Development of Capillary Loop Convective Polymerase Chain Reaction Platform with Real-Time Fluorescence Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Equipment
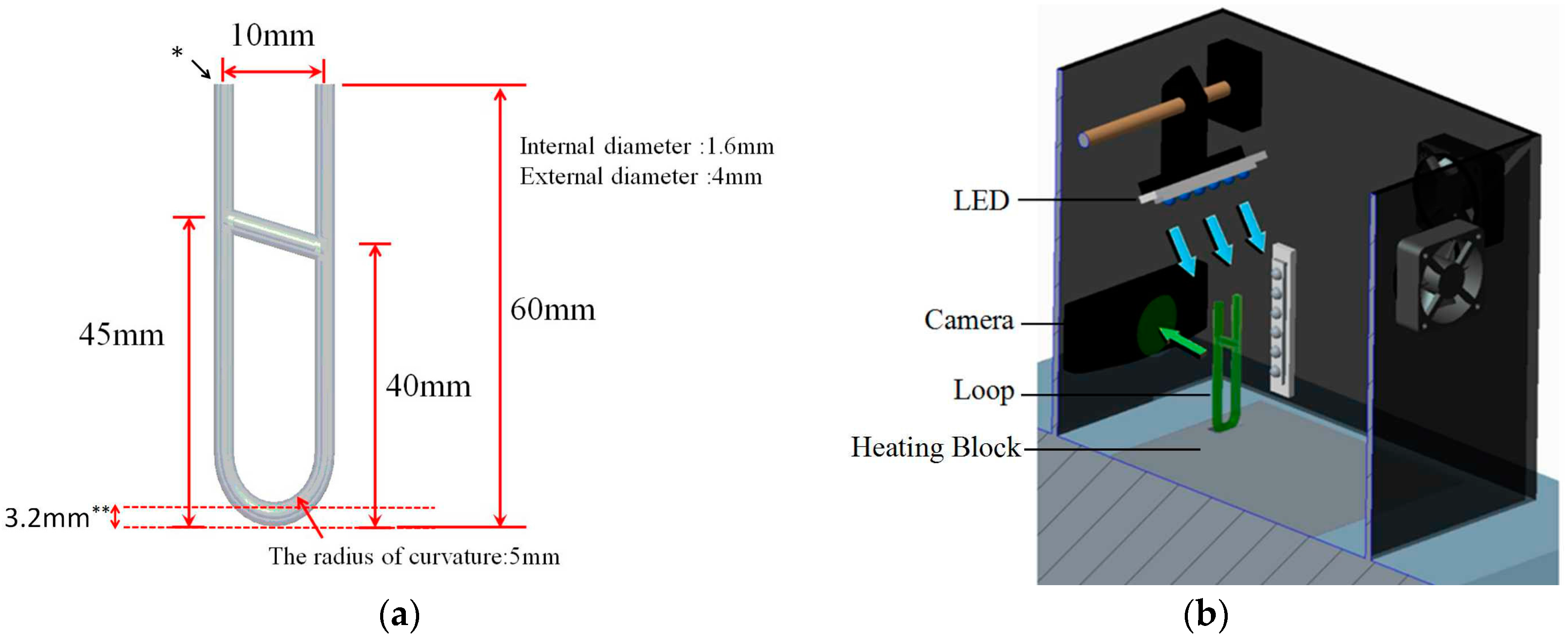
2.1.1. U-Shaped Loop Capillaries
2.1.2. Heating System
2.1.3. Fluorescence Detection
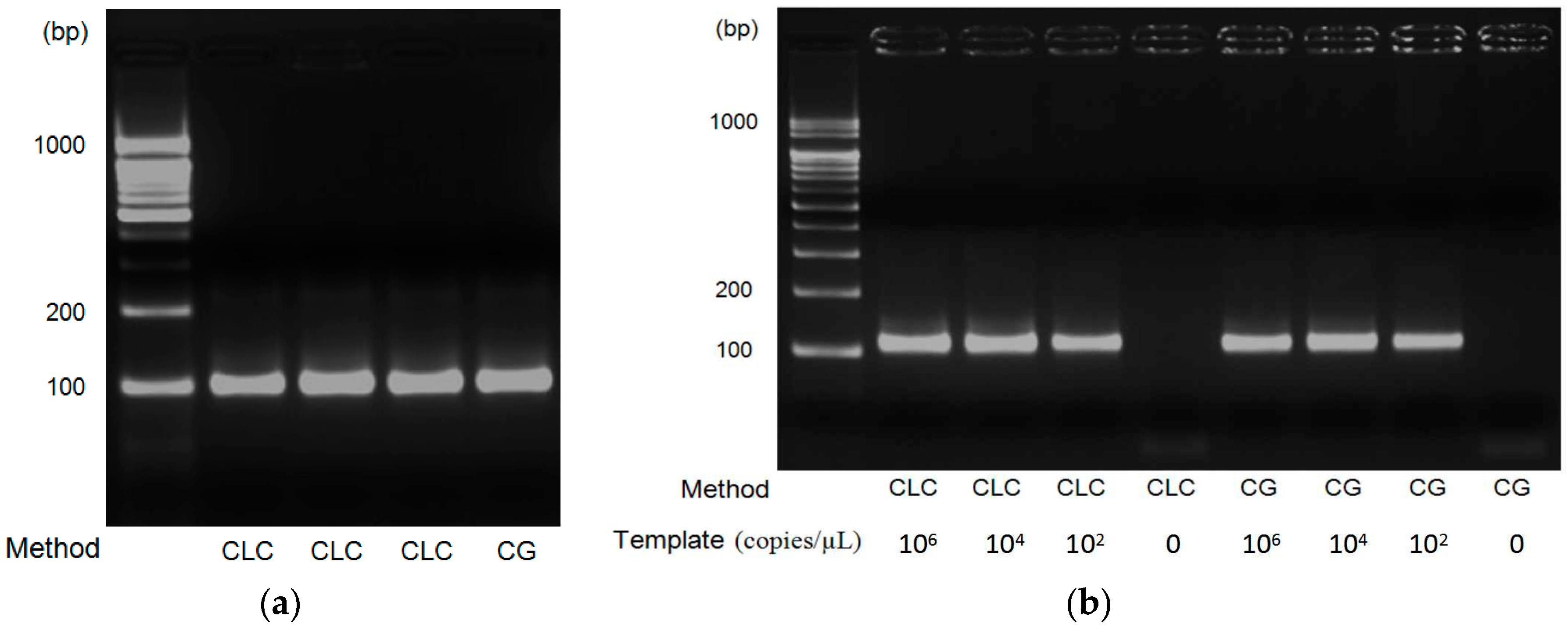
2.1.4. Reagent
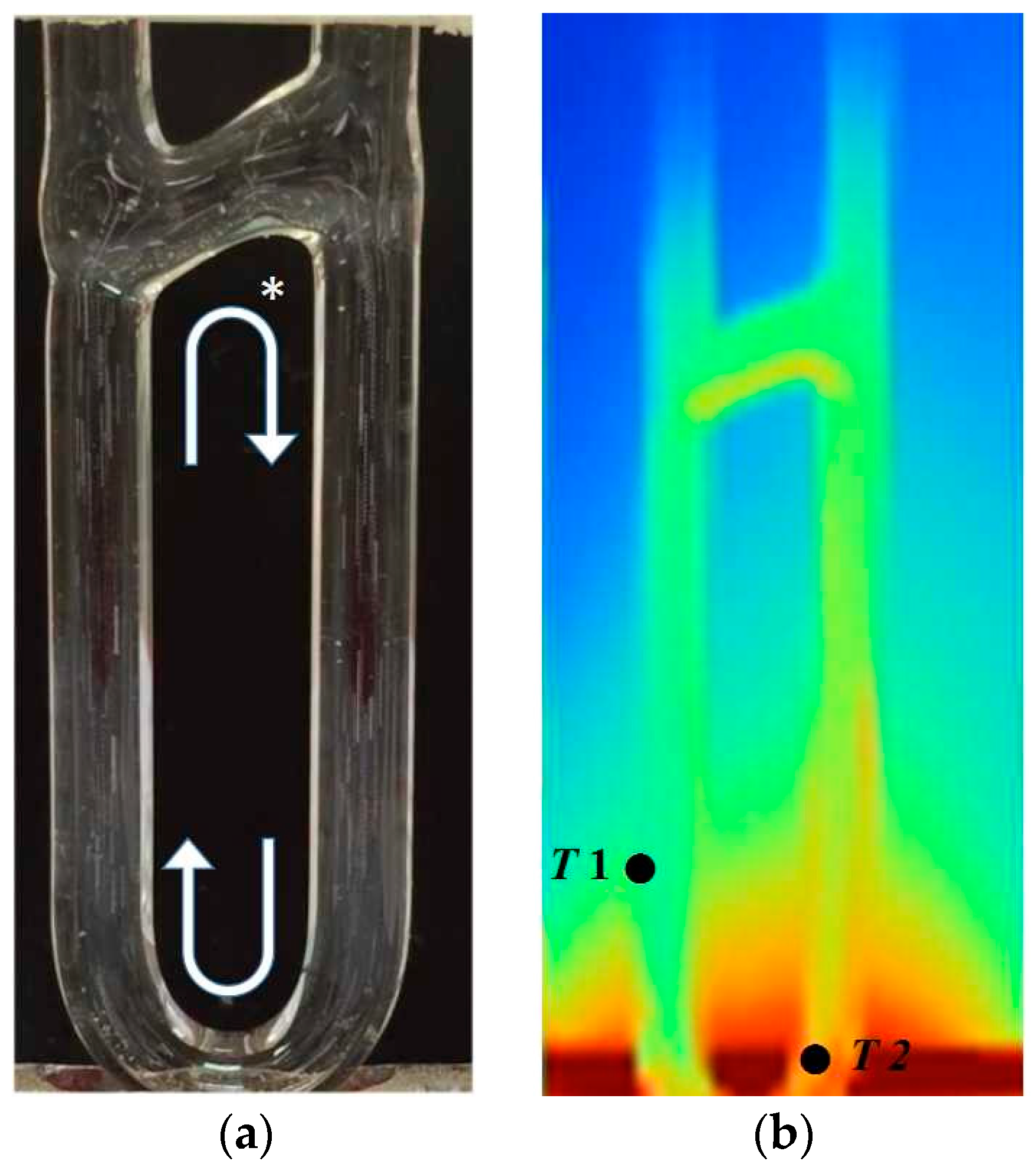
2.2. The Observation of Flow and Temperature Field
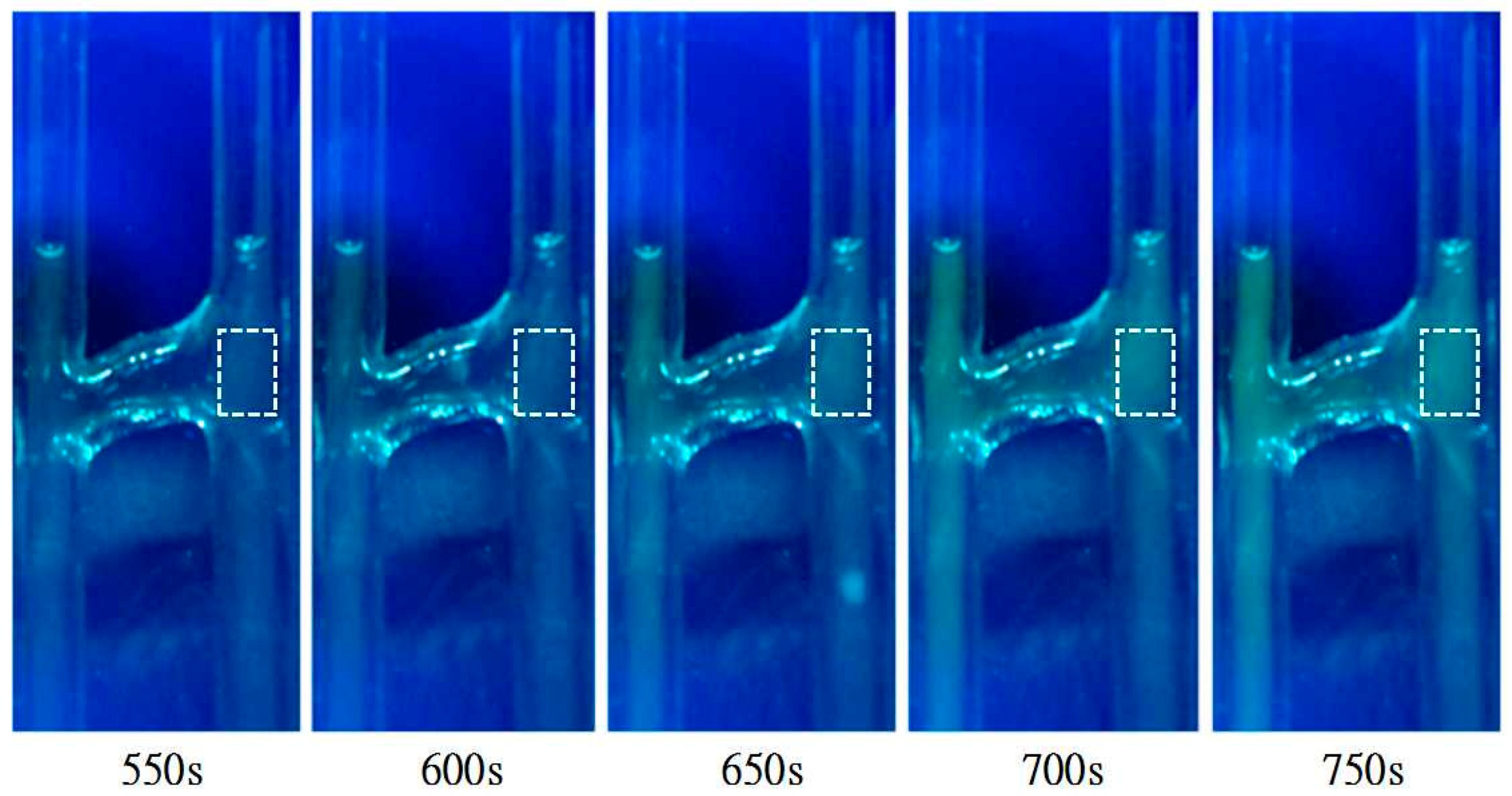
2.2.1. The Internal Flow Field
2.2.2. Temperature Measurement
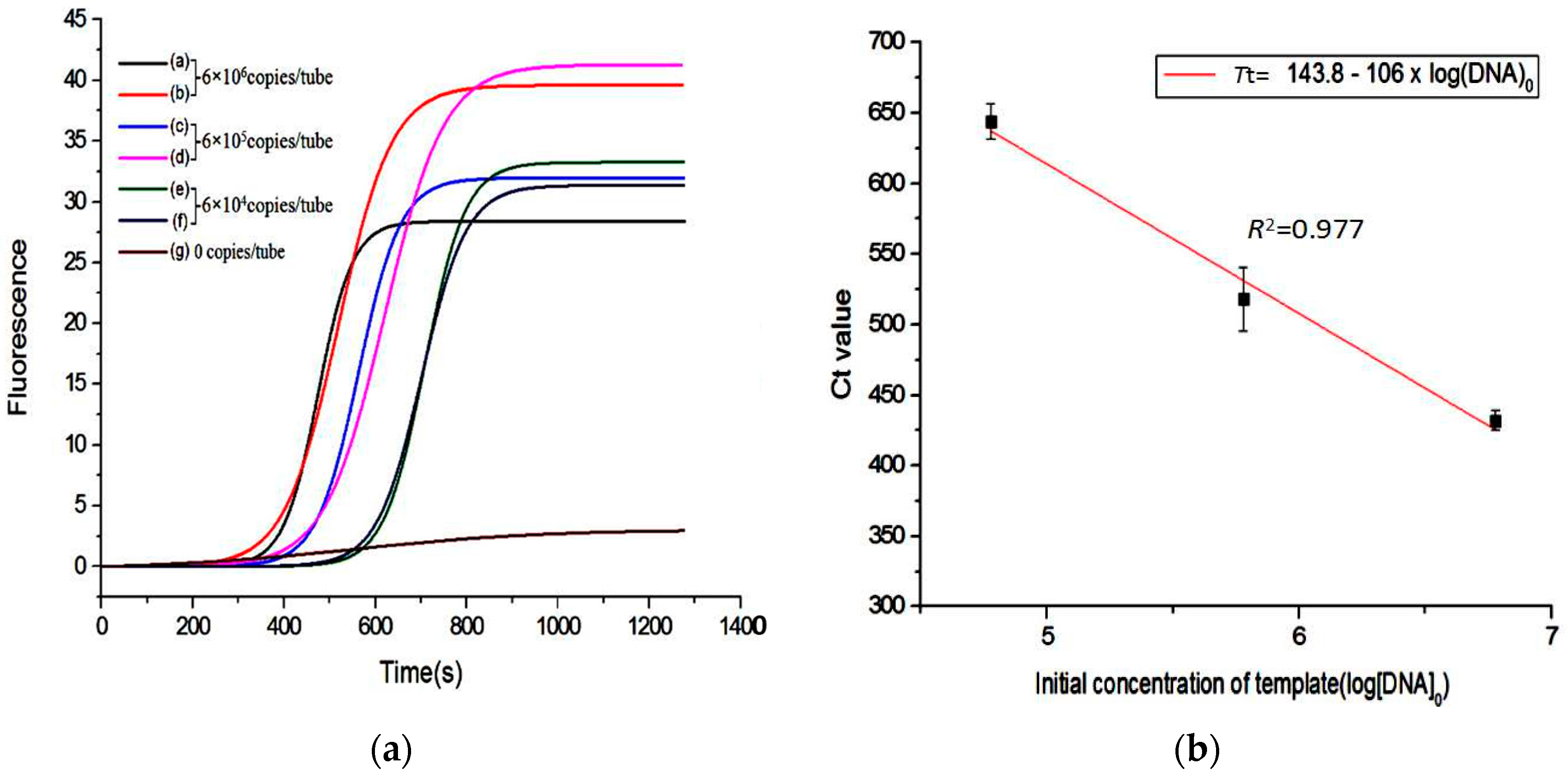
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Espinosa de los Monteros, L.E. Polymerase chain reaction (PCR) in clinical diagnosis. Review of the use of the technique. Rev. Latinoam. Microbiol. 1993, 35, 225–230. [Google Scholar] [PubMed]
- Wittbrodt, J.; Erhardt, W. An inexpensive and versatile computer-controlled PCR machine using a peltier element as a thermoelectric heat pump. Trends Genet. 1989, 5, 202–203. [Google Scholar] [CrossRef]
- Fang, T.H.; Ramalingam, N.; Xian-Dui, D.; Ngin, T.S.; Xianting, Z.; Lai Kuan, A.T.; Peng Huat, E.Y.; Hai-Qing, G. Real-time PCR microfluidic devices with concurrent electrochemical detection. Biosens. Bioelectron. 2009, 24, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- Khandurina, J.; McKnight, T.E.; Jacobson, S.C.; Waters, L.C.; Foote, R.S.; Ramsey, J.M. Integrated system for rapid PCR-based DNA analysis in microfluidic devices. Anal. Chem. 2000, 72, 2995–3000. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Kurosawa, Y.; Furui, S.; Kerman, K.; Kobayashi, M.; Rao, S.R.; Yonezawa, Y.; Nakano, K.; Hino, A.; Yamamura, S.; et al. Circumventing air bubbles in microfluidic systems and quantitative continuous-flow PCR applications. Anal. Bioanal. Chem. 2006, 386, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, J.; Ma, W.; Zheng, W. PCR microfluidic devices for DNA amplification. Biotechnol. Adv. 2006, 24, 243–284. [Google Scholar] [CrossRef] [PubMed]
- Ottesen, E.A.; Hong, J.W.; Quake, S.R.; Leadbetter, J.R. Microfluidic digital PCR enables multigene analysis of individual environmental bacteria. Science 2006, 314, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Schell, W.A.; Benton, J.L.; Smith, P.B.; Poore, M.; Rouse, J.L.; Boles, D.J.; Johnson, M.D.; Alexander, B.D.; Pamula, V.K.; Eckhardt, A.E.; et al. Evaluation of a digital microfluidic real-time PCR platform to detect DNA of candida albicans in blood. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.; Ugaz, V.M.; Burns, M.A. PCR in a rayleigh-benard convection cell. Science 2002, 298, 793. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Hassan, Y.A.; Ugaz, V.M. A pocket-sized convective PCR thermocycler. Angew. Chem. Int. Ed. Engl. 2007, 46, 4316–4319. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.P.; Chen, P.H.; Miao, M., Jr.; Kuo, L.S.; Yeh, S.H.; Chen, P.J. Rapid DNA amplification in a capillary tube by natural convection with a single isothermal heater. Biotechniques 2011, 50, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Qian, S.; Abrams, W.R.; Malamud, D.; Bau, H.H. Thermosiphon-based PCR reactor: Experiment and modeling. Anal. Chem. 2004, 76, 3707–3715. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, E.K.; Benett, W.; Stratton, P.; Richards, J.; Chen, A.; Christian, A.; Ness, K.D.; Ortega, J.; Li, L.G.; Weisgraber, T.H.; et al. Convectively driven polymerase chain reaction thermal cycler. Anal. Chem. 2004, 76, 4011–4016. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.H.; Park, S.H.; Choi, Y.H. A palmtop PCR system with a disposable polymer chip operated by the thermosiphon effect. Lab Chip 2010, 10, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lin, Y.; Wang, J.; Wang, P.; Chen, J.; Xue, M.; He, S.; Zhou, W.; Xu, F.; Liu, P.; et al. A convenient nucleic acid test on the basis of the capillary convective PCR for the on-site detection of enterovirus 71. J. Mol. Diagn. 2014, 16, 452–458. [Google Scholar] [CrossRef] [PubMed]
| Primer Name | Sequence |
|---|---|
| HBV 96bp-F | 5′-CCGGAAACTACTGTTGTTAGACGACGGGACCGAGGCAGG-3′ |
| HBV 96bp-R | 5′-GCGACGCGGCGATTGAGATCTGCGTCTGCGAGG C-3′ |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, W.-P.; Lee, C.; Hsu, Z.-J.; Lai, M.-H.; Kuo, L.-S.; Chen, P.-H. Development of Capillary Loop Convective Polymerase Chain Reaction Platform with Real-Time Fluorescence Detection. Inventions 2017, 2, 3. https://doi.org/10.3390/inventions2010003
Chou W-P, Lee C, Hsu Z-J, Lai M-H, Kuo L-S, Chen P-H. Development of Capillary Loop Convective Polymerase Chain Reaction Platform with Real-Time Fluorescence Detection. Inventions. 2017; 2(1):3. https://doi.org/10.3390/inventions2010003
Chicago/Turabian StyleChou, Wen-Pin, Chien Lee, Zong-Jyun Hsu, Mei-Hui Lai, Long-Sheng Kuo, and Ping-Hei Chen. 2017. "Development of Capillary Loop Convective Polymerase Chain Reaction Platform with Real-Time Fluorescence Detection" Inventions 2, no. 1: 3. https://doi.org/10.3390/inventions2010003






