Simulation results and relevant discussions are provided in this part. We first introduce the first stage of the computation for one specific case as well as the following stage, namely, CO2 drop flowing stage. Also introduced in the first part is the formation of a complete water film near the wall spanning from the front meniscus to the end meniscus of the drop. Secondly, flow fields within the CO2 drop and near the interface during the second stage of all computations are shown and analyzed. Lastly, we show the interfacial distribution profile of CO2 during the second stage which is subject to collaborating interphase diffusion and local convections.
3.1. CO2 Drop Preparation and Thin Film Formation
None of the commonly used geometries in microfluidics are used to produce the CO
2 drop in our simulation work. Instead, an initialized CO
2 drop by adapting one specific region that is close to the inlet of the channel with CO
2 properties (i.e., density, viscosity, diffusivity, velocity) is employed to provide the CO
2 drop (see
Figure 1,
Table 1 and
Table 2). Since interfacial tension between pure CO
2 and water is considered in the simulation that treated as a source term in the momentum equation, as shown by Equations (6)–(10), a typical cylindrical drop profile is expected. Nevertheless, a certain period of computation time is required for the interfacial tension to show effects.
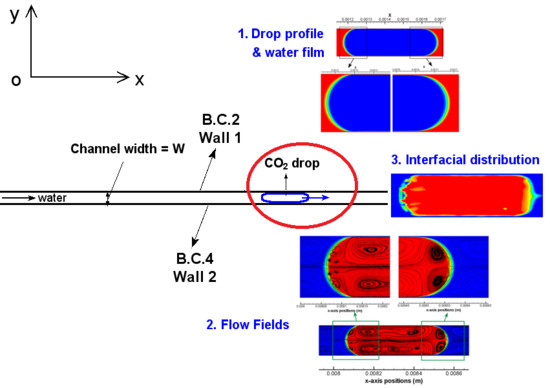
Taking Case 1 as an example, the first stage of computation for preparing a completely cylindrical profile of the drop is presented in
Figure 2a. The initialized (liquid) CO
2 drop is 0.3 mm from its left boundary to the channel inlet and assigned a velocity of 0.1 m/s which equals to that of water. The initial profile of this drop is a perfect quadrilateral. After 0.25 ms, the front boundary of the drop shows a bending effect, so does the back boundary with a slightly lower degree. As the computation proceeds, water films start to form at the front part of the drop near the channel wall which separate the drop from contact with the wall. The films continue to grow and extend to the back part of the drop. At
t = 9.2 ms (i.e., Δt
0 = 9.2 ms), a completely cylindrical profile of the CO
2 drop is shaped (see
Figure 2b). Both the front and back of the drop are of a meniscus shape. The drop is separated from contact with the channel wall by two thin water films, as shown by the zoom-in views in
Figure 2b. The thin film thickness is measured 2~2.3 µm, i.e., 1.3~1.5% of the channel width (W). Based on the water properties in
Table 2, the capillary number (Ca = µu/σ) of flowing water is 2.8 × 10
−3 which results in a film thickness of ~2% W.
Three additional frames of the liquid CO
2 drop at
t = 10.2 ms,
t = 11.2 ms, and
t = 12.2 ms are provided in
Figure 2a. The purpose is to showcase a stabilized thin film as well as stabilized front and back meniscus following the formation of these drop features at
t = 9.2 ms, which legitimates dedicating
t = 9.2 ms as an indication of the end of the CO
2 drop preparation stage.
Figure 3a shows the profile of the liquid CO
2 drop at
t = 12.2 ms, the drop length from the back to the front vertex of the meniscus is approximately equivalent to that at
t = 9.2 ms (in
Figure 2a). The thin film thickness (measured from the two bottom frames in
Figure 3a) is 2.09 ± 0.06 µm on average which agrees with the 2~2.3 µm which has been measured at
t = 9.2 ms.
Based on the above strategy to define the completion of the CO
2 drop preparation, the other five simulation cases are analyzed as well. Three key parameters at the moment of the end of the first stage, i.e., the time duration (Δt
0) of drop preparation stage, drop length (L
0) at the end of this stage, and the film thickness (t
film), are summarized (see
Table 3). As introduced above, (x
1 − x
0) is the length of the initialized drop in a quadrilateral shape. However, during the drop preparation stage, there are certain expansions of initialized drops due to the effects of interfacial tension and pressure difference across the interface. Thus, the length of the prepared CO
2 drop is generally greater than the initial one, as shown in
Table 3. Furthermore, the deviation between (x
1 − x
0) and L
0 is less significant for scCO
2 drops than for liquid CO
2 drops, which is attributed to the slightly higher interfacial tension of the scCO
2 and water.
Besides, time durations (Δt0) of the CO2 drop preparation stage are also listed. The determination of Δt0 is analogous as in simulation Case 1. Regardless of the value of Δt0, they are small compared with the expected time duration of the second stage of the computation, namely, the drop flowing stage. The comparison can be carried out by Δt0/(Lt/ − Δt0) which is well below 7.5% for all cases.
The thin film thickness in all simulation cases are measured and shown in
Table 3. They generally range from 2 µm to 2.5 µm for most cases. Uniquely, Case 6 is characterized by a minimum film thickness of 3.36 µm at the narrowest position. This increased film thickness results from a short and bullet-shaped scCO
2 drop in this case, which is rooted in an increased Ca (approaching 10
−2) due to a higher fluid velocity.
Figure 3b presents the drop profile of the prepared scCO
2 drop in Case 6. It shows a difference in curvature between the front and the back meniscus of the drop and thus showcases the bullet-like drop profile. Two circles are added in the image to demonstrate the positions at which the film thickness is measured. Note that the two positions provide the minimum film thickness in this simulation case, even though it is larger than those of the drops in the other five cases. Although Case 6 is only one case of a high flow velocity being considered, the same drop profile and increased film thickness are anticipated for even higher flow velocities resulting in capillary numbers ~10
−2.
3.2. Flow Fields within CO2 Drops and Near the Interface
Segmented flow or Taylor flow in microchannels has been placed with great expectations to enhance heat and mass transfer between phases such as gas–liquid and liquid–liquid. One important reason among others (e.g., high surface–volume ratio, short transport distance) is the convective hydrodynamics within the drop or bubble as well as those lying in both sides of the interface between phases. This feature is helpful in enhancing the transport of thermal energy and mass by collaborating with diffusion. Thus, flow fields within discrete segments and slugs particularly in the vicinity of the segment meniscus have become an interesting research topic in recent years.
CO
2 molecules are able to enter into the solvent phase (e.g., water) driven by diffusion and convection. As a consequence, the shrinkage of CO
2 drop occurs, as reported previously [
18,
19]. However, it is technically challenging and demanding to experimentally probe the flow fields within droplets. An alternative is numerical simulation.
Figure 4 shows the flow streamlines within the scCO
2 drop and in the vicinity of the interface at the drop meniscus at eight time moments during the drop flowing stage. The streamlines are plotted in a frame of reference of the CO
2 drop based on: (1) relative
x-axis velocities that are calculated by subtracting the mean flow velocity (i.e.,
= 0.11 m/s) from computed
x-axis velocities; and (2)
y-axis velocities.
Focusing on the drop region (colored by red), there are generally four toroidal flow regions and one front region that can be identified. Two large vortex regions which are x-axisymmetric are located in the center of the CO2 drop. Along x-axis, these two regions share a same flow path that is identical to the bulk flow. However, the vortex direction are converse with each other—i.e., one is clockwise and the other is counter-clockwise—which has been caused by the local shear stress at the interface due to the presence of thin films. The shear stress of the drop at the interface are opposite to the drop flow and results in the tangential flow within the drop near the interface. Besides, there are two other vortices at the back meniscus of the CO2 drop that are x-axisymmetric as well and these two small vortices are in opposite directions too. At the back meniscus, the tangential flow velocity are dominated by the interfacial tension where shear stress becomes a weak role, as quantified by the local capillary number. At the front meniscus of the drop, no vortex region is observed and flows are in an opposite direction to the bulk drop flow. Due to the presence of the two larger vortex regions, the internal flow in the front region of the drop tends to be split and squeezed towards the thin film regions.
Out of the drop region, water slug regions in the vicinity of the back and the front meniscus of the drop are characterized by toroidal flow streamlines too, which can be found in all of the images in
Figure 4. Near either the front or the back meniscus of the drop, there exist two vortex regions in the water slug which are in opposite directions and
x-axisymmetric with each other. These vortices are formed due to combined effects of the shear stress (in negative-
x direction) at the near-wall region and the net bulk flow (in positive-
x direction) along the channel centerline under a presence of a drop meniscus. This elucidation is further clarified by the contour of relative
x-axis velocities (partly based on which the flow streamlines are plotted) as shown in
Figure 5. The blue region indicates that the thin water film as well as the boundary layer region (corner regions in
Figure 5) near the wall are characterized by negative relative
x-axis velocities. On the other hand, within the water region and on the axis, positive relative
x-axis velocities are presented which indicate net bulk flows. These findings verify that shear stresses in near-wall regions are reverse to the bulk flow which further lead to the formation of flow vortices in water slugs.
According to
Figure 4, the pattern of the flow field streamline within CO
2 drop and near the interface are overall uniform among all studied time moments of simulation Case 4. It reflects that governing forces as well as their relative strengths during the drop flowing stage in the straight channel, quantified by a group of dimensionless numbers—such as Ca, Weber, and Re numbers—are overall constants in a steady hydrodynamic scenario in terms of flow velocities. The same situation in terms of a uniform pattern of flow streamlines within CO
2 drops and near the interface, therefore, can be applicable to other five simulation cases (i.e., Case 1, 2, 3, 5, and 6) as well.
In view of a uniform pattern of flow streamlines for each simulation case, one time moment roughly during the middle of the a full computation process is chosen to present the characteristic flow streamline pattern for all cases, as shown in
Figure 6. For liquid CO
2 drops as shown by
Figure 6a–c, a non-dimensional drop length (L
0/W) has a range from 2.4 to 6.3. In addition, the capillary number of these three cases has an order of magnitude of 10
−3. It is noticed that within both the front and the back meniscus of the drop there exists a pair of vortex regions, thus there are six vortex regions identified in total for these liquid CO
2 drops. It is also clear that the front pair of vortex features a larger area than the back pair of vortex. Taking Case 1 as an example,
y-axis velocities may be helpful to elucidate the vortex directions of the front vortex pair as well as the back vortex pair, as shown in
Figure 7. It can be seen that across the interface there are always reverse
y-axis velocities. In
Figure 7a, colored small regions show a positive and a negative
y-axis velocity in a neighboring domain across the meniscus. The reverse direction between these two small regions further results in a small vortex region in between of them. Note that this resulted small vortex region is still located within the CO
2 drop. This inference (i.e., small vortex region in the drop meniscus resulting from the shear between inside of the drop and outside of the drop) is also applicable to the other pair of vortexes in the front meniscus domain, as that shown in
Figure 7b. Based on observations from
Figure 5,
Figure 6 and
Figure 7, it can be concluded that flow streamlines within the drop and near the interface are mainly caused by the shear including the shear stress in the thin film and that at the drop meniscus.
According to
Figure 6, it can also be observed that there are multiple smaller vortex regions within the two larger vortexes in the middle of each Taylor liquid CO
2 drop. These smaller vortexes may have been formed due to possible uneven shears from place to place along the interface of the long drop on the
x-dimension while strong inertia always maintain as a constant on the axis of the drop. Similarly, six vortex regions are found in Case 5 (see
Figure 6d), where the scCO
2 drop has a non-dimensional length of 3.45 and a Ca number of 1.66 × 10
−3. Despite a Ca number of 2.15 × 10
−3 for Case 4, the Weber number (We = ρv
2L/σ) is approximately 5.4 × 10
−2. By comparison, the inertia is 25 times larger than the viscous force, which can be actually quantified by the Re number (Re = We/Ca). Increased inertia find its influence on whether can vortex arise in the front meniscus of the drop. An extreme condition of scCO
2 drop flowing at a very high velocity (~0.37 m/s) is provided by Case 6, as shown in
Figure 3b and
Figure 6e. The velocity increase has much more profound effects on inertia than on viscous forces, as formulated by Re number approaching 100 for the scCO
2 drop in Case 6. The strong inertia does not lead to any formations of vortex in the drop meniscus domain. Nevertheless, shear stresses still dominate in the thin film region which are able to cause vortexes in the middle of the drop, as shown in
Figure 6e.
3.3. Interfacial Profile of CO2 Drop
Mass diffusivity of liquid CO
2 and scCO
2 in water has been considered in the numerical simulation, see
Table 2. These diffusivities are determined according to the Stokes–Einstein equation. Based on Equations (3) and (4), the diffusion of CO
2 into water through the assumed “sharp interface” is considered in all simulation cases. Nevertheless, the diffusion should not be viewed as the only mechanism of CO
2 transport.
Using Case 1 as an example,
Figure 8 presents comparisons of the drop profile of volume fraction (of CO
2) and that of molar concentration of CO
2 in water at three moments (i.e., at the beginning
t = 9.2 ms, at the middle of this stage
t = 83.85 ms, and at the end of the drop flowing stage
t = 156 ms) of the drop flowing stage. Diffusion is generally weaker in mass transport compared with advection when these two mechanisms are collaborating in the transport process in the same direction. Here, the discussion of the CO
2 transport are limited to the
x-axis direction. Despite strong effects of the drop advection in the straight microchannel, the drop generally moves at the same pace as the continuously flowing water based on assigned velocities of these two phases, see
Table 2. Therefore, the CO
2 transport from its pure phase to water should first rely on diffusion (actually dissolution and diffusion, but dissolution is considered instant). At the front meniscus of the scCO
2 drop, as shown in
Figure 8b, the concentration gradient of CO
2 which drives the diffusion is evident, as shown by the color change from red, yellow, green, and eventually to blue. However, evidenced by the vortex on the water slug side, as shown in
Figure 6a, relative convections of the water flow from the channel wall towards the channel axis tend to flush the interface of the meniscus. Consequently, a distortion of the diffusional profile results from the neighboring convections. The distortion is justified by a triangular convex at the front meniscus of the drop (see
Figure 8b). At the back meniscus of the drop, the profile of CO
2 molar concentration is rather irregular. Different from at the front meniscus of the drop, convections here near the back meniscus are generally counteractive to the diffusion in terms of CO
2 transport. Specifically, they are exactly opposite to diffusion on the axis, and at a bit deviated distance (a quarter of the channel width) from the axis, the opposing effects of convections are weakened due to a reduced
x-axis velocity component and the diffusion here is less suppressed. Because of the varying relative strength of the diffusion compared with the local convections, the profile of CO
2 molar concentration at the back meniscus generally presents a wavy pattern. In addition to the profile at the meniscus, the concentration as well as the concentration gradient of CO
2 in the thin film region are almost zeros. This result is not very surprising since the convections in the thin film region are extremely rapid in transporting the diffused CO
2 though the flow near the wall is typically slow than that in the middle of the channel.
Although not shown here, the CO
2 concentration profile at the interface between the CO
2 drop and water for all other CO
2 drops in Cases 2, 3, 4, and 5 are the same based on the flow field streamline in
Figure 6. A different interfacial profile at the front meniscus of the scCO
2 drop in Case 4 may exist, for which flow streams originate from the water side and proceed into the drop through the meniscus, see
Figure 4 and
Figure 6. Since the flow streams are opposing to the diffusion in this region, the CO
2 molar concentration profile could be significantly suppressed by the reverse convections.
The other distinct CO
2 distribution profile emerges in simulation Case 6. According to
Figure 6e. There are only two vortex regions in the middle of the drop and no vortex is found in the meniscus region. This is due to the relative small
y-axis velocity compared with the
x-axis velocity. As discussed in previous sections, the neglected
y-axis velocity, despite some differences of it across the meniscus, is not likely to induce vortex inside the meniscus. In fact, relative
x-axis velocities dominate the flow stream in the central region of the scCO
2 drop. Additionally, there are very rare relative convections between water and CO
2 due to the quite uniform
x-axis velocity.
Figure 9 shows the scCO
2 drop in Case 6 at three different moments. Instead of CO
2 molar concentration, the volume fraction of CO
2 is utilized to identify the scCO
2 drop. As soon as the scCO
2 drop is prepared during the first stage of the computation, the diffusive ring surrounding the scCO
2 drop is quite thin. However, as computation continues, the diffusive ring becomes wider over the
x-dimension. Also, significant wide regions of volume fraction gradients at the front and the back meniscus of the drop can be observed, as shown in
Figure 9b,c. The unidirectional gradient indicates that diffusion may be the only effective transport mechanism for CO
2 into water as scCO
2 drop moves at a very high velocity. This diffusion-only scenario can be comprehended based on above discussions about the involved flow streamlines. As now, relative
x-axis velocities override the difference of
y-axis velocities, resulting in no formations of vortex in the meniscus region of the drop. On the other hand, the dominant
x-axis velocity is so uniform that any significant relative convections between water and CO
2 do not exist. Thus, the effects of convection become subtle in a relative sense and diffusion becomes the only effective mechanism of CO
2 transfer into water.
According to
Figure 9, the length (L
drop,x) of scCO
2 drop at these three time moments are determined based on a nominal drop defined by a 0.5 cut-off volume fraction. The decreasing length demonstrates the shrinkage of this scCO
2 drop over time. The computed drop length is first normalized by the channel width (W = 150 µm) and is then compared to the experimental result in the case Q
CO2/Q
H2O = 50/280, as presented in
Figure 10. Generally, the simulated drop length overestimate the experimental drop length. However, the decreasing tendency over time is well predicted. Note that the first drop length in the simulation originates from the drop initialization in which an initialized drop length is referred to the experimental drop length and is applied in our simulation. If the computed scCO
2 drop length is precisely predicted, it may enhance the predication further.