chem. 2013, 85, 11826–11834. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Roth, M.J.; Keil, A.D.; Grossenbacher, J.W.; Justes, D.R.; Patterson, G.E.; Barket, D.J. Implementation of DART and DESI ionization on a fieldable mass spectrometer. J. Am. Soc. Mass Spectrom. 2008, 19, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Song, Q.; Patterson, G.E.; Cooks, R.G.; Ouyang, Z. Handheld rectilinear ion trap mass spectrometer. Anal. Chem. 2006, 78, 5994–6002. [Google Scholar] [CrossRef] [PubMed]
- Badman, E.R.; Graham Cooks, R. Miniature mass analyzers. J. Mass Spectrom. 2000, 35, 659–671. [Google Scholar] [CrossRef]
- Cooks, R.G.; Ouyang, Z.; Takats, Z.; Wiseman, J.M. Ambient mass spectrometry. Science 2006, 311, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Takáts, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Cody, R.B.; Laramée, J.A.; Durst, H.D. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal. Chem. 2005, 77, 2297–2302. [Google Scholar] [CrossRef] [PubMed]
- Takats, Z.; Cotte-Rodriguez, I.; Talaty, N.; Chen, H.; Cooks, R.G. Direct, trace level detection of explosives on ambient surfaces by desorption electrospray ionization mass spectrometry. Chem. Commun. 2005, 1950–1952. [Google Scholar] [CrossRef] [PubMed]
- Justes, D.R.; Talaty, N.; Cotte-Rodriguez, I.; Cooks, R.G. Detection of explosives on skin using ambient ionization mass spectrometry. Chem. Commun. 2007, 2142–2144. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, A.E.; Hall, S.E.; Vircks, K.E.; Mulligan, C.C. Monitoring the clandestine synthesis of methamphetamine in real-time with ambient sampling, portable mass spectrometry. Anal. Methods 2015, 7, 7156–7163. [Google Scholar] [CrossRef]
- Steiner, R.R.; Larson, R.L. Validation of the direct analysis in real time source for use in forensic drug screening. J. Forensic Sci. 2009, 54, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Keil, A.; Talaty, N.; Janfelt, C.; Noll, R.J.; Gao, L.; Ouyang, Z.; Cooks, R.G. Ambient mass spectrometry with a handheld mass spectrometer at high pressure. Anal. Chem. 2007, 79, 7734–7739. [Google Scholar] [CrossRef] [PubMed]
- Lawton, Z.E.; Traub, A.; Fatigante, W.L.; Mancias, J.; O’Leary, A.E.; Hall, S.E.; Wieland, J.R.; Oberacher, H.; Gizzi, M.C.; Mulligan, C.C. Analytical validation of a portable mass spectrometer featuring interchangeable, ambient ionization sources for high throughput forensic evidence screening. J. Am. Soc. Mass Spectrom. 2017, 28, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Pavlovich, M.J.; Musselman, B.; Hall, A.B. Direct analysis in real time—Mass spectrometry (DART-MS) in forensic and security applications. Mass Spectrom. Rev. 2018, 37, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Correa, D.N.; Santos, J.M.; Eberlin, L.S.; Eberlin, M.N.; Teunissen, S.F. Forensic chemistry and ambient mass spectrometry: A perfect couple destined for a happy marriage? Anal. Chem. 2016, 88, 2515–2526. [Google Scholar] [CrossRef] [PubMed]
- Ifa, D.R.; Jackson, A.U.; Paglia, G.; Cooks, R.G. Forensic applications of ambient ionization mass spectrometry. Anal. Bioanal. Chem. 2009, 394, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Morelato, M.; Beavis, A.; Kirkbride, P.; Roux, C. Forensic applications of desorption electrospray ionisation mass spectrometry (DESI-MS). Forensic Sci. Int. 2013, 226, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Green, F.M.; Salter, T.L.; Stokes, P.; Gilmore, I.S.; O’Connor, G. Ambient mass spectrometry: Advances and applications in forensics. Surf. Interface Anal. 2010, 42, 347–357. [Google Scholar] [CrossRef]
- Jjunju, F.P.M.; Maher, S.; Li, A.; Badu-Tawiah, A.K.; Taylor, S.; Graham Cooks, R. Analysis of Polycyclic Aromatic Hydrocarbons Using Desorption Atmospheric Pressure Chemical Ionization Coupled to a Portable Mass Spectrometer. J. Am. Soc. Mass Spectrom. 2015, 26, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, J.; Zhang, X.; Luo, M.; Wang, Z.; Qiao, X. Surface desorption atmospheric pressure chemical ionization mass spectrometry for direct ambient sample analysis without toxic chemical contamination. J. Mass Spectrom. 2007, 42, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Manicke, N.E.; Lin, J.-M.; Cooks, R.G.; Ouyang, Z. Development, characterization, and application of paper spray ionization. Anal. Chem. 2010, 82, 2463–2471. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Cha, S. Paper cone spray ionization mass spectrometry (PCSI MS) for simple and rapid analysis of raw solid samples. Analyst 2015, 140, 5868–5872. [Google Scholar] [CrossRef] [PubMed]
- Fedick, P.W.; Bain, R.M. Swab touch spray mass spectrometry for rapid analysis of organic gunshot residue from human hand and various surfaces using commercial and fieldable mass spectrometry systems. Forensic Chem. 2017, 5, 53–57. [Google Scholar] [CrossRef]
- Harper, J.D.; Charipar, N.A.; Mulligan, C.C.; Zhang, X.; Cooks, R.G.; Ouyang, Z. Low-temperature plasma probe for ambient desorption ionization. Anal. Chem. 2008, 80, 9097–9104. [Google Scholar] [CrossRef] [PubMed]
- Shelley, J.T.; Wiley, J.S.; Hieftje, G.M. Ultrasensitive ambient mass spectrometric analysis with a pin-to-capillary flowing atmospheric-pressure afterglow source. Anal. Chem. 2011, 83, 5741–5748. [Google Scholar] [CrossRef] [PubMed]
- Javanshad, R.; Venter, A.R. Ambient ionization mass spectrometry: Real-time, proximal sample processing and ionization. Anal. Methods 2017, 9, 4896–4907. [Google Scholar] [CrossRef]
- Weston, D.J. Ambient ionization mass spectrometry: Current understanding of mechanistic theory; analytical performance and application areas. Analyst 2010, 135, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Monge, M.E.; Harris, G.A.; Dwivedi, P.; Fernández, F.M. Mass spectrometry: Recent advances in direct open air surface sampling/ionization. Chem. Rev. 2013, 113, 2269–2308. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Jjunju, F.P.M.; Taylor, S. Colloquium: 100 years of mass spectrometry: Perspectives and future trends. Rev. Mod. Phys. 2015, 87, 113–135. [Google Scholar] [CrossRef]
- Mulligan, C.C.; Talaty, N.; Cooks, R.G. Desorption electrospray ionization with a portable mass spectrometer: In situ analysis of ambient surfaces. Chem. Commun. 2006, 1709–1711. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, S.F.; Fedick, P.W.; Berendsen, B.J.A.; Nielen, M.W.F.; Eberlin, M.N.; Graham Cooks, R.; van Asten, A.C. Novel selectivity-based forensic toxicological validation of a paper spray mass spectrometry method for the quantitative determination of eight amphetamines in whole blood. J. Am. Soc. Mass Spectrom. 2017, 28, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- McKenna, J.; Jett, R.; Shanks, K.; Manicke, N.E. Toxicological drug screening using paper spray high-resolution tandem mass spectrometry (HR-MS/MS). J. Anal. Toxicol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gurdak, E.; Green, F.M.; Rakowska, P.D.; Seah, M.P.; Salter, T.L.; Gilmore, I.S. VAMAS interlaboratory study for desorption electrospray ionization mass spectrometry (DESI MS) intensity repeatability and constancy. Anal. Chem. 2014, 86, 9603–9611. [Google Scholar] [CrossRef] [PubMed]
- SWGDRUG. SWGDRUG Recommendations Edition 7.1. Available online: http://www.swgdrug.org/Documents/SWGDRUG%20Recommendations%20Version%207-1.pdf (accessed on 20 March 2018).
- Fedick, P.W.; Bills, B.J.; Manicke, N.E.; Cooks, R.G. Forensic sampling and analysis from a single substrate: Surface-enhanced Raman spectroscopy followed by paper spray mass spectrometry. Anal. Chem. 2017, 89, 10973–10979. [Google Scholar] [CrossRef] [PubMed]
- Giannoukos, S.; Brkić, B.; Taylor, S.; Marshall, A.; Verbeck, G.F. Chemical Sniffing Instrumentation for Security Applications. Chem. Rev. 2016, 116, 8146–8172. [Google Scholar] [CrossRef] [PubMed]
- Vircks, K.E.; Mulligan, C.C. Rapid screening of synthetic cathinones as trace residues and in authentic seizures using a portable mass spectrometer equipped with desorption electrospray ionization. Rapid Commun. Mass Spectrom. 2012, 26, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Damon, D.E.; Davis, K.M.; Moreira, C.R.; Capone, P.; Cruttenden, R.; Badu-Tawiah, A.K. Direct biofluid analysis using hydrophobic paper spray mass spectrometry. Anal. Chem. 2016, 88, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, J.; Cooks, R.G.; Ouyang, Z. Paper spray for direct analysis of complex mixtures using mass spectrometry. Angew. Chem. Int. Ed. 2010, 49, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-W.; Tipple, C.A.; Yost, R.A. Application of paper spray ionization for explosives analysis. Rapid Commun. Mass Spectrom. 2017, 31, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- McKenna, J.; Dhummakupt, E.S.; Connell, T.; Demond, P.S.; Miller, D.B.; Michael Nilles, J.; Manicke, N.E.; Glaros, T. Detection of chemical warfare agent simulants and hydrolysis products in biological samples by paper spray mass spectrometry. Analyst 2017, 142, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, A.E.; Oberacher, H.; Hall, S.E.; Mulligan, C.C. Combining a portable, tandem mass spectrometer with automated library searching—An important step towards streamlined, on-site identification of forensic evidence. Anal. Methods 2015, 7, 3331–3339. [Google Scholar] [CrossRef]
- Damon, D.E.; Maher, Y.S.; Yin, M.; Jjunju, F.P.M.; Young, I.S.; Taylor, S.; Maher, S.; Badu-Tawiah, A.K. 2D wax-printed paper substrates with extended solvent supply capabilities allow enhanced ion signal in paper spray ionization. Analyst 2016, 141, 3866–3873. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Jjunju, F.P.M.; Damon, D.E.; Gorton, H.; Maher, Y.S.; Syed, S.U.; Heeren, R.M.A.; Young, I.S.; Taylor, S.; Badu-Tawiah, A.K. Direct Analysis and Quantification of Metaldehyde in Water Using Reactive Paper Spray Mass Spectrometry. Sci. Rep. 2016, 6, 35643. [Google Scholar] [CrossRef] [PubMed]
- Pavlic, M.; Libiseller, K.; Oberacher, H. Combined use of ESI–QqTOF-MS and ESI–QqTOF-MS/MS with mass-spectral library search for qualitative analysis of drugs. Anal. Bioanal. Chem. 2006, 386, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Jun, G.; Park, T.-M.; Cha, S. Fast and simple chemical fingerprinting analysis of medicinal herbs by paper cone spray ionization mass spectrometry (PCSI MS). Bull. Korean Chem. Soc. 2016, 37, 1337–1343. [Google Scholar] [CrossRef]
- Fang, W.B.; Chang, Y.; McCance-Katz, E.F.; Moody, D.E. Determination of Naloxone and Nornaloxone (Noroxymorphone) by High-Performance Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry. J. Anal. Toxicol. 2009, 33, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Moody, D.E.; Slawson, M.H.; Strain, E.C.; Laycock, J.D.; Spanbauer, A.C.; Foltz, R.L. A Liquid Chromatographic-Electrospray Ionization-Tandem Mass Spectrometric Method for Determination of Buprenorphine, Its Metabolite, norBuprenorphine, and a Coformulant, Naloxone, That Is Suitable for in Vivo and in Vitro Metabolism Studies. Anal. Biochem. 2002, 306, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Jarmusch, A.K.; Pirro, V.; Kerian, K.S.; Cooks, R.G. Detection of strep throat causing bacterium directly from medical swabs by touch spray-mass spectrometry. Analyst 2014, 139, 4785–4789. [Google Scholar] [CrossRef] [PubMed]
- Pirro, V.; Jarmusch, A.K.; Vincenti, M.; Cooks, R.G. Direct drug analysis from oral fluid using medical swab touch spray mass spectrometry. Anal. Chim. Acta 2015, 861, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Feng, Y.; Wei, Y.; Wang, Y.; Xu, W. Development of a miniature mass spectrometer with continuous atmospheric pressure interface. Analyst 2015, 140, 3406–3414. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, B.C.; Mulligan, C.C.; Cooks, R.G. Atmospheric pressure ionization in a miniature mass spectrometer. Anal. Chem. 2005, 77, 2928–2939. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.C.; Justes, D.R.; Noll, R.J.; Sanders, N.L.; Laughlin, B.C.; Cooks, R.G. Direct monitoring of toxic compounds in air using a portable mass spectrometer. Analyst 2006, 131, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Gao, L.; Duncan, J.; Harper, J.D.; Sanders, N.L.; Ouyang, Z.; Cooks, R.G. Direct detection of benzene, toluene, and ethylbenzene at trace levels in ambient air by atmospheric pressure chemical ionization using a handheld mass spectrometer. J. Am. Soc. Mass Spectrom. 2010, 21, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.E.; Mulligan, C.C. Application of ambient sampling portable mass spectrometry toward on-site screening of clandestine drug operations. LCGC 2014, 12, 8–13. [Google Scholar]
- Horning, E.C.; Horning, M.G.; Carroll, D.I.; Dzidic, I.; Stillwell, R.N. New picogram detection system based on a mass spectrometer with an external ionization source at atmospheric pressure. Anal. Chem. 1973, 45, 936–943. [Google Scholar] [CrossRef]
- Proctor, C.J.; Todd, J.F.J. Atmospheric pressure ionization mass spectrometry. Org. Mass Spectrom. 1983, 18, 509–516. [Google Scholar] [CrossRef]
- Lehrle, R.S.; Parsons, I.W.; Rollinson, M. Thermal degradation mechanisms of nylon 6 deduced from kinetic studies by pyrolysis-g.c. Polym. Degrad. Stab. 2000, 67, 21–33. [Google Scholar] [CrossRef]
- Jjunju, F.P.M.; Maher, S.; Li, A.; Syed, S.U.; Smith, B.; Heeren, R.M.A.; Taylor, S.; Cooks, R.G. Hand-held portable desorption atmospheric pressure chemical ionization ion source for in situ analysis of nitroaromatic explosives. Anal. Chem. 2015, 87, 10047–10055. [Google Scholar] [CrossRef] [PubMed]
- Dalgleish, J.K.; Wleklinski, M.; Shelley, J.T.; Mulligan, C.C.; Ouyang, Z.; Cooks, R.G. Arrays of low-temperature plasma probes for ambient ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Kauppila, T.J.; Syage, J.A.; Benter, T. Recent developments in atmospheric pressure photoionization-mass spectrometry. Mass Spectrom. Rev. 2017, 36, 423–449. [Google Scholar] [CrossRef] [PubMed]
- Lalli, P.M.; Sanvido, G.B.; Garcia, J.S.; Haddad, R.; Cosso, R.G.; Maia, D.R.J.; Zacca, J.J.; Maldaner, A.O.; Eberlin, M.N. Fingerprinting and aging of ink by easy ambient sonic-spray ionization mass spectrometry. Analyst 2010, 135, 745–750. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedick, P.W.; Fatigante, W.L.; Lawton, Z.E.; O’Leary, A.E.; Hall, S.E.; Bain, R.M.; Ayrton, S.T.; Ludwig, J.A.; Mulligan, C.C. A Low-Cost, Simplified Platform of Interchangeable, Ambient Ionization Sources for Rapid, Forensic Evidence Screening on Portable Mass Spectrometric Instrumentation. Instruments 2018, 2, 5. https://doi.org/10.3390/instruments2020005
Fedick PW, Fatigante WL, Lawton ZE, O’Leary AE, Hall SE, Bain RM, Ayrton ST, Ludwig JA, Mulligan CC. A Low-Cost, Simplified Platform of Interchangeable, Ambient Ionization Sources for Rapid, Forensic Evidence Screening on Portable Mass Spectrometric Instrumentation. Instruments. 2018; 2(2):5. https://doi.org/10.3390/instruments2020005
Chicago/Turabian StyleFedick, Patrick W., William L. Fatigante, Zachary E. Lawton, Adam E. O’Leary, Seth. E. Hall, Ryan M. Bain, Stephen T. Ayrton, Joseph A. Ludwig, and Christopher C. Mulligan. 2018. "A Low-Cost, Simplified Platform of Interchangeable, Ambient Ionization Sources for Rapid, Forensic Evidence Screening on Portable Mass Spectrometric Instrumentation" Instruments 2, no. 2: 5. https://doi.org/10.3390/instruments2020005